Abstract
OBJECTIVE
Low vitamin D levels predict the development of diabetes. This double-blind, randomized, control study in subjects with prediabetes and hypovitaminosis D evaluated whether high doses of vitamin D for 1 year affected insulin secretion, insulin sensitivity, and the development of diabetes.
RESEARCH DESIGN AND METHODS
A total of 1,551 subjects ≥40 years of age not known to have diabetes were screened with A1C levels. Subjects with A1C levels of 5.8–6.9% underwent an oral glucose tolerance test (OGTT). Subjects with prediabetes and 25-OH vitamin D (25-OHD) levels <30 ng/mL were randomized to receive weekly placebo (n = 53) or vitamin D (n = 56) with doses based on body weight and baseline 25-OHD levels. OGTTs were performed 3, 6, 9, and 12 months later. Insulin secretion and sensitivity were measured, and the proportion of subjects developing diabetes was assessed.
RESULTS
25-OHD levels rapidly rose from 22 to nearly 70 ng/mL after vitamin D supplementation with a mean weekly dose of 88,865 IU. There were no differences between the placebo and vitamin D groups regarding fasting plasma glucose, 2-h glucose, or insulin secretion and sensitivity or in the percent developing diabetes or returning to normal glucose tolerance. No subjects experienced increased serum or urinary calcium levels. At 12 months, A1C levels were significantly slightly less (0.2%) in the vitamin D group.
CONCLUSIONS
In individuals with prediabetes and hypovitaminosis D, doses of vitamin D supplementation designed to raise serum 25-OHD levels into the upper-normal range for 1 year had no effect on insulin secretion, insulin sensitivity, or the development of diabetes compared with placebo administration.
Individuals with diabetes (1–6) and prediabetes (6–8) have lower serum 25-OH vitamin D (25-OHD) levels than those without either. Furthermore, higher levels of 25-OHD are associated with lower rates of development of diabetes (9–12). Of course, associations do not prove causation; they can only generate hypotheses. A randomized intervention study is necessary to prove causation. African Americans, Hispanic/Latino Americans, American Indians, Alaska Natives, some Asian Americans, Native Hawaiians, and other Pacific Islanders are at particularly high risk for the development of type 2 diabetes (13). Diabetes in ethnic and racial minorities is increasing at an alarming rate. Although access to care or genetic variations may play a role, an intriguing biological explanation to explain this health disparity is racial differences in vitamin D levels. Racial/ethnic minorities have reduced serum 25-OHD levels, with African Americans having lower levels than Caucasians and Hispanic/Latino Americans having intermediate levels (3). To determine whether high doses of vitamin D supplementation for 1 year would affect the development of diabetes in minority subjects, we carried out a double-blind, placebo-controlled trial treating subjects with prediabetes and hypovitaminosis D with either vitamin D (designed to raise 25-OHD into the high-normal range) or placebo. We also evaluated insulin secretion and insulin sensitivity in these subjects.
RESEARCH DESIGN AND METHODS
The Charles R. Drew University institutional review board approved the study and determined that an informed consent was not necessary for individuals screened with a point-of-care A1C test because screening for diabetes often takes place at community sites, including health fairs, churches, and senior citizen centers. Informed consent was obtained for those undergoing an oral glucose tolerance test (OGTT). Separate informed consent describing the study was obtained for subjects before randomization.
