Abstract
OBJECTIVE
—Visceral adiposity is an important risk factor for cardiovascular disease and type 2 diabetes. We sought to determine whether change in intraabdominal fat area (IAF) over time predicts subsequent development of diabetes.
RESEARCH DESIGN AND METHODS
—We followed up 436 nondiabetic Japanese-American subjects (mean age 51.9 years, mean BMI 24.2 kg/m2, 54% male) for development of diabetes. We fit a logistic regression model to examine the association over a 10-year follow-up between change in IAF at 5-year follow-up and other fat areas (measured by computed tomography) and development of incident diabetes, adjusted for age, sex, family history of diabetes in a first-degree relative, second-generation versus third-generation Japanese American (Nisei vs. Sansei), baseline IAF, BMI, weight change over time, smoking status, physical activity level, and subcutaneous fat (SCF) depot areas.
RESULTS
—Cumulative incidence of diabetes was 20.4% at 10 years. Mean change in IAF was 10.9 cm2. An increase of 1 SD in IAF was associated with a 1.65-fold increase in the odds of diabetes over 10 years (OR = 1.65, 95% CI 1.21–2.25) after adjusting for the above covariates. This association was also independent of changes in thoracic, thigh, and abdominal SCF, as well as change in weight.
CONCLUSIONS
—We conclude that baseline IAF and accumulation of fat in this area over time are independent predictors of the development of type 2 diabetes in Japanese Americans.
Central obesity is well established as an independent risk factor for type 2 diabetes. This association is strong and has been demonstrated in cross-sectional and longitudinal analyses with a number of different metrics, including surface measurements such as waist circumference and waist-to-hip ratio in a variety of populations (1,2), and imaging of the visceral fat depot. A higher risk of incident type 2 diabetes over 5 to 10 years of follow-up was associated with larger baseline visceral fat area measured using single-slice computed tomography (CT) scans in Japanese Americans (3).
The association between change in regional or overall adiposity and risk of diabetes is less clear with a smaller number of investigations reported and inconsistent results. Several investigations document a higher prevalence of diabetes with greater duration of overweight or obesity (4), and an obesity-years metric was recently proposed to refer to this phenomenon (5) in relation to mortality. Measurements at baseline may reflect size of adipose depots over a number of years, whereas recent change in depot size might be expected to have less influence on health outcomes because of a shorter exposure period. A number of studies have investigated recent weight change in relation to diabetes risk, finding it to be inconsistently associated, whereas baseline adiposity is consistently associated with this outcome (6). Although studies of diabetes risk have been performed in relation to weight change that presumably reflects change in overall adiposity, to our knowledge only one observational study has examined increase in regional adiposity as measured by waist circumference in relation to diabetes risk; that study found an independent association even after adjusting for weight gain (7). To our knowledge, no observational research has examined whether increase in size of a directly measured fat depot (visceral or otherwise) is associated with diabetes risk. In a substudy of the Diabetes Prevention Program (a randomized trial of diabetes prevention in persons identified as being at high risk for this outcome based on elevated levels of fasting and 2-h plasma glucose), diabetes risk was assessed in relation to a decrease in CT-measured fat depots over a brief 1-year period. In that intervention, a decline in body fat depot size over the first year was shown to predict diabetes risk over a subsequent mean 1.5 years of follow-up (8). In addition, a randomized clinical trial in Japanese Americans examined whether lifestyle changes improved measurements of adiposity in individuals with impaired glucose tolerance. In that trial, the treatment arm showed significantly greater reduction in percent body fat, BMI, and CT-measured subcutaneous fat (SCF) depots at six and 24 months, with at least one occurrence of a normal oral glucose tolerance test occurring significantly more frequently in the intervention arm during follow-up. Both treatment arms, however, showed similar decreases in intraabdominal fat area (IAF) (9).
Given the paucity of information on the effects of change in size of regional adipose depots in general and the visceral depot in particular in relation to diabetes risk, we investigated the association between 5-year change in CT-measured adipose depots and diabetes occurrence over 10 years in a prospective study of Japanese Americans.
RESEARCH DESIGN AND METHODS
We followed up 436 nondiabetic Japanese-American subjects for the development of diabetes at 10 years. Study subjects were taken from the Japanese-American Community Diabetes Study, a cohort of second- (Nisei) and third-generation (Sansei) Japanese Americans of 100% Japanese ancestry who were representative of Japanese-American residents of King County, WA, in age, residential distribution, and parental immigration pattern (10). A comprehensive mailing list and telephone directory that included almost 95% of the Japanese-American population of the county were used to identify and contact potential study subjects. Subjects returned for follow-up 5–6 years and then again 10–11 years after a baseline evaluation. Selection and recruitment have been described in greater detail previously (11). The study received approval from the University of Washington Human Subjects Division, and all subjects provided written informed consent.
Overall, 80% of subjects eligible at baseline completed the 10-year follow-up assessment. Evaluations were done at the General Clinical Research Center at the University of Washington, Seattle, WA. Presence of diabetes was assessed at the 5- and 10-year follow-up visits using the oral glucose tolerance test (75-g load) and defined as fasting glucose ≥126 mg/dL and/or 2-h glucose ≥200 mg/dL (12), or use of diabetes medication. BMI was defined as weight in kilograms divided by height in meters squared. Family history of diabetes was considered positive if a parent or sibling had diabetes. Alcohol consumption was ascertained by questionnaire and measured in grams/week. Individuals were categorized at baseline as never, former, or current smokers. To quantify physical activity levels, the physical activity index was applied to responses to the Paffenbarger Physical Activity Questionnaire to estimate physical activity in kilocalories/week (13).
Single (1-cm) CT scan slices were obtained at the chest at the level of the nipples, abdomen (umbilicus level), and thigh (halfway between the greater trochanter and the superior margin of the patella). CT scans were analyzed using density contour software. Areas corresponding to a density of −250 to −50 Hounsfield units were classified as adipose tissue (14). The following cross-sectional fat areas (centimeters squared) were measured at baseline and 5-year follow-up: subcutaneous thoracic fat, subcutaneous abdominal fat, IAF (within the confines of the transversalis fascia), and left thigh SCF (14). Change in fat area was calculated as the difference between the 5-year and baseline values. The intraobserver variability for multiple measurements by a single observer of a single CT scan ranged from 0.2 to 1.4%.
Logistic regression was used to estimate the association between change in IAF and odds of incident diabetes while adjusting for covariates known to be associated with type 2 diabetes (age, family history of diabetes, and baseline BMI), for sex (15–17), and for the baseline SCF depots discussed above. Additional covariates included weight change between baseline and 5- to 6-year follow-up and baseline smoking and physical activity. We tested whether associations between baseline IAF and IAF change differed by age, sex, and family history of diabetes through insertion of first-order multiplicative interaction terms into the regression model. Odds ratios (OR) and 95% CI for continuous variables are shown for a 1-SD-magnitude increase. The variance inflation factor was used to assess presence of collinearity in multivariable models, with a value >4 suggesting presence of this problem. A P value of <0.05 was considered statistically significant. Analyses were performed with Stata (version 12.0; StataCorp, College Station, TX).
RESULTS
