Abstract
OBJECTIVE
Preclinical data suggest that peroxisome proliferator–activated receptor γ (PPARγ) agonists have antineoplastic effects in colorectal cancer. We aimed to assess the association between the use of synthetic PPARγ agonists, represented by thiazolidinediones (TZDs), and the risk of developing colorectal cancer.
RESEARCH DESIGN AND METHODS
We conducted a nationwide, population-based, case-control study using the Taiwan National Health Insurance Research Database. Case subjects were defined as patients who were diagnosed with diabetes at least 365 days prior to a new diagnosis of colorectal cancer between 2000 and 2008. We randomly selected diabetic control subjects for each case subject, which were matched by sex, age, and the duration of diabetes. Among the 24,496 eligible case subjects and control subjects, we used conditional logistic regression to assess the risk of colorectal cancer in association with the use of TZDs. An additional analysis was conducted to assess the effects of concomitant use of TZDs and low-dose aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) on the risk of colorectal cancer.
RESULTS
A decreased risk of colorectal cancer was observed in patients who had used TZDs compared with those who had never used TZDs (adjusted odds ratio 0.86 [95% CI 0.79–0.94]). Furthermore, the benefit of a decreased colorectal cancer risk was also found with concomitant use of TZDs and low-dose aspirin or NSAIDs.
CONCLUSIONS
The use of TZDs may be associated with a decreased risk of colorectal cancer in patients with diabetes. Further studies are warranted to confirm our findings.
Peroxisome proliferator–activated receptors (PPARs) are members of the nuclear hormone receptor superfamily. The three PPAR isoforms are PPARα, PPARβ/δ, and PPARγ. The PPARs are ligand-activated transcription factors that modulate gene expression (1,2). PPARγ is activated by several natural and synthetic ligands, and its activation elicits cell differentiation and induces cell cycle arrest and apoptosis (3,4). PPARγ is expressed at high levels in adipose tissue and the mucosa of the colon, as well as in adenocarcinoma and human colon cancer cell lines (4–6). At present, the majority of the available preclinical data suggests that PPARγ agonists have antineoplastic effects on colon cancer (7). It has been shown that PPARγ agonists induce the differentiation of human colon cancer cells and reduce tumor growth (4). In the azoxymethane-induction animal model, PPARγ agonists were found to suppress colon carcinogenesis and inhibit aberrant cryptal foci or precursor lesions of colon malignancy (8,9). However, epidemiologic data and clinical human studies on the effect of PPARγ agonists and the risk of colorectal cancer are limited (10–14). Thiazolidinediones (TZDs) are synthetic insulin-sensitizing PPARγ agonists that are widely used for controlling blood glucose concentration in diabetes patients. A previous clinical study conducted in a population of male veteran diabetic patients in the U.S. demonstrated that the use of TZDs was associated with a significant reduction in lung cancer risk (11). Additionally, in the subgroup analysis of that study, there was a decrease in the incidence of colon cancer among African American TZD users. A few additional clinical studies have been conducted to investigate the association between the use of TZDs and the risk of cancer (12–15). However, the results from these studies were inconclusive and did not provide clear evidence of an antineoplastic effect of TZDs on colorectal cancer. Furthermore, recent data indicated a slightly increased risk of bladder cancer associated with long-term use of pioglitazone (16). We aimed to assess the association between the use of TZDs, as representative PPARγ agonists, and the risk of colorectal cancer.