The following four criteria were used to identify subjects ≥40 years of age who may have prediabetes: 1) waist circumference measured at the umbilicus of ≥40 inches in men and ≥35 inches in women, 2) family history of diabetes in first-degree relatives, 3) hypertension (either being treated or newly diagnosed at screening of ≥140/90 mmHg), and 4) history of gestational diabetes mellitus. A total of 1,551 Latino and African American individuals with one or more of these risk factors were evaluated at 37 churches, 10 health fairs, and 7 other types of community events or in the Clinical Translational Resource Center after referral from two clinics or responding to flyers distributed in the community surrounding Charles R. Drew University. All recruitment sites were located in the south-central region of Los Angeles, an area populated by Latino and African American individuals. Prospective subjects received point-of-care A1C tests using A1CNow+ (Bayer HealthCare, Tarrytown, NY). A direct comparison of 178 A1C levels in 110 individuals with and without diabetes comparing point-of-care A1C with a TOSOH ion-exchange high-performance liquid chromatography method revealed that the point-of-care method was 0.135% higher than the laboratory method (14). Individuals with A1C values <5.8% (40 mmol/mol) were considered at low risk for diabetes (15), and those with A1C ≥7.0% (53 mmol/mol) had diabetes. This study was initiated before the American Diabetes Association (ADA) recommended diagnosing diabetes with a confirmed A1C level of ≥6.5% (47.5 mmol/mol) (16). Subjects with point-of-care A1C levels of 5.8% (40 mmol/mol) through 6.9% (52 mmol/mol) consented and then underwent a 75-g OGTT to determine whether they had prediabetes. The OGTT criteria for prediabetes used in this study were a fasting plasma glucose (FPG) concentration of 110–125 mg/dL or a 2-h glucose value of 140–199 mg/dL. The World Health Organization lower limit of 110 mg/dL (17) was used instead of the ADA lower limit of 100 mg/dL (16) because reversion to normal, as defined by the WHO criterion, would be easier to demonstrate than the ADA criterion. The criterion for the diagnosis of diabetes was an FPG ≥126 mg/dL or a 2-h value on the OGTT of ≥200 mg/dL (16).
After an overnight fast, a baseline blood sample was obtained. Glucola (75 g) was ingested within 5 min, and further blood samples were obtained 30, 60, 90, and 120 min later. Glucose concentrations were measured by a glucose oxidase method in a YSI machine. If the subject met the criteria for prediabetes, a 25-OHD level was measured by high-performance liquid chromatography/tandem spectrometry in the baseline sample. If that value were <30 ng/mL, the subject was randomized by a computer-generated code to receive either weekly doses of vitamin D3 or a placebo. (Only the research pharmacist knew the allocation.) Subjects were given a blinded, identical-appearing and -smelling solution in a prefilled syringe containing the weekly dose of either placebo (medium-chain triglycerides) or vitamin D3 dissolved in the triglyceride at a concentration of 1,000 IU/drop or 35,714 IU/mL. According to the number of empty syringes returned at each visit, there was 100% compliance; i.e., none were lost, and subjects claimed that they took the contents of each one.
The goal of vitamin D supplementation was to achieve a serum 25-OHD level of 65–90 ng/mL. On the basis of a pharmacokinetic study in which three oral doses of vitamin D and its metabolites were given to healthy men (18), the initial vitamin D supplementation regimen was calculated as follows: (80 − baseline serum 25-OHD) × kg body wt × 15.7 = IU/week. After the first 20 subjects, it was apparent that the dose was too low because few subjects attained a serum 25-OHD level >65 ng/mL. Therefore, 100 was substituted for 80 in the dosing formula, which subsequently allowed most subjects to reach the desired serum 25-OHD level. If a serum 25-OHD level of ≥80 ng/mL were achieved at any visit, the dose was reduced by 25% by the research pharmacist who was unblinded during the study. The mean ± SD weekly dose of vitamin D3 was 88,865 ± 16,154 IU (range 64,731–134,446).
Additional calcium beyond the subjects’ usual intake was not given, as vitamin D is expected to enhance intestinal calcium absorption. In addition, the large Women’s Health Initiative Calcium/Vitamin D Trial showed that supplementation with 1,000 mg calcium (and 400 IU vitamin D3) did not affect the development of diabetes (19). Therefore, subjects were allowed to continue (and encouraged not to change) any current vitamin/mineral supplements that they were taking. Given that subjects were followed for 1 year, seasonal influences on the results were not a factor.
