Abstract
OBJECTIVE
To determine if partial wound closure surrogate markers proposed for neuropathic, small diabetic foot ulcers (DFUs) can be extended to advanced lesions and if the development of granulation tissue can be used to predict complete healing.
RESEARCH DESIGN AND METHODS
Data from two multicenter, double-blind, randomized clinical trials (one of them placebo controlled) that used intralesional recombinant human epidermal growth factor (rhEGF) to promote granulation and healing were used. For confirmation in a larger sample from common clinical practice, the results of an active postmarketing surveillance of rhEGF treatment of DFUs in 60 healthcare units was included. The surrogates evaluated were percent area change, log healing rate, ratio of log areas, and percent of granulation tissue covering the wound area. The tests used were surrogate final end point correlation, receiver operating characteristic curves to discriminate healers from nonhealers, validation tests using logistic regression models, and the proportion-mediated estimation.
RESULTS
Two weeks >50% granulation, end of treatment >75% granulation, and 16.1% area change showed significant predictive value (>70% correct classification) for final wound closure. The granulation-based variables fulfilled the criterion that the effect of rhEGF treatment on wound closure was mediated by the surrogate.
CONCLUSIONS
This work provides the first evidence for the use of granulation tissue development as a predictor of wound healing in advanced DFUs. These results can be useful for clinical trial design, particularly during the exploratory phase of new products.
Drug development is a continuous and complicated decision-making process. The use of surrogate end points provides a powerful tool to accelerate and make decisions more efficient. It reduces the cost and duration of clinical trials during the exploratory phase of drug development and can be accepted by regulatory agencies (1). Specifically for diseases with risk of death or severe incapacity, this issue acquires vital importance. Surrogate end points can be measured earlier and more frequently, so the adherence to treatment is greater and less influenced by concomitant interventions than the final outcome.
Some authors (2–4) have demonstrated that variables based on early wound area reduction (i.e., after 4 weeks of treatment), such as the percent change in area, log healing rate, or log area ratio, can be used as surrogate end points for later complete wound re-epithelization (i.e., at 12 or 20 weeks of care) in patients that receive different treatments for diabetic foot ulcers (DFUs). However, these analyses were performed for neuropathic, relatively small (median size <5 cm2) ulcers. It is not clear whether they can be extrapolated to more advanced, larger lesions, including ischemic ones. In a more complicated scenario (large, chronic, nonischemic diabetic foot wounds after partial foot amputation), the percent of wound area reduction at 1 and 4 weeks was proposed as predictor of healing at 16 weeks (5). Advanced DFUs that fall in Wagner classification grades 3 and 4 (6,7), sometimes ischemic, and >10 cm2 are an unmet medical need that frequently lead to limb amputation (8). For these advanced ulcers, the study of surrogate end points is more difficult because of complications, possible evolution to amputation, and the scarcity of effective treatments.
The use of surrogate markers in this particular area, due to the large and complex process to achieve complete healing, is recognized (9) as well as the necessity to facilitate the product development cycle, specifying in each case the true efficacy on the treated wounds and adjusting the regulatory requirements. These authors identified some potential intermediate end points, such as the development of granulation tissue, readiness for grafting, reduction of colonization, and others, depending on the wound therapy evaluated.
Granulation has been used as an outcome in clinical trials on DFUs (10–15) since it is part of the healing process and should necessarily precede the final lesion closure. Besides, granulation over the ulcer area permits skin grafting to attain final healing, so some patients do not reach complete wound closure to be evaluated. In a randomized, placebo-controlled trial, recombinant human epidermal growth factor (rhEGF) was applied intralesionally in Wagner 3 or 4 DFUs up to complete wound granulation or a maximum of 8 weeks. In these subjects, a highly significant correlation between complete healing and >50% granulation at 2 weeks of treatment or >75% granulation at the end of treatment (EOT) (median, 5 weeks) was found (16). This granulation-healing correlation suggested that these variables could be used as surrogates. Multivariate analyses in this trial identified a significant influence of rhEGF treatment, ulcer etiology (neuropathic or ischemic), and initial size on wound closure.
The purpose of this work was to determine if the surrogate markers proposed for the neuropathic, small DFUs can be extended to advanced, Wagner grade 3 and 4 ulcers, including ischemic ones, and if the development of granulation tissue can be used to predict complete healing.