RESEARCH DESIGN AND METHODS
Source population
The population for this study was derived from the National Health Insurance Research Database (NHIRD) in Taiwan between 1 January 1997 and 31 December 2008. The National Health Insurance (NHI) program was implemented in Taiwan in March 1995. By the end of 2008, 99.48% of the entire Taiwanese population was enrolled in this program (17,18). In accordance with the Regulations Governing the Review of the Medical Services, the Bureau of National Health Insurance (BNHI) performs a review system conducted by a panel of related medical experts to inspect reimbursement claims filed by contracted medical institutions and to screen the type, volume, quality, and appropriateness of medical services provided under the NHI program. The claims review system can identify those that do not conform to the NHI fee schedule, drug list, clinical guidelines, and patient conditions (such as age, sex, and indications). According to the NHI Act, false reports of diagnosis or inappropriateness of medical services will yield a severe penalty (17,19). The National Health Research Institute (NHRI) maintains and safeguards the privacy of all accumulative administrative and claims data from the BNHI reimbursement data files, and it has established a comprehensive computerized database, the NHIRD, from this system (20). Specific data subsets were also constructed for research purposes within the NHIRD, and these databases are provided to researchers after ethical approval is obtained. In Taiwan, diagnoses of cancer, including colorectal cancer, are usually accurate and must be confirmed by tissue pathology. Insured patients with colorectal cancer are eligible to register with the Catastrophic Illness Registry and apply for a catastrophic illness certificate. Under the NHI system, holders of catastrophic illness certificates are entitled to a subsidy from the government, which allows them to waive outpatient and inpatient copayments. The issuance of the certificate requires a diagnosis of catastrophic illness by physicians and a formal review by the BNHI. Every enrollee of NHI was assigned a unique personal identification number in NHIRD, which enables electronic data linkage between different databases (20). With approval from the Ethics Review Board at the National Taiwan University College of Public Health, we conducted a retrospective, nationwide, population-based, case-control study among all enrollees in the NHI between 1 January 1997 and 31 December 2008.
Case subjects and control subjects
Cases of colorectal cancer were defined according to codes (153.xx and 154.xx) from the International Classification of Diseases (ICD-9), and further linkage to the Catastrophic Illness Registry Dataset aimed to confirm the identification of a cancer diagnosis. Participants with diabetes were identified by the following conditions: the existence of primary hospital discharge diagnosis including diabetes, three or more outpatient visits for the diagnosis of diabetes in a 1-year period, or at least two prescriptions of any antidiabetic medication filled in the preceding 6-month period. The TZDs rosiglitazone and pioglitazone were approved for use by the Taiwan Department of Health in 2000. Thus, considering the availability date of TZD prescriptions, case subjects that were newly diagnosed with colorectal cancer between 1 January 2000 and 31 December 2008 and concomitantly diagnosed with diabetes >365 days prior to the index date (i.e., the date of colorectal cancer diagnosis) were selected. Participants with any cancer diagnosis preceding the index date were excluded. The dataset for the control population of one million samples was randomly culled from the entire NHI population, and members who were free of a cancer diagnosis were selected for the control population. We randomly selected diabetic control subjects for each case, which were matched by sex, age (i.e., with the same birth calendar year), and the age at the time of diabetes diagnosis (the same calendar year of the initial diabetes diagnosis date).
Exposure assessment
The data for exposure to TZDs for all participants were confirmed with the NHIRD. Exposure to TZDs after the index date was not taken into account. Furthermore, each patient’s exposure to TZDs was determined with the following categories: the cumulative dose of TZDs (quantified by a defined daily dose [DDD], according to the World Health Organization definition) (21), the duration of TZD therapy (the number of cumulative days for which TZDs were prescribed), and the time since starting TZDs (the duration of time from the initial TZD prescription date to the index date). The administration of other antidiabetic medications and potentially confounding drugs (including low-dose aspirin, nonsteroidal anti-inflammatory drugs [NSAIDs], statins, fibrates, angiotensin-converting enzyme inhibitors [ACEIs], and hormone replacement therapy agents) prescribed before the index date was also confirmed. We excluded patients who took drugs, including TZDs, that were prescribed during the 90 days preceding the index date to reduce the possibility of confounding due to indication. In addition, the previous medical conditions, which were reviewed as systemic covariates, of all the participants were complied. These covariate medical conditions were identified based on the presence of three outpatient diagnoses in the period of 1 year or the existence of an inpatient diagnosis preceding the index date as well as the use of hormone replacement therapy, which was defined by the cumulative duration of the therapy before the index date.