Subjects were seen monthly for the first 3 months and subsequently every 3 months for up to a year. Vitamin D levels, serum calcium, and urinary calcium-to-creatinine ratios were measured at each visit, and A1C levels were measured at baseline and at the trimonthly visits. Follow-up OGTTs were carried out at 3, 6, 9, and 12 months. Glucose concentrations were measured by the YSI glucose oxidase method and insulin levels by an enzyme-linked immunosorbent assay in the five samples from each OGTT. Insulin secretion was assessed by five methods: 1) homeostasis model assessment (HOMA) of β-cell function (20); 2) insulin area under the curve (AUC)/glucose AUC (trapezoidal rule); Stumvoll 3) first-phase insulin secretion equations; and 4) second-phase insulin secretion equations (21) [first-phase insulin secretion = 1,283 + 1.829(Ins30) – 138.7(Gluc30) + 3.772(Ins0); second-phase insulin secretion = 287 + 0.4164(Ins30) – 26.07(Gluc30) + 0.9226(Ins0), where Ins30 and Gluc30 are insulin (picomoles per liter) and glucose (millimoles per liter) levels, respectively, at 30 min of the OGTT and Ins0 is basal insulin (picomoles per liter) level]; and 5) the insulinogenic index (IGI) (22) (Ins30 − Ins0/Gluc30 – Gluc0). Insulin sensitivity was assessed by two methods: HOMA of insulin resistance (20) and the Matsuda insulin sensitivity index (ISI):
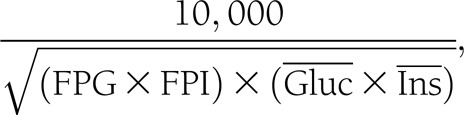 |
where FPI is fasting plasma insulin (microunits per milliliter) and FPG is measured in milligrams per deciliter and where  and
and  are the mean of glucose (milligrams per deciliter) and insulin (microunits per milliliter) levels obtained during the OGTT (23). An oral disposition index was assessed by two methods: insulin secretion-sensitivity index-2 (ISSA-2) (ISI × insulin AUC/glucose AUC) (24) and IGI × 1/FPI as previously described by Utzschneider et al. (25) (which predicted the development of diabetes over 10 years in a Japanese population).
are the mean of glucose (milligrams per deciliter) and insulin (microunits per milliliter) levels obtained during the OGTT (23). An oral disposition index was assessed by two methods: insulin secretion-sensitivity index-2 (ISSA-2) (ISI × insulin AUC/glucose AUC) (24) and IGI × 1/FPI as previously described by Utzschneider et al. (25) (which predicted the development of diabetes over 10 years in a Japanese population).
No studies could be found describing the effect of vitamin D supplementation on an OGTT in subjects with prediabetes to use for a power calculation. The effect of vitamin D supplementation on insulin secretion, insulin sensitivity, and the oral disposition index was analyzed by two-way repeated-measures ANOVA. The primary analysis was based on a modified intention-to-treat (mITT) principle. The last-value-carried-forward paradigm was used to fill in missing values in order to perform the mITT analysis that was carried out for all subjects who had at least the 3-month OGTT after randomization. The baseline FPG, 2-h glucose, and A1C levels were analyzed by Student t test for unpaired means. The baseline categorical demographics and the effect on changing the OGTT diagnostic classification (diabetes, prediabetes, and normal) were analyzed by the χ2 test for homogeneity. Significance was determined at P < 0.05 (two tailed). Two sensitivity analyses were carried out to determine whether the mITT failed to detect a change in outcomes; a per-protocol analysis for the 99 subjects who completed 1 year of placebo or vitamin D supplementation and an mITT analysis on the subset of 28 subjects whose baseline vitamin D levels were <20 ng/mL.