RESEARCH DESIGN AND METHODS
Data from two multicenter, double-blind, randomized clinical trials were used to test the surrogate variables proposed for neuropathic DFUs and to explore the correlation between granulation tissue development (the main efficacy variable of the studies) and complete healing during follow-up for 1 year after the EOT (median time to complete closure, 15 weeks; 25th percentile, 9.6 weeks; 75th percentile, 21.4 weeks). In the first study, a dose-exploratory trial in five hospitals (13), patients were randomized to receive intralesional injections of rhEGF at 75 (23 subjects) or 25 µg (18 subjects), three times per week. In the second study, a confirmatory trial in 20 hospitals (16), 53 subjects were randomized to rhEGF 75 µg, 48 to rhEGF 25 µg, and 48 to a placebo group. Each patient had only one lesion, so the total number of ulcers included in the analyses was 190. For confirmation in a larger sample from common clinical practice, the results of an active postmarketing surveillance of rhEGF treatment in 1,440 DFUs in 60 healthcare units (41 hospitals and 19 primary care polyclinics) were taken into account (unpublished data). In this series, the 25- and 75-µg dose levels were used, according to the product label (17). In all series, rhEGF treatment was administered up to complete granulation or a maximum of 8 weeks.
Diabetic patients (type 1 or 2), both sexes, >18 years of age were included in the trials, with Wagner grade 3 or 4 DFUs >1 cm2. Intralesional rhEGF was administered adjuvant to standard care, which included thorough debridement (sometimes minor amputation), moist dressing, pressure offloading, and antibiotics, if necessary. Patients with chronic, uncontrolled illness, psychiatric or neurologic diseases that could impair proper reasoning for consent, or presence or suspicion of neoplasia or who were pregnant or breastfeeding were excluded.
Analyses
Variables proposed by Margolis et al. (2) as surrogate markers of complete healing were generated: percent change in area, log healing rate, and ratio of log areas at the 2nd week and EOT (median, 5 weeks).
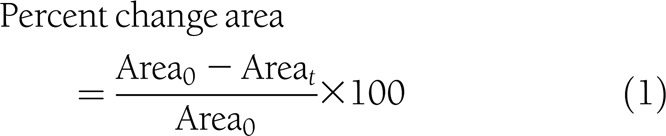 |
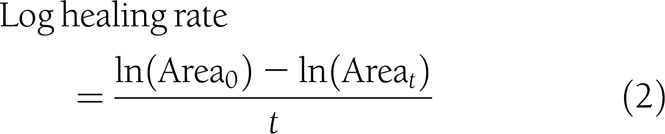 |
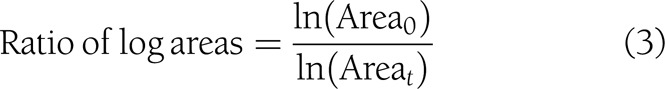 |
Additionally the variable
 |
was taken into account.
Considering that the median treatment duration was 5 weeks, and that there was no area evaluation at 4 weeks for most of the patients (>50% missing values), the EOT data were taken as the comparison points with the 4-week area change proposed by other authors (2–4).
Ulcer areas and percent granulation were measured by planimetry from manual tracing on a transparent grid sheet, using a validated portable wound measurement device (Visitrack; Smith & Nephew, London, U.K.) (18). For comparability with the results in Margolis et al. (2), the variables defined in Eqs. 2 and 3 were calculated using the area in mm2 and t in days.
Statistical analysis
Descriptive analysis.
Continuous variables are expressed as mean ± SD or median (25th and 75th percentiles). Categorical variables are given as absolute values and percentages. Comparison between healed and nonhealed was assessed by the χ2 test for categorical variables and Mann-Whitney U test for continuous variables. Since missing data can constitute deviations from the intention-to-treat principle, clinical trial analyses require the use of imputation (19). For the purpose of this work, 18 missing data at the 2nd week (9.5%) were imputed using the last observation carried forward (LOCF) or the average between the two contiguous evaluations that included the missing one.
Performance of the classification with surrogate end points.
The area under the receiver operating characteristic (ROC) curve was calculated (20) for each candidate surrogate variable to evaluate its capacity and quality of discrimination among individuals. Cutoff points were generated in order to maximize both sensitivity (probability to detect a positive result if the outcome is positive) and specificity (probability to detect a negative result if the outcome is negative). The candidate surrogates were then codified, and their association with the final outcome (complete ulcer closure) was assessed using the sensitivity, specificity, positive predictive values (PV+, conditional probability to have a positive result if the test was positive), negative PVs (PV−, conditional probability to have a negative result if the test was negative), and the correct classification index (percent of individuals correctly classified), also named validity index. The corresponding 95% CIs were estimated for all of them.
Test validation and sensitivity were performed only for the placebo-controlled trial (16) for the sake of homogeneity and balance of the samples compared.