Statistical analyses
We used conditional logistic regression to assess the risk of colorectal cancer according to each category of exposure to TZDs. The odds ratios (ORs) and 95% CIs for colorectal cancer were calculated and estimated as unadjusted and adjusted for covariates (including urbanization, income, inflammatory bowel disease, colorectal polyp disorder, alcohol-related disease, biliary stone disease, chronic kidney disease, diabetes-related complications, hypertension, hyperlipidemia, ischemic heart disease, cerebrovascular disease, heart failure, chronic obstructive pulmonary disease, and hormone therapy). We assessed the dose- or duration-response effects according to the category of exposure and tested the trend by assessing the significance of the β coefficients (i.e., P value for trend) of exposure, which was regarded as a continuous variable. Sensitivity analyses based on the duration of therapy of TZDs were conducted to assess whether the effect of TZDs would be altered by adding potentially confounding drugs, including low-dose aspirin, NSAIDs, statins, fibrates, ACEIs, and other antidiabetic medications. Furthermore, considering that low-dose aspirin and NSAIDs were the most widely studied agents for the chemoprevention of colorectal cancer, we conducted additional analyses to assess the effects of concomitant use of low-dose aspirin or NSAIDs with TZDs in the risk of colorectal cancer. All analyses were conducted using the SAS version 9.2 software package (SAS Institute, Cary, NC).
RESULTS
We identified 12,469 case subjects with a diabetes diagnosis at least 365 days prior to a new diagnosis of colorectal cancer between 1 January 2000 and 31 December 2008 and 62,016 diabetic subjects who were free of a cancer diagnosis from the control population. By matching with sex, age, and age at the time of the diabetes diagnosis, there were 12,248 case subjects and 12,248 control subjects included in our study. Table 1 summarizes the demographic and clinical characteristics of the study population. The median age at the time of colorectal cancer diagnosis for this study population was 71.2 years, and males comprised 54.64% of the sample. The median age at the time of the initial diabetes diagnosis was 65.4 years, and the mean duration of time between the diagnosis of diabetes and the diagnosis of colorectal cancer was 2,042.5 days. There are 1,634 (13.34%) colorectal cancer case subjects and 1,753 (14.31%) control subjects that had ever been exposed to TZDs. The