RESULTS
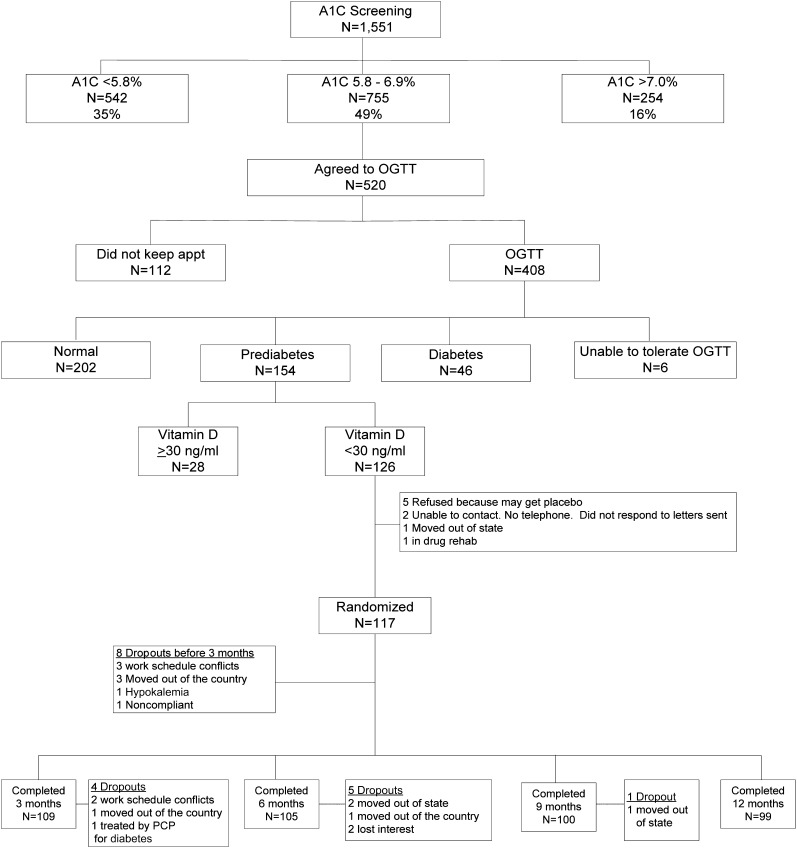
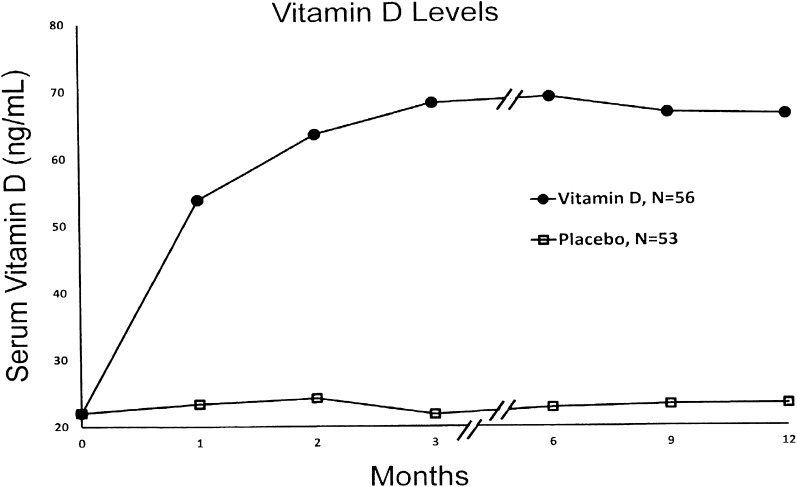
The results of screening, subsequent OGTTs, serum 25-OHD levels, randomization, and completion rates are shown in Fig. 1. One hundred and seventeen subjects with prediabetes and hypovitaminosis D were randomized with 8 dropping out before the 3-month OGTT leaving 53 in the placebo group and 56 receiving vitamin D for an ITT analysis. The baseline demographic (age, sex, ethnicity/race, and BMI), glycemic (FPG, 2-h OGTT glucose, and A1C), and 25-OHD status were similar in the two groups (Supplementary Table 1). Serum 25-OHD levels are shown in Fig. 2. There was a rapid rise in those receiving vitamin D, achieving a mean serum 25-OHD level of nearly 70 ng/mL by 3 months (which was sustained), and no change in those receiving placebo.
Figure 1.
Flow diagram of the study. appt, appointment; PCP, primary care physician; rehab, rehabilitation.
Figure 2.
Serum 25-OHD levels.