To quantify the contribution of the candidate surrogates to the final outcome, the product coefficient (PC) was estimated (21,22), considering these variables as continuous. This is one of the methods to assess mediation in the case of binary-dependent outcome that treats it as the product of two regression coefficients: the regression of the independent treatment variable on the mediator (αt in Eq. 6, below) and the partial regression coefficient of the independent treatment variable on the final outcome adjusted for the mediator (γs in Eq. 7, below). In order to estimate the PC, the following variables were taken into consideration. Let Y, a binary variable, be the outcome “closure” (1, yes; 0, no), X the baseline covariates X1 (etiology: neuropathic and ischemic) and X2 (initial area), T the intervention status (1 for rhEGF treatment and 0 for placebo), and S the granulation tissue covering the wound area (at 2nd week or at the EOT). The following models were adjusted:
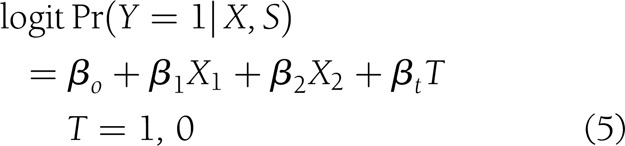 |
which measures the effect of treatment on healing without the influence of the surrogate,
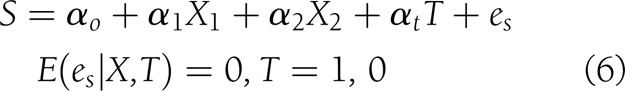 |
which measures the effect of treatment on the surrogate, taking into account the same covariates, and
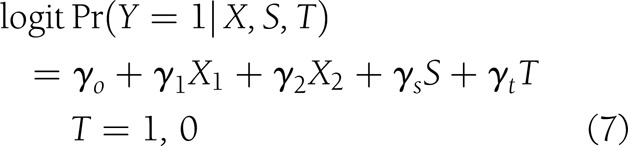 |
which measures the effect of treatment on wound closure, mediated by the surrogate, taking into account the covariates.
The PC estimator 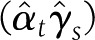 and the proportion mediated
and the proportion mediated 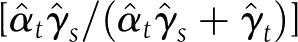 and its 95% CI (21) were used to quantify the contribution of the surrogate (S: granulation and area change variables) on ulcer closure.
and its 95% CI (21) were used to quantify the contribution of the surrogate (S: granulation and area change variables) on ulcer closure.
The association with some baseline influencing factors between healing and candidate surrogate variables was estimated using the odds ratio and the 95% CIs in binary logistic regression.
Confirmation was performed only for the granulation variable percent of granulation tissue covering the ulcer area (>75% or not), since during medical practice it was not possible to standardize a precise measurement of the wound area and partial percent of granulation.
Statistical analyses were performed using SPSS version 15 and Epidat softwares for Windows.
RESULTS