prevalence of TZD use was higher in control subjects than in the colorectal cancer case subjects. The median cumulative dose of TZDs was 180.5 DDD in exposed case subjects and 190.0 DDD in exposed control subjects; the median duration of TZD therapy was 254.5 days in exposed case subjects and 270.0 days in exposed control subjects; and the median time since starting treatment with TZDs to the index date was 913 days in exposed case subjects and 924 days in exposed control subjects. Regarding the potential confounders, the case subjects were more likely to have colorectal polyps and inflammatory bowel disease than the control subjects, as expected, and the prevalence of most of the covariates for the medical conditions in the case subjects appeared to be slightly higher than in the control subjects. Table 2 shows the frequency and ORs for colorectal cancer risk and the use of TZDs in the case subjects and control subjects. We noted a decreased risk of colorectal cancer in patients who had used TZDs compared with those who had never used TZDs (crude OR 0.91 [95% CI 0.84–0.99]; adjusted OR 0.86 [0.79–0.94]). The trends of dose- or duration-response relationships between the use of TZDs and a decreased colorectal cancer risk were observed whether estimated based on the cumulative dose of the TZDs, duration of the TZD therapy, or time since the initiation of TZDs.
Table 1.
Characteristics in case subjects and control subjects
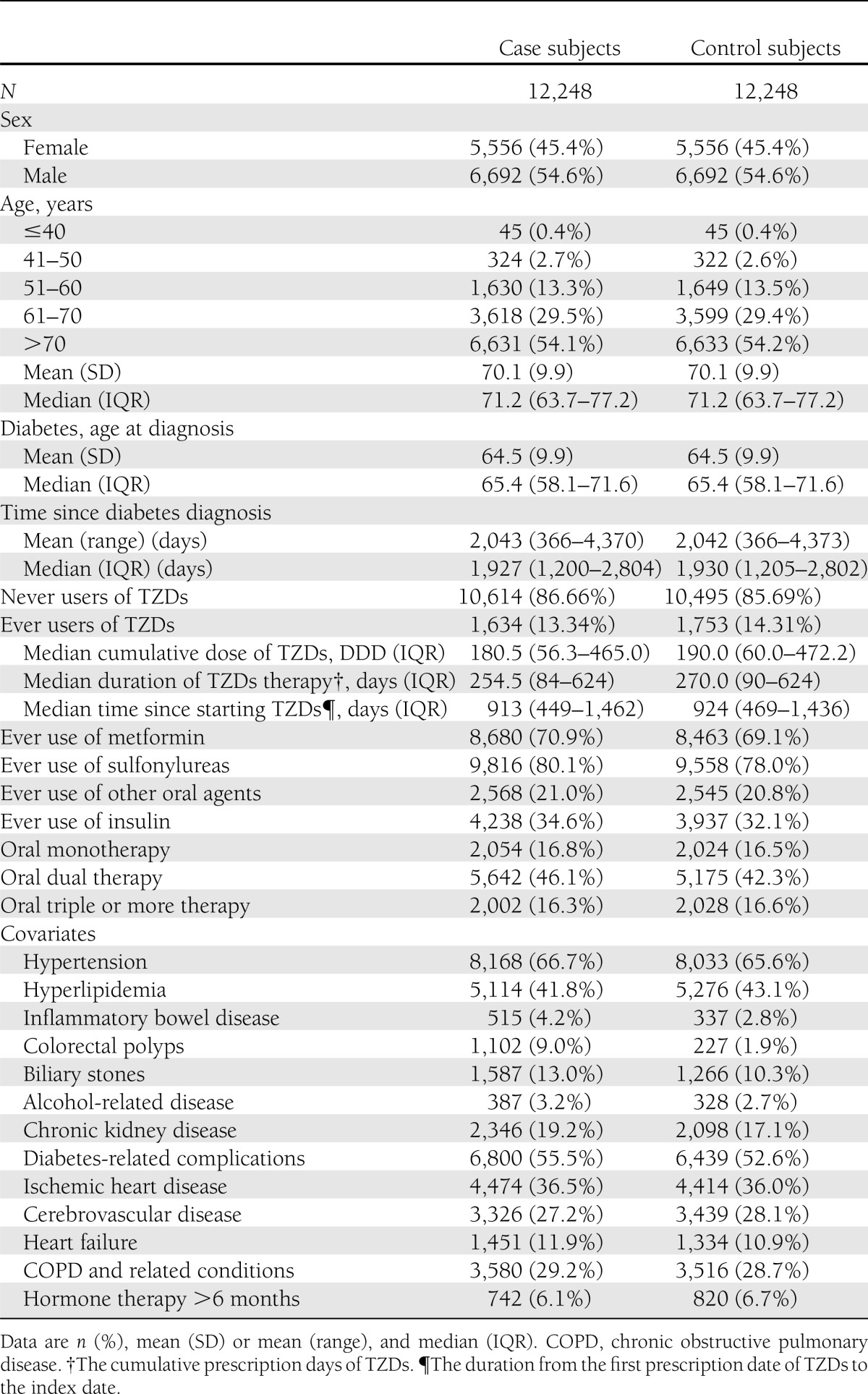
Table 2.
Use of TZDs and ORs of colorectal cancer among patients with diabetes

In sensitivity tests, the significance of the decreased risks of colorectal cancer in the TZD users was not altered by adding potentially confounding drugs or other antidiabetic agents, and the values of the ORs in each TZD treatment duration category showed little change upon the addition of each drug covariate to the model (Table 3). Furthermore, in additional analyses (Fig. 1), the antineoplastic effect of decreased colorectal cancer risk in the use of low-dose aspirin or NSAIDs was observed in our study design, and the result was consistent with prior studies. Moreover, a longer duration of TZD use resulted in a significant decreased risk of colorectal cancer within the groups that concomitantly used low-dose aspirin or NSAIDs. However, there is no significant additive effect of lowering the colorectal cancer risk with combined use of TZDs and low-dose aspirin (P for interaction = 0.854) or NSAIDs (P for interaction = 0.781).
Table 3.