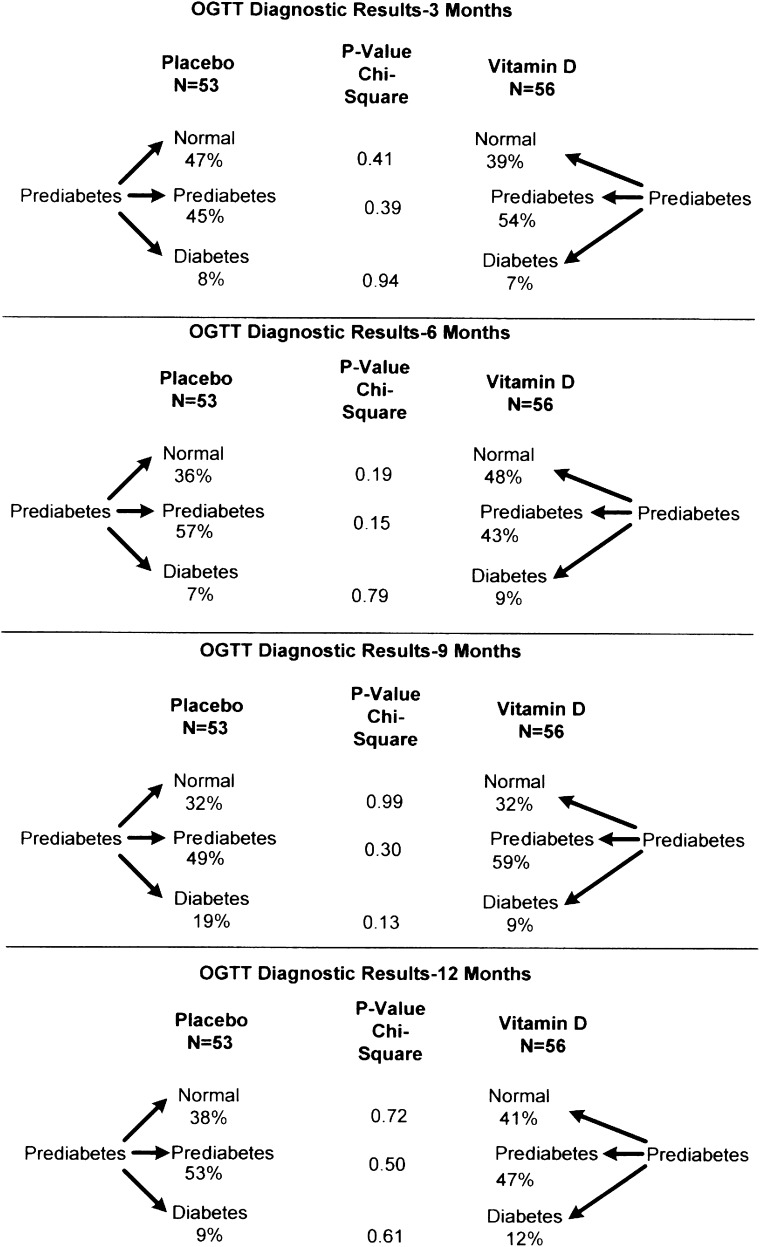
Changes in the glycemic indices and BMIs are shown in Table 1. There were no differences in FPG, 2-h glucose, or BMI between the two groups during the year after randomization. A1C levels at 1 year were slightly (0.2%), but significantly, less in the vitamin D group .There were also no differences in the five indices of insulin secretion, two indices of insulin sensitivity, or two measures of the oral disposition index during the year of placebo or vitamin D supplementation between the two groups in the mITT analysis. These results remained nonsignificant in the per-protocol analysis comparing 47 subjects in the placebo group and 52 receiving vitamin D for the entire year. Likewise, in subjects with baseline serum 25-OHD levels <20 ng/mL, the results were not significantly different between 13 subjects in the placebo group (baseline serum 25-OHD levels 15.5 ± 3.4 ng/mL) and 15 subjects receiving vitamin D (baseline serum 25-OHD levels 16.0 ± 2.0 ng/mL). Likewise, there were no differences in those developing diabetes or whose OGTTs reverted to normal at 3, 6, 9, and 12 months (Fig. 3).
Table 1.