A total of 190 patients were included in the clinical trials analyzed. The median age was >60 years, median ulcer size was 22 cm2 (75% >10 cm2), lesions were 57% ischemic, and Wagner classification grades 3 and 4 were 73.7 and 26.3%, respectively. Treatments lasted 5 weeks (median duration). All demographic and baseline variables were similar among treatment groups. They are shown in detail in Supplementary Table 1.
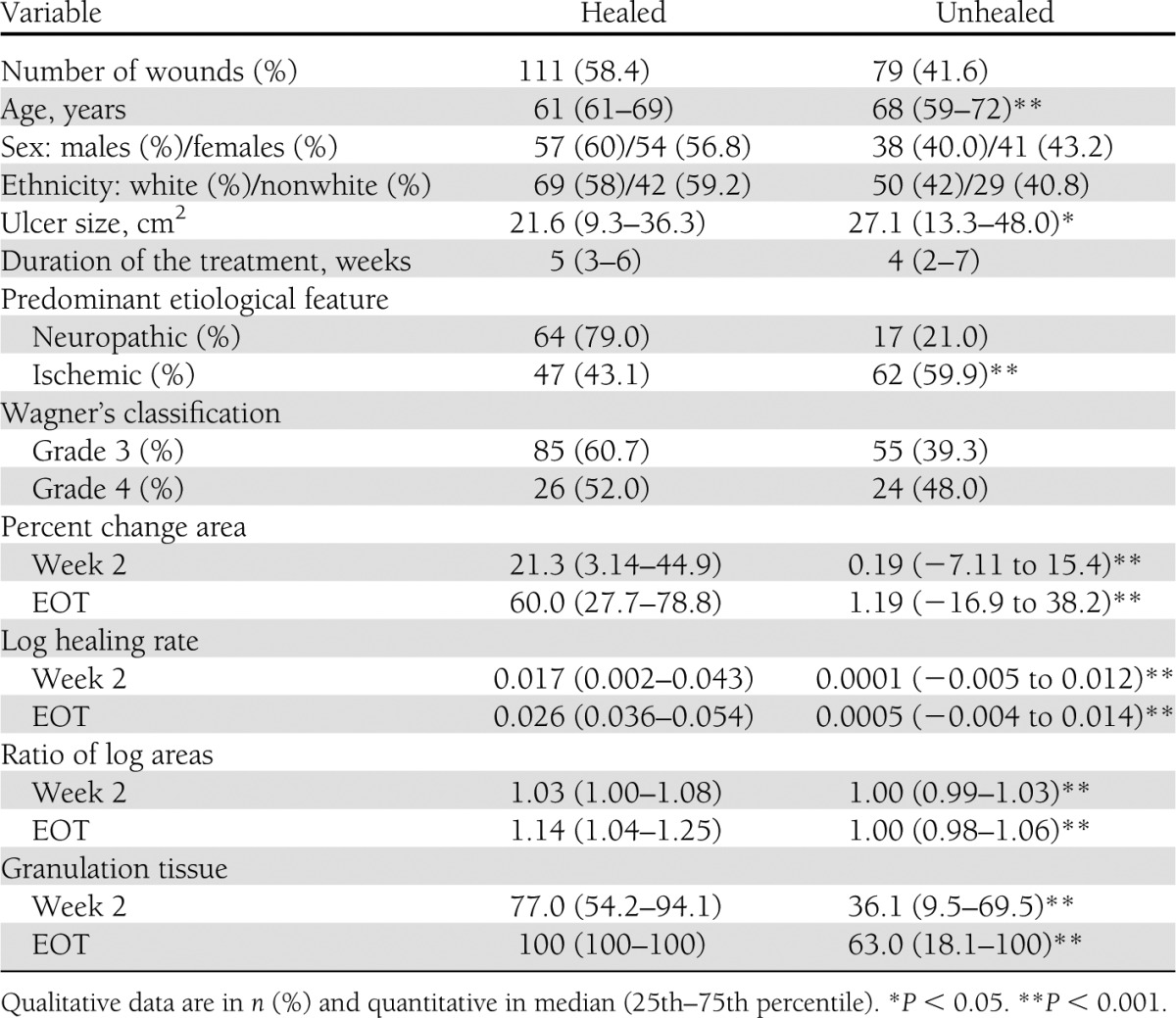
The inferential summary of baseline characteristics and candidate surrogates by healing is shown in Table 1. Globally, 58.4% of the wounds achieved closure. Complete healing showed dependence on all variables except for sex, ethnicity, treatment duration, and Wagner classification.
Table 1.
Patient characteristics in terms of healing (all treatment groups combined)
All variables tested had a good discrimination capacity, with ROC curve areas >0.7 at the 2nd week and >0.8 at the EOT (see Supplementary Table 2 for details). Those with the lower limit of the 95% CI >0.7 were selected as the best ones: the three variables based on the area change at the EOT and granulation tissue formation at the 2nd week and at the EOT. Supplementary Fig. 1 shows the ROC curves for granulation end points as examples.
Cutoff points were selected for each variable to maximize both sensitivity and specificity. Table 2 shows these, as well as the sensitivity, specificity, positive and negative PVs, and percent correct classification calculated from them. The 95% CIs were not shown for simplicity of the table, but they were all <2% around the estimators, showing high precision.
Table 2.
Diagnostic test for candidate surrogate variables and complete healing
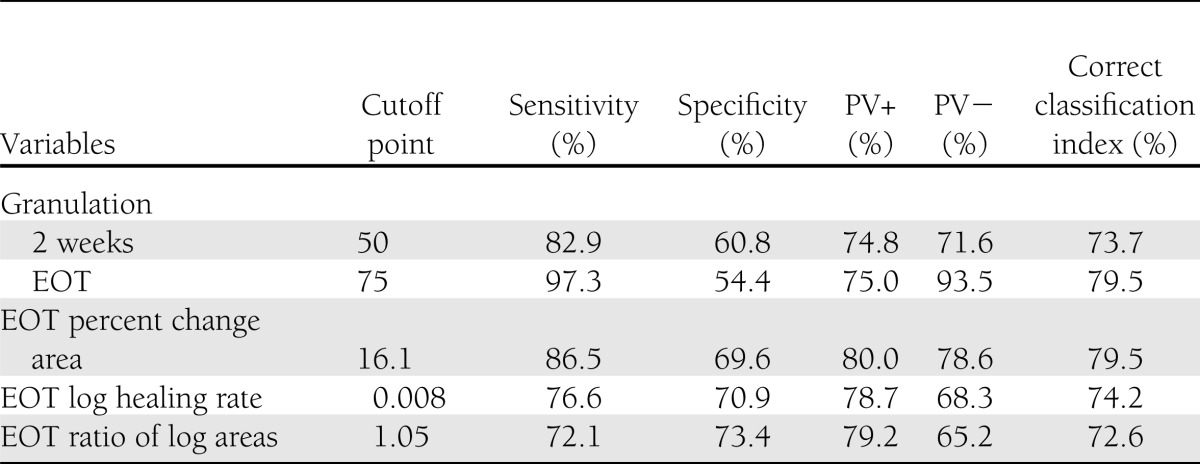
The most efficient predictor was the granulation tissue covering >75% of the ulcer area at the end of the treatment, with 79.5% correct classification and a high negative PV (93.5%). An earlier evaluation of this surrogate marker (granulation tissue covering >50% of the ulcer area at 2 weeks) was also adequate, with 73.7% correct classification, PVs (positive and negative) >70%, and acceptable sensitivity and specificity values. The best cutoff points for variables based on the area change at the EOT were selected too. The correct classification was also high (>70%), with suitable diagnostic indicators. At this point, all candidates can be used to discriminate healing.