Sensitivity test for adjusted ORs of colorectal cancer in potentially confounding drugs
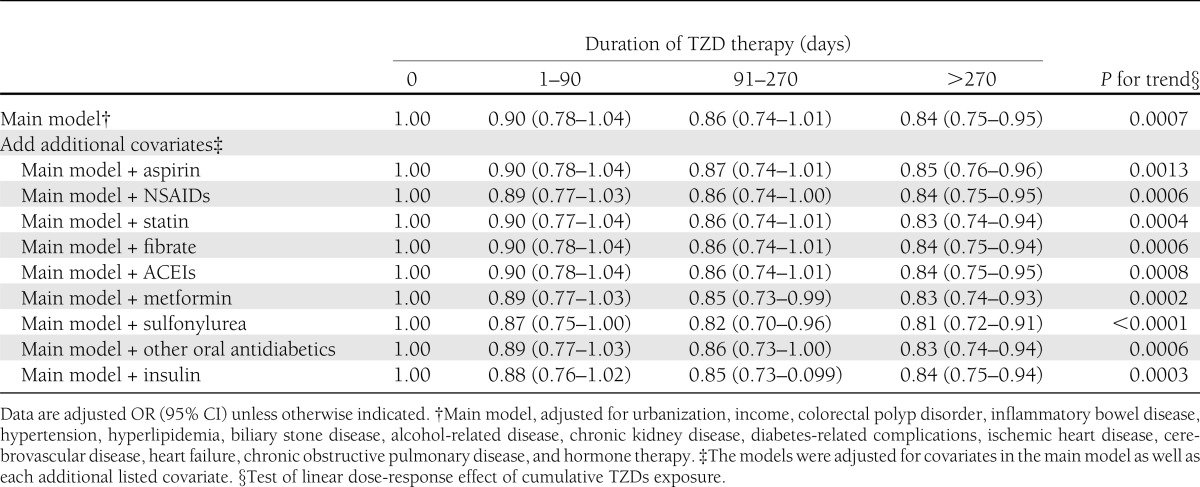
Figure 1.
Adjusted ORs for colorectal cancer treated with a combination of TZDs and low-dose aspirin (A) or a combination of TZDs and NSAIDs (B). CRC, colorectal cancer. Group Ia, never used TZDs and the duration of low-dose aspirin use was <270 days; group IIa, duration of TZD use was between 1 and 270 days, and the duration of low-dose aspirin use was <270 days; group IIIa, duration of TZD use was >270 days, and duration of low-dose aspirin use was <270 days; group IVa, never used TZDs, and duration of low-dose aspirin use was >270 days; group Va, duration of TZD use was between 1 and 270 days, and the duration of low-dose aspirin use was > 270 days; group VIa, duration of TZD use was >270 days, and the duration of low-dose aspirin use was >270 days; group Ib, never used TZDs, and the duration of NSAID use was <90 days; group IIb, duration of TZD use was between 1 and 270 days, and the duration of NSAIDs use was <90 days; group IIIb, duration of TZD use was >270 days, and the duration of NSAID use was <90 days; group IVb, never used TZD, and the duration of NSAID use was >90 days; group Vb, duration of TZD use was between 1 and 270 days, and the duration of NSAID use was >90 days; group VIb, duration of TZD use was >270 days, and the duration of NSAID use was >90 days. *Adjusted for urbanization, income, colorectal polyp disorder, inflammatory bowel disease, hypertension, hyperlipidemia, biliary stone disease, alcohol-related disease, chronic kidney disease, diabetes-related complications, ischemic heart disease, cerebrovascular disease, heart failure, chronic obstructive pulmonary disease, and hormone therapy. P for interaction between low-dose aspirin and TZD = 0.854. P for interaction between NSAID and TZD = 0.781.
CONCLUSIONS
The results of this large population-based study indicate that the use of TZDs may be associated with a decreased risk of colorectal cancer in patients with diabetes. The majority of the published preclinical studies have suggested that PPARγ agonists have antineoplastic effects in colon cancer (7). The possible mechanism by which PPARγ agonists cause cell differentiation and induce cancer cell apoptosis has been demonstrated in prior studies (2–6,8,9,22,23). Our results are consistent with prior in vitro studies and provide clinical evidence for a decreased risk of colorectal cancer that is associated with the use of PPARγ agonists in patients with diabetes.