Effect of vitamin D on glycemic indices and BMI
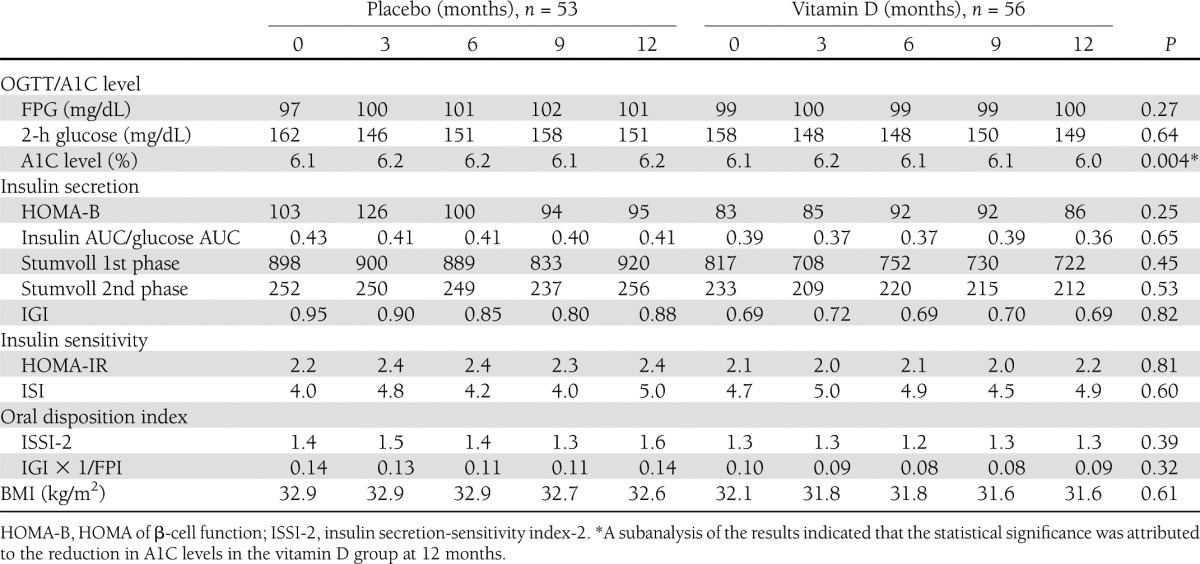
Figure 3.
Percent of subjects who developed diabetes or reverted to normal. OGTT diagnostic results at 3, 6, 9, and 12 months.
It is noteworthy that no subject receiving vitamin D manifested hypercalcemia or increased urinary calcium measured in a morning urine sample. With the attained sample size in each group, there was a >95% power to detect a 1-SD change in BMI, FPG, 2-h glucose, or A1C levels between the study groups.
CONCLUSIONS
The literature surrounding the association of vitamin D levels and glycemia is conflicting. Many studies found a significant positive association between insulin sensitivity and serum 25-OHD levels, whereas a few found no such association. Fewer studies evaluated insulin secretion with the majority unable to show a positive association (Supplementary Table 2). Similarly, there are conflicting data on the effects of vitamin D supplementation on glycemia (Supplementary Table 3). Most studies showed no effect on insulin sensitivity, while a few showed an increase. Insulin secretion was unaffected by vitamin D supplementation in the majority of studies, increased in a substantial minority, and decreased in one. Vitamin D supplementation with 700 (26) or 400 IU (19) per day for 3 and 7 years, respectively, did not affect the development of diabetes. In patients with type 2 diabetes, fructosamine levels were no different 1 month after an intramuscular injection of either 300,000 IU of vitamin D or distilled water (27). Similarly, fructosamine and A1C levels were no different in type 2 diabetic patients receiving either 40,000 IU per week or a placebo for 6 months (28).
The lack of an effect of vitamin D supplementation in the published studies could be due to one or more of the following: too small a dose of vitamin D, too short a period of time to show an effect, or including subjects with normal vitamin D levels. None of these can explain the negative results of this study in which subjects with hypovitaminosis D received large amounts of vitamin D (mean weekly dose of 88,865 IU) for 1 year and rapidly achieved serum 25-OHD levels of nearly 70 ng/mL. There were no significant differences between the placebo and vitamin D supplementation groups with regard to five indices of insulin secretion, two indices of insulin sensitivity, or two oral disposition indexes or in the proportion of subjects with prediabetes developing diabetes or whose OGTT returned to normal.