The proportion-mediated (using PC estimators) 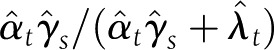 for 2 weeks >50% granulation and EOT >75% granulation were 1.008 (95% CI 0.933–1.083) and 1.043 (0.946–1.142), respectively, both very close to 1, suggesting that almost 100% of the effect of the treatment on complete healing may have been mediated by the formation of granulation tissue. With the area change–dependent candidate surrogates, the estimation was only possible for log healing rate. The mediation explained by this variable was very low: 0.003 (95% CI −0.002 to 0.008). Coefficients and PC values can be seen in Supplementary Table 3. The effect of the treatment on healing of this type of complicated ulcers is not necessarily mediated by an area reduction in the earlier weeks. The models given by Eqs. 6 and 7 for percent area change and ratio of log areas were not adequate.
for 2 weeks >50% granulation and EOT >75% granulation were 1.008 (95% CI 0.933–1.083) and 1.043 (0.946–1.142), respectively, both very close to 1, suggesting that almost 100% of the effect of the treatment on complete healing may have been mediated by the formation of granulation tissue. With the area change–dependent candidate surrogates, the estimation was only possible for log healing rate. The mediation explained by this variable was very low: 0.003 (95% CI −0.002 to 0.008). Coefficients and PC values can be seen in Supplementary Table 3. The effect of the treatment on healing of this type of complicated ulcers is not necessarily mediated by an area reduction in the earlier weeks. The models given by Eqs. 6 and 7 for percent area change and ratio of log areas were not adequate.
Table 3 shows the association between healing and candidate surrogate variables with some baseline influencing factors. All the models with the granulation-based candidates were adequate (Hosmer-Lemeshow goodness-of-fit statistics). Treatment had a significant influence on healing and the two granulation-dependent variables considered. For simplicity, only the 75-µg rhEGF is shown, since the 25-µg dose had no significant effect on healing in the clinical trial (16). The effect of the intervention on healing was lost when either 2 weeks >50% granulation or EOT >75% granulation is incorporated in the model. The odds ratios for the influence of the covariates (initial area and etiology) were similar for the three dependent outcomes (2-week and EOT granulation and healing).
Table 3.
Association between covariates and outcomes (granulation and healing)
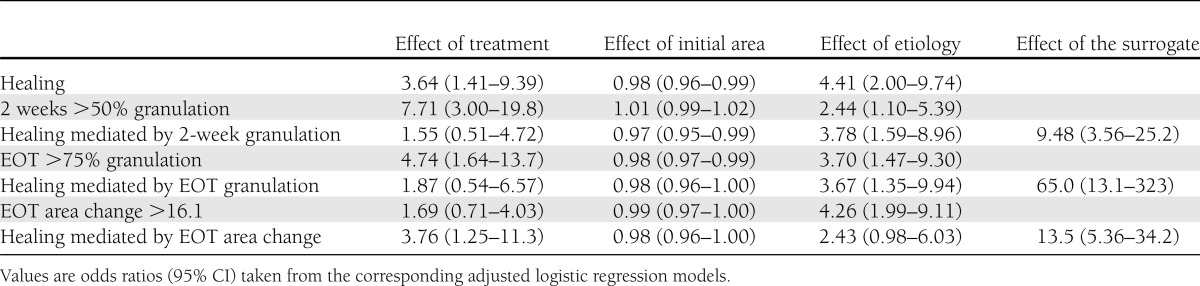
The results of the model with the EOT percent area change are also shown in Table 3 as an example. Models with the log healing rate variable are not shown, but their interpretation was the same. No effect of treatment on the surrogate was found (95% CI 0.71–4.03), and the effect of treatment on complete healing was not lost when the percent area change was incorporated in the model. The odds ratios for the covariates were similar for healing and percent area change.