Few previous clinical studies have been conducted to investigate the association between the use of TZDs and the risk of developing cancer (10–14); most of these clinical studies had short-term follow-up periods, and the results appeared to be neutral in regard to colorectal cancer. We searched the PubMed database for articles published up to July 2011 and identified three original clinical studies focusing on the assessment of cancer risks, including colorectal cancer, in patients exposed to TZDs (11,12,14). Govindarajan et al. (11) conducted a retrospective analysis in a male population using databases from 10 veteran affairs medical centers in the U.S. to assess the influence of TZDs on the risk of lung, prostate, and colorectal cancers in patients with diabetes. Mild trends indicating a reduction of risk for colorectal cancer in patients treated with TZDs were observed (adjusted HR 0.88 [95% CI 0.74–1.05]); however, this result did not attain statistical significance. Nevertheless, in their subgroup analysis, in which the sample was segmented based on race/ethnicity, the use of TZDs was associated with a reduced risk for colorectal cancer among male African American patients (adjusted HR 0.53 [0.31–0.93]). Another nested case-control study conducted by Koro et al. (12) evaluated the risk of breast, colon, and prostate cancers in patients exposed to TZDs compared with exposure to other antidiabetic agents using data from the U.S. Integrated Healthcare Information Services database. The findings suggested that the effect of TZDs compared with other antidiabetic agents on the likelihood of development of colon cancer was neutral. However, we observed a decreased risk of colorectal cancer associated with TZD use compared with an untreated group (adjusted OR 0.70 [0.50–0.96]) in their study. The most recent study was conducted by Ferrara et al. (14) using data from the Kaiser Permanente Northern California Diabetes Registry and approved by the European Medicines Agency to explore whether pioglitazone was associated with a risk of incident cancer in the 10 most common sites, including the colon and rectum. The authors found no suggestion of an association between the use of pioglitazone and the risk of colon (adjusted HR 0.90 [0.72–1.13]) or rectal cancer (1.20 [0.80–1.80]). We have also conducted a meta-analysis with a fixed-effect model to pool data across studies, including ours (Supplementary Fig. 1); this meta-analysis suggested an overall trend toward the reduction of risk for colorectal cancer associated with TZD use.
Govindarajan et al. (11) suggested that the use of TZDs was associated with a reduced risk for colon cancer among male African American patients but not among white patients. The difference in the association between the risk of colorectal cancer and the use of TZDs among different racial/ethnic groups, whether mediated by genetic factors or common environmental triggers, is not easy to explain, and this issue would be difficult to clarify using our database due to the almost homogenous racial/ethnic composition of Taiwan. However, our study has several strengths, including the large, nationwide population of study subjects with a relatively longer duration of potential exposure to TZDs. Recall and selection bias were not likely in our study. The case subjects were identified in the NHIRD and further confirmed using the Catastrophic Illness Registry dataset, which included almost all cases of colorectal cancer in the population. The control subjects were randomly selected from the entire NHI population and matched with predefined risk factors for each case.
According to our analysis, exposure of TZDs was associated with a lower risk of colorectal cancer risk whether estimated by the cumulative dose or the duration and initiation time of TZD therapy. Dose- or duration-response relationships between the use of TZDs and decreased colorectal cancer risk were observed; however, the ORs did not appear to drop substantially with increasing dose or duration. The results of our sensitivity analysis also demonstrated a consistent antineoplastic effect, and the trend was not altered by adding potential confounding drugs, including low-dose aspirin, NSAIDs, and other antidiabetic agents. Detection or recall bias for the exposure of TZDs and other potential confounding drugs is not likely in our study because the NHI reimbursement database collects virtually complete prescription information for all participants. Moreover, missing information regarding the use of over-the-counter drugs is not likely to bias our results because most of the individuals in our study were residents of Taiwan and were enrolled in the NHI program, which has comprehensive and universal coverage for the prescription of these drugs. There is no evidence to suggest that the prescription of these drugs differs between colorectal cancer case subjects and control subjects.
Low-dose aspirin was widely recommended for the primary prevention of cardiovascular events in patients with diabetes, and it has been suggested to reduce incidence and mortality due to colorectal cancer (24,25). In addition, NSAIDs were also widely studied agents for the chemoprevention of colorectal cancer (26–28). As concern about low-dose aspirin or NSAIDs might be a major confounder in our study, we conducted an additional analysis to investigate the effect of concomitant use of TZDs and low-dose aspirin or NSAIDs. In our additional analysis, the association between the use of low-dose aspirin or NSAIDs and the decreased risk of colorectal cancer in patients with diabetes was consistent with prior studies. Nevertheless, longer TZD use also showed a significant decrease in the risk of colorectal cancer within the groups that concomitantly used low-dose aspirin or NSAIDs. However, there was no significant additive effect of lowering the colorectal cancer risk in combining the use of TZDs and low-dose aspirin or NSAIDs.