There are three potential limitations to this study. The number of subjects is relatively small, but the results in the two groups are so similar that it is doubtful that more subjects would have found any significant differences. The percent of subjects developing diabetes after 1 year in the placebo and vitamin D groups of 9 and 12%, respectively, was similar to the 11% yearly rate of development of diabetes in the placebo arm of the Diabetes Prevention Program (DPP) (29), strongly suggesting that the natural history of the development of diabetes was seen in both groups. Some might argue that 1 year of vitamin D supplementation is not enough time to see a protective effect against the development of diabetes. However, compared with the placebo group in the DPP, there was a marked difference in the development of diabetes at 1 year in persons with prediabetes exposed to intensive lifestyle change (29), metformin (29), or troglitazine (30). Finally, the OGTTs in the current study were not repeated and the literature shows that a repeat OGTT within 2–6 weeks in individuals with IGT confirms that diagnosis in only half (31–33). However, in the clinical setting, the ADA does not recommend confirmation of IGT (16) and the variability of the OGTT should have affected both groups equally.
Our study was carried out in ethnic minorities—a group with high rates of hypovitaminosis D and prediabetes/diabetes. We showed that vitamin D supplementation did not affect glycemic parameters in this group. Although a Caucasian population was not studied, we expect generalizability to that population.
In conclusion, although hope had been expressed that vitamin D supplementation might at least delay the development of diabetes (34), this was not the case. High doses of vitamin D for 1 year in Latinos (mostly) and African Americans with prediabetes and hypovitamosis D did not affect insulin secretion, insulin sensitivity, the oral disposition index, or the proportion of subjects who developed diabetes or whose OGTT returned to normal. As Thomas Huxley so aptly stated, “The great tragedy of science—the slaying of a beautiful hypothesis by an ugly fact.”
Supplementary Material
Acknowledgments
This study was wholly supported by American Diabetes Association Grant 1-09CR-15. M.B.D. also received partial salary support from National Institutes of Health–National Institute on Minority Health and Health Disparities Grant U54MD007598 (Accelerating Excellence in Translational Science [AXIS] Grant, formerly U54 RR026138), and T.C.F. received salary support from R24DA017298 and S06GM068510.
Bayer HealthCare, LLC/Bayer Diabetes Care supplied the A1CNow+ assay kits, and D Drops Company in Woodbridge, Ontario, Canada, provided vitamin D and the placebo. No other potential conflicts of interest relevant to this article were reported.
M.B.D. designed the study, researched the literature, and wrote the manuscript. P.D. carried out the study and established the screening approach, M.L.L. decided on the appropriate statistical analyses and analyzed the data. T.C.F. worked out the vitamin D dosing, researched the literature, and helped write the manuscript. All authors reviewed and edited the manuscript. M.B.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank Indrani Sinha-Hikim, PhD, of the Technology Core of the AXIS Grant for measuring insulin levels, and the staff of the Clinical Translational Resource Center. The authors also thank Troy and Teresa Campbell for recruiting subjects; Maria Navar, NP, and Phyllis Kirkpatrick for both recruiting and screening subjects; and Elizabeth Montiel, Jessica Fermin, Christine Turner, PA, Lianna Navar, Joselyn Munoz, and Peter Villarreal for screening subjects.
Footnotes
Clinical trial reg. no. NCT00876928, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1204/-/DC1.