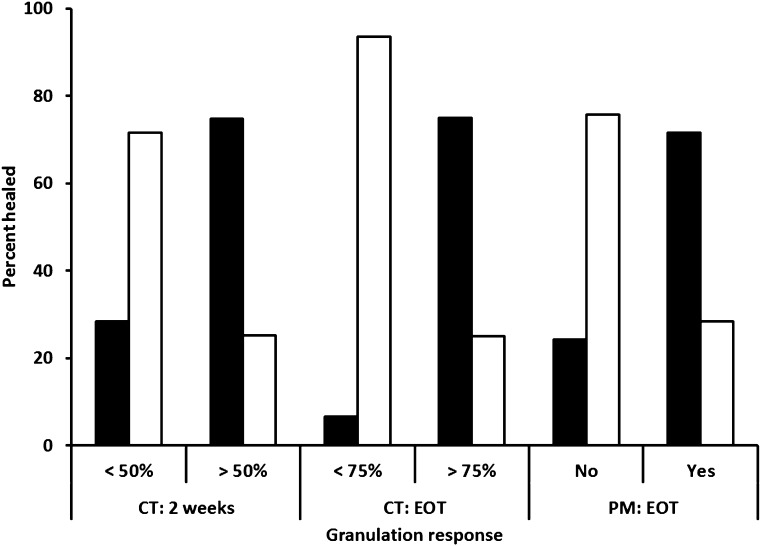
The results of the granulation-healing correlation analysis in the postmarketing study in current medical practice were obtained from the data of 1,440 ulcers. The result confirms that granulation tissue development at the EOT was an early predictor of final healing, with a 72.9% certainty and positive/negative PVs of 71.7/75.8%, respectively (details in Supplementary Table 4). Figure 1 illustrates the discrimination capacity of the granulation variables in clinical trials and in the postmarketing series.
Figure 1.
Discrimination capacity of the granulation variables in clinical trials (CTs) and in the postmarketing (PM) series. Black bars, healed; white bars, not healed.
CONCLUSIONS
The results of this study show that several surrogate variables can be taken into consideration for the final outcome in advanced DFUs, including ischemic, Wagner grade 3 or 4, and median area >20 cm2. There are no previous reports of predictive variables in this kind of lesion.
Granulation tissue development has been taken previously as a secondary outcome in DFU trials (14,15). It was also proposed as a potential intermediate variable (9), but its use as a surrogate of complete wound closure has not been proposed before, despite being part of the healing process and necessarily preceding final re-epithelization. The granulation process showed good capacity to discriminate healers from nonhealers with ROC curve areas >70% at the 2nd week and EOT. The diagnostic indexes of the dichotomized surrogate markers 2 weeks >50% and EOT >75% granulation had high correct classification percentages.
The validation tests using multivariate analyses were developed with the data of the randomized placebo-controlled clinical trial of intralesional rhEGF in advanced DFUs (16). The proportion mediated for granulation variables (at 2 weeks and at the EOT) was very high, which agrees with the idea that granulation constitutes an essential part of the healing process, particularly in larger and advanced ulcers. The EGF-induced mitogenic, motogenic, and cytoprotective actions are instrumental for healing events that may be summarized as follows: 1) stimulation of productive cell migration toward the injured area; 2) stimulation of granulation tissue outgrowth, including extracellular matrix accumulation, maturation, and de novo angiogenesis; 3) stimulation of wound contraction by myofibroblast activation and proliferation; and 4) stimulation of the damaged area resurfacing by epithelial cell migration and proliferation (23). Since treatment with rhEGF was given only up to complete granulation, mechanisms 1 and 2 took place completely but not 3 and 4, which require a previous granulating wound bed. For area change–dependent candidates, the proportion mediated was small, which could be explained by a more exigent condition for these lesions. Had the treatment continued after complete granulation, maybe a better proportion mediated would be obtained, given that mechanisms 3 and 4 could have worked longer.