A potential bias that may result from confounding indications is the association between diabetes and colorectal cancer risk. Type 2 diabetes and cancer have many mutual risk factors, and diabetes is suggested to be associated with an increased risk of certain cancers. A recent study also indicated that the fasting glucose level was related to the risk of colorectal cancer and certain other cancer-related causes of death (29). Appropriately, glucose control plays a role in effective diabetes management, which may lessen morbidity and mortality by reducing the risk of diabetes-associated complications (30). However, the potential causality or association between diabetes and cancer has not been clearly established, and it remains unclear whether the association between these two diseases is direct or indirect, and whether the cancer risk would be influenced by the different conditions of diabetes. Apart from their clinical application for improving glucose and lipid metabolism in diabetes patients, TZDs may also have antineoplastic effects on colorectal cancer and other cancers through activation of PPARγ (31). There are limitations of our database in that the NHIRD did not include detailed results from laboratory tests; thus, we were unable to provide exact measurements of serum glucose level or diabetes control status.
We also had incomplete data on several variables known to be associated with colorectal cancer, such as obesity, a family history of colorectal cancer, smoking, alcohol consumption, or other unhealthy lifestyle factors. However, there is no evidence to suggest that the subjects who used TZDs had either a worse or better diabetes status. Moreover, in our study design, we matched the case and control subjects not only by age and sex but also by the duration of diabetes, and the associated potential confounding medical conditions, including diabetes-related complications, were adjusted in our statistical analysis. Moreover, in our sensitivity tests, it can be considered a strength that the values of the decreased ORs in each duration group showed little change upon the addition of each drug covariate to the model, and the significance of the decreased risk of colorectal cancer in TZD users was not altered by including potentially confounding drugs or other antidiabetic agents and insulin in the analysis. For example, most of our study populations were with cumulative duration of insulin use <30 days, and there is no statistical difference in the distribution of insulin use between case and control populations in our study (Supplementary Table 1). Longer TZD use was still associated with decreased risk of colorectal cancer within the group of less insulin use (Supplementary Table 2).
Finally, a detection bias could occur in our study in that patients taking TZDs might have access to a better quality of health care and an increased opportunity to receive cancer screening, which would result in higher cancer detection rates, and early resection of polyps during routine screening would also decrease subsequent cancer rates. We investigated the records of colorectal polyp diagnosis before the index date between the TZD users and nonusers in our study population, and there was no statistical difference in the distribution of TZD use and record of colorectal polyp in our study population (Supplementary Table 3). Besides, longer TZD use was still associated with decreased risk of colorectal cancer within the group of never diagnosed with colorectal polyp (Supplementary Table 4). However, the possibility of higher cancer detection rates in TZD users would potentially lead to an underestimation of the antineoplastic effect of TZDs.
This study provided clinical evidence that PPARγ agonists had an antineoplastic effect on colorectal cancer among patients with diabetes. However, concerns regarding drug safety and cardiovascular events and the possible elevated risk of bladder cancer associated with the use of TZDs have been raised in recent studies (16). The complexity of the links between diabetes, cancer, and PPARγ activation warrants clarification through further studies.
Supplementary Material
Acknowledgments
The data for analysis were provided by the BNHI, the Department of Health, and the NHRI.
No potential conflicts of interest relevant to this article were reported.
S.-W.C. designed the study, drafted the manuscript, and collected, arranged, filtered, and managed data. Y.-T.T. collected, arranged, filtered, and managed data. J.-D.C. drafted the manuscript. H.-I.H. drafted the manuscript and contributed to the statistical analysis. C.-H.L. collected, arranged, filtered, and managed data and contributed to the statistical analysis. H.-H.L. contributed to the statistical analysis. J.-D.W. designed the study. P.-C.C. designed the study and contributed to the statistical analysis. All authors revised the manuscript. P.-C.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2197/-/DC1.