References
- 1.Scragg R, Sowers MF, Bell C, Third National Health and Nutrition Examination Survey Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2813–2818 [DOI] [PubMed] [Google Scholar]
- 2.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care 2006;29:722–724 [DOI] [PubMed] [Google Scholar]
- 3.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2007;167:1159–1165 [DOI] [PubMed] [Google Scholar]
- 4.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008;168:1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalgård C, Petersen MS, Weihe P, Grandjean P. Vitamin D status in relation to glucose metabolism and type 2 diabetes in septuagenarians. Diabetes Care 2011;34:1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta AK, Brashear MM, Johnson WD. Prediabetes and prehypertension in healthy adults are associated with low vitamin D levels. Diabetes Care 2011;34:658–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinelli NR, Jaber LA, Brown MB, Herman WH. Serum 25-hydroxy vitamin D and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care 2010;33:1373–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankar A, Sabanayagam C, Kalidindi S. Serum 25-hydroxyvitamin D levels and prediabetes among subjects free of diabetes. Diabetes Care 2011;34:1114–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knekt P, Laaksonen M, Mattila C, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 2008;19:666–671 [DOI] [PubMed] [Google Scholar]
- 10.Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care 2010;33:2021–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagnon C, Lu ZX, Magliano DJ, et al. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: results from a national, population-based prospective study (the Australian Diabetes, Obesity and Lifestyle study). Diabetes Care 2011;34:1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pittas AG, Nelson J, Mitri J, et al. Diabetes Prevention Program Research Group Plasma 25-hydroxyvitamin D and progression to diabetes in patients at risk for diabetes: an ancillary analysis in the Diabetes Prevention Program. Diabetes Care 2012;35:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Diabetes Data Group. Diabetes in America 2nd ed. National Institutes of Health, Bethesda, MD, 1995 (NIH publ. no.) [Google Scholar]
- 14.Chang A, Frank J, Knaebel J, Fullam J, Pardo S, Simmons DA. Evaluation of an over-the-counter glycated hemoglobin (A1C) test kit. J Diabetes Sci Tech 2010;4:1495–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buell C, Kermah D, Davidson MB. Utility of A1C for diabetes screening in the 1999–2004 NHANES population. Diabetes Care 2007;30:2233–2235 [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl 1.):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization Definitions, Diagnosis, and Classification of Diabetes Mellitus and its Complication: Report of a WHO consultation. Part 1. Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Org., 1999 [Google Scholar]
- 18.Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int 1998;8:222–230 [DOI] [PubMed] [Google Scholar]
- 19.de Boer IH, Tinker LF, Connelly S, et al. Women’s Health Initiative Investigators Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care 2008;31:701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 21.Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000;23:295–301 [DOI] [PubMed] [Google Scholar]
- 22.Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE; American Diabetes Association GENNID Study Group. B-Cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes 2002;51:2170–2178 [DOI] [PubMed]
- 23.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 24.Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring) 2008;16:1901–1907 [DOI] [PubMed] [Google Scholar]
- 25.Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007;30:980–986 [DOI] [PubMed] [Google Scholar]
- 27.Parekh D, Sarathi V, Shivane VK, Bandgar TR, Menon PS, Shah NS. Pilot study to evaluate the effect of short-term improvement in vitamin D status on glucose tolerance in patients with type 2 diabetes mellitus. Endocr Pract 2010;16:600–608 [DOI] [PubMed] [Google Scholar]
- 28.Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr 2009;48:349–354 [DOI] [PubMed] [Google Scholar]
- 29.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowler WC, Hamman RF, Edelstein SL, et al. Diabetes Prevention Program Research Group Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 2005;54:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mooy JM, Grootenhuis PA, de Vries H, et al. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia 1996;39:298–305 [DOI] [PubMed] [Google Scholar]
- 32.Ko GTC, Chan JCN, Woo J, et al. The reproducibility and usefulness of the oral glucose tolerance test in screening for diabetes and other cardiovascular risk factors. Ann Clin Biochem 1998;35:62–67 [DOI] [PubMed] [Google Scholar]
- 33.Brohall G, Behre CJ, Hulthe J, Wikstrand J, Fagerberg B. Prevalence of diabetes and impaired glucose tolerance in 64-year-old Swedish women: experiences of using repeated oral glucose tolerance tests. Diabetes Care 2006;29:363–367 [DOI] [PubMed] [Google Scholar]
- 34.Friedman TC. Vitamin D supplementation to prevent the progression of prediabetes to diabetes: getting closer to a recommendation. Transl Res 2011;158:273–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.