The models for final cicatrization and the granulation surrogates were adequate and fulfilled the original Prentice (24) criteria for the definition of a surrogate end point: 1) treatment had a significant effect upon the surrogate end point; 2) treatment had a significant effect upon the true end point; 3) the surrogate end point had a significant effect upon the true end point; and 4) the full effect of treatment upon the true end point was mediated by the surrogate. Additionally, the effect of the covariates (wound area and ulcer etiology) was similar in all models. Criterion 4 is highly dependent on the power of the model and the sample size used. These are still limitations of this work. Patients with such advanced DFUs are usually not included in clinical trials, which limits the possibility of meta-analysis at present. The larger sample of the postmarketing series could not be included since it was not a controlled study. Nevertheless, it was useful to confirm the predictive character of the EOT granulation on final wound closure. On the other hand, as a measure of statistical power, ≥10 events per predictor is considered suitable, taking all the candidates and the other covariates (25,26). In this work, only in the placebo-controlled confirmatory trial (16), there were 93 healing events and 9 variables, so the criterion is fulfilled.
EOT >75% granulation had a higher PV with respect to final re-epithelization and thus should be a better surrogate for clinical trial design. This can be useful since it would not be necessary to wait for complete re-epithelization due to treatment, and alternative wound closure procedures could be used after complete granulation, such as grafts or engineered skin. The 2 weeks >50% granulation, being an earlier surrogate, may also have practical value for treatment algorithm decisions and/or pharmacoeconomic considerations.
Several variables based on wound area change have been proposed as surrogates for complete healing in neuropathic noncomplicated lesions. Margolis et al. (2) described the predictive capacity of 61% area reduction at 4 weeks as well as several transformations of the area change. Sheehan (27), based on previous work (3), proposes 50% wound closure at 4 weeks as a good surrogate for final healing and thus a decision point in a foot ulcer management algorithm. This cutoff point is also proposed by Snyder et al. (4).
The results of the present work suggest that the predictive property of percent area change, log healing rate, and ratio of log area can be extrapolated to more complicated, ischemic, larger, Wagner grade 3 or 4 ulcers. However, the cutoff points for classification were not the same. With the cutoff proposed by the above-mentioned authors, the correct classification percent in the present series was <70%, with negative PVs <60. This is reasonable since larger and more complex ulcers take longer to heal, and thus the value to expect as predictive around 4 weeks should be smaller, ∼16% area change, and much lower for the other transformed variables. It was not possible to validate the surrogate property for these variables in this series since the percent area change did not fulfill the Prentice criteria (24) 1 and 4. Additionally, the proportion mediated by these variables was very low (discussed above). The extrapolation of the cutoff points proposed in the present work has the limitation of the small sample size analyzed, so it requires further research. It could not be performed in the confirmatory, postmarketing series since the partial areas were not measured in routine clinical practice.
This work provides the first evidence for the use of granulation tissue development as a predictor of wound healing in advanced DFUs. These results can be useful for clinical trial design, particularly during the exploratory phase of development of new products. The surrogate markers that we report can easily be applied by clinicians because the measurement of the proportion of wound area covered by granulation tissue is part of routine clinical care. Correctly classifying patients with advanced diabetic foot ulcers into those who will heal or not heal by the 2nd week of care or at the end of granulation may have relevant implications in new wound healing products and for healthcare programs managing diabetic foot ulcers. Further research is needed to finally validate these results.
Supplementary Material
Acknowledgments
C.M.V.-S., A.D.T.-I., E.G.-I., A.d.R.-M., O.G.-D., I.B.Y.A., and P.A.L.-S. are employees of the Center for Genetic Engineering and Biotechnology (CIGB), where rhEGF is produced. CIGB sponsored the clinical trials and postmarketing study that provided the data for this study. J.I.F.-M. is coauthor of the product patent. No other potential conflicts of interest relevant to this article were reported.
C.M.V.-S. wrote the manuscript, performed the statistical analyses for this study, and participated in the protocol designs, statistical analyses, and article writing of the clinical trials included. A.D.T.-I. was in charge of the statistical analyses for the post-marketing study and analyzed this work. E.G.-I. contributed to the design and statistical analyses of the two clinical trials included. O.G.-D. and A.d.R.-M. designed the protocols and procured the data of the clinical trials. I.B.Y.A. organized and acquired data for the post-marketing study. J.I.F.-M. was the principal investigator of the clinical trials included. P.A.L.-S. wrote the manuscript, reviewed and contributed to protocol designs, analyzed results, and wrote study reports and articles for the clinical trials and the post-marketing study. C.M.V.-S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Eng. Leovaldo Alvarez and Reinier Hernández and technicians Ma. Adela Delgado, Yunia Delgado, Grettel Melo, and Ketty Cruz (Center for Genetic Engineering and Biotechnology) for their assistance in data management and processing.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1323/-/DC1.