References
- 1.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell 1995;83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature 2000;405:421–424 [DOI] [PubMed] [Google Scholar]
- 3.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 1995;83:803–812 [DOI] [PubMed] [Google Scholar]
- 4.Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med 1998;4:1046–1052 [DOI] [PubMed] [Google Scholar]
- 5.Mansén A, Guardiola-Diaz H, Rafter J, Branting C, Gustafsson JA. Expression of the peroxisome proliferator-activated receptor (PPAR) in the mouse colonic mucosa. Biochem Biophys Res Commun 1996;222:844–851 [DOI] [PubMed] [Google Scholar]
- 6.DuBois RN, Gupta R, Brockman J, Reddy BS, Krakow SL, Lazar MA. The nuclear eicosanoid receptor, PPARgamma, is aberrantly expressed in colonic cancers. Carcinogenesis 1998;19:49–53 [DOI] [PubMed] [Google Scholar]
- 7.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor γ agonists. Lancet Oncol 2004;5:419–429 [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T, Kohno H, Yoshitani S, et al. Ligands for peroxisome proliferator-activated receptors alpha and gamma inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Res 2001;61:2424–2428 [PubMed] [Google Scholar]
- 9.Osawa E, Nakajima A, Wada K, et al. Peroxisome proliferator-activated receptor γ ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology 2003;124:361–367 [DOI] [PubMed] [Google Scholar]
- 10.Kulke MH, Demetri GD, Sharpless NE, et al. A phase II study of troglitazone, an activator of the PPARgamma receptor, in patients with chemotherapy-resistant metastatic colorectal cancer. Cancer J 2002;8:395–399 [DOI] [PubMed] [Google Scholar]
- 11.Govindarajan R, Ratnasinghe L, Simmons DL, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol 2007;25:1476–1481 [DOI] [PubMed] [Google Scholar]
- 12.Koro C, Barrett S, Qizilbash N. Cancer risks in thiazolidinedione users compared to other anti-diabetic agents. Pharmacoepidemiol Drug Saf 2007;16:485–492 [DOI] [PubMed] [Google Scholar]
- 13.Lewis JD, Capra AM, Achacoso NS, et al. Thiazolidinedione therapy is not associated with increased colonic neoplasia risk in patients with diabetes mellitus. Gastroenterology 2008;135:1914–1923, 1923, e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrara A, Lewis JD, Quesenberry CP, Jr, et al. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care 2011;34:923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monami M, Lamanna C, Marchionni N, Mannucci E. Rosiglitazone and risk of cancer: a meta-analysis of randomized clinical trials. Diabetes Care 2008;31:1455–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011;34:916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bureau of National Health Insurance. Available from http://www.nhi.gov.tw/Resource/webdata/Attach_13767_1_National%20Health%20Insurance%20in%20Taiwan%202010.pdf Accessed 20 October 2011
- 18.Chiang TL. Taiwan’s 1995 health care reform. Health Policy 1997;39:225–239 [DOI] [PubMed] [Google Scholar]
- 19.Lu JF, Hsiao WC. Does universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff (Millwood) 2003;22:77–88 [DOI] [PubMed] [Google Scholar]
- 20.Institutes NHR. National Health Insurance Research Database. Available from http://w3.nhri.org.tw/nhird/en/index.htm Accessed 20 October 2011
- 21.WHO. The ATC and DDD system. Available from http://www.whocc.no/atc_ddd_index/ Accessed 20 October 2011
- 22.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem 1999;274:9116–9121 [DOI] [PubMed] [Google Scholar]
- 23.Panigrahy D, Singer S, Shen LQ, et al. PPARgamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J Clin Invest 2002;110:923–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flossmann E, Rothwell PM, British Doctors Aspirin Trial and the UK-TIA Aspirin Trial Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 2007;369:1603–1613 [DOI] [PubMed] [Google Scholar]
- 25.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741–1750 [DOI] [PubMed] [Google Scholar]
- 26.Smalley W, Ray WA, Daugherty J, Griffin MR. Use of nonsteroidal anti-inflammatory drugs and incidence of colorectal cancer: a population-based study. Arch Intern Med 1999;159:161–166 [DOI] [PubMed] [Google Scholar]
- 27.Rostom A, Dubé C, Lewin G, et al. U.S. Preventive Services Task Force Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med 2007;146:376–389 [DOI] [PubMed] [Google Scholar]
- 28.Ruder EH, Laiyemo AO, Graubard BI, Hollenbeck AR, Schatzkin A, Cross AJ. Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol 2011;106:1340–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seshasai SR, Kaptoge S, Thompson A, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanquicett C, Roman J, Hart CM. Thiazolidinediones as anti-cancer agents. Cancer Ther 2008;6(A):25–34 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.