References
- 1.Fleming TR. Surrogate endpoints and FDA’s accelerated approval process. Health Aff (Millwood) 2005;24:67–78 [DOI] [PubMed] [Google Scholar]
- 2.Margolis DJ, Gelfand JM, Hoffstad O, Berlin JA. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2003;26:1696–1700 [DOI] [PubMed] [Google Scholar]
- 3.Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care 2003;26:1879–1882 [DOI] [PubMed] [Google Scholar]
- 4.Snyder RJ, Cardinal M, Dauphinée DM, Stavosky J. A post-hoc analysis of reduction in diabetic foot ulcer size at 4 weeks as a predictor of healing by 12 weeks. Ostomy Wound Manage 2010;56:44–50 [PubMed] [Google Scholar]
- 5.Lavery LA, Barnes SA, Keith MS, Seaman JW, Armstrong DG. Prediction of healing for postoperative diabetic foot wounds based on early wound area progression. Diabetes Care 2008;31:26–29 [DOI] [PubMed] [Google Scholar]
- 6.Frykberg RG, Zgonis T, Armstrong DG, et al. American College of Foot and Ankle Surgeons Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006;45(Suppl.):S1–S66 [DOI] [PubMed] [Google Scholar]
- 7.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998;21:855–859 [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DG, Cohen K, Courric S, Bharara M, Marston W. Diabetic foot ulcers and vascular insufficiency: our population has changed, but our methods have not. J Diabetes Sci Tech 2011;5:1591–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong DG, Boulton AJM, Andros G, et al. Defining success in clinical trials of diabetic foot wounds: the Los Angeles DFCon consensus. Int Wound J 2009;6:211–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung PC, Wong MW, Wong WC. Limb salvage in extensive diabetic foot ulceration: an extended study using a herbal supplement. Hong Kong Med J 2008;14:29–33 [PubMed] [Google Scholar]
- 11.Sepúlveda G, Espíndola M, Maureira M, et al. Negative-pressure wound therapy versus standard wound dressing in the treatment of diabetic foot amputation. A randomised controlled trial. Cir Esp 2009;86:171–177 [in Spanish] [DOI] [PubMed] [Google Scholar]
- 12.EudraCT Register. A feasibility study to evaluate the effect of Vivostat platelet rich fibrin (PRF) in the treatment of diabetic foot ulcers [Internet]. Available from https://www.clinicaltrialsregister.eu/ctr-search/trial/2009-011755-47/DE Accessed 25 March 2012
- 13.Fernández-Montequín JI, Infante-Cristiá E, Valenzuela-Silva C, et al. Cuban Citoprot-P Study Group Intralesional injections of Citoprot-P (recombinant human epidermal growth factor) in advanced diabetic foot ulcers with risk of amputation. Int Wound J 2007;4:333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong DG, Lavery LA, Diabetic Foot Study Consortium Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–1710 [DOI] [PubMed] [Google Scholar]
- 15.Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008;31:631–636 [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Montequín JI, Valenzuela-Silva CM, González-Díaz O, et al. Cuban Diabetic Foot Study Group Intralesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers. Multicenter, randomized, placebo-controlled, double blind study. Int Wound J 2009;6:432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heberprot P. Product information. Available from http://heberprot-p.cigb.edu.cu/ Accessed 26 June 2012
- 18.Sugama J, Matsui Y, Sanada H, Konya C, Okuwa M, Kitagawa A. A study of the efficiency and convenience of an advanced portable Wound Measurement System (VISITRAK). J Clin Nurs 2007;16:1265–1269 [DOI] [PubMed] [Google Scholar]
- 19.European Medicines Agency. Committee for Medicinal Products for Human Use. Guideline on Missing Data in Confirmatory Clinical Trials London, European Medicines Agency, 2009 (CPMP/EWP/1776/99 rev. 1 corr. p. 1–13)
- 20.Burgueño MJ, García-Bastos JL, González-Buitrago JM. ROC curves in the evaluation of diagnostic tests. Med Clin (Barc) 1995;104:661–670 [in Spanish] [PubMed] [Google Scholar]
- 21.MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval Rev 1993;17:144–158 [Google Scholar]
- 22.MacKinnon DP, Lockwood CM, Brown CH, Wang W, Hoffman JM. The intermediate endpoint effect in logistic and probit regression. Clin Trials 2007;4:499–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–870 [DOI] [PubMed] [Google Scholar]
- 24.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989;8:431–440 [DOI] [PubMed] [Google Scholar]
- 25.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–1379 [DOI] [PubMed] [Google Scholar]
- 26.Bouwmeester W, Zuithoff NPA, Mallett S, et al. Reporting and methods in clinical prediction research: a systematic review. PLoS Med 2012;9:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheehan P. Early change in wound area as a predictor of healing in diabetic foot ulcers: knowing “when to say when”. Plast Reconstr Surg 2006;117(Suppl.):245S–247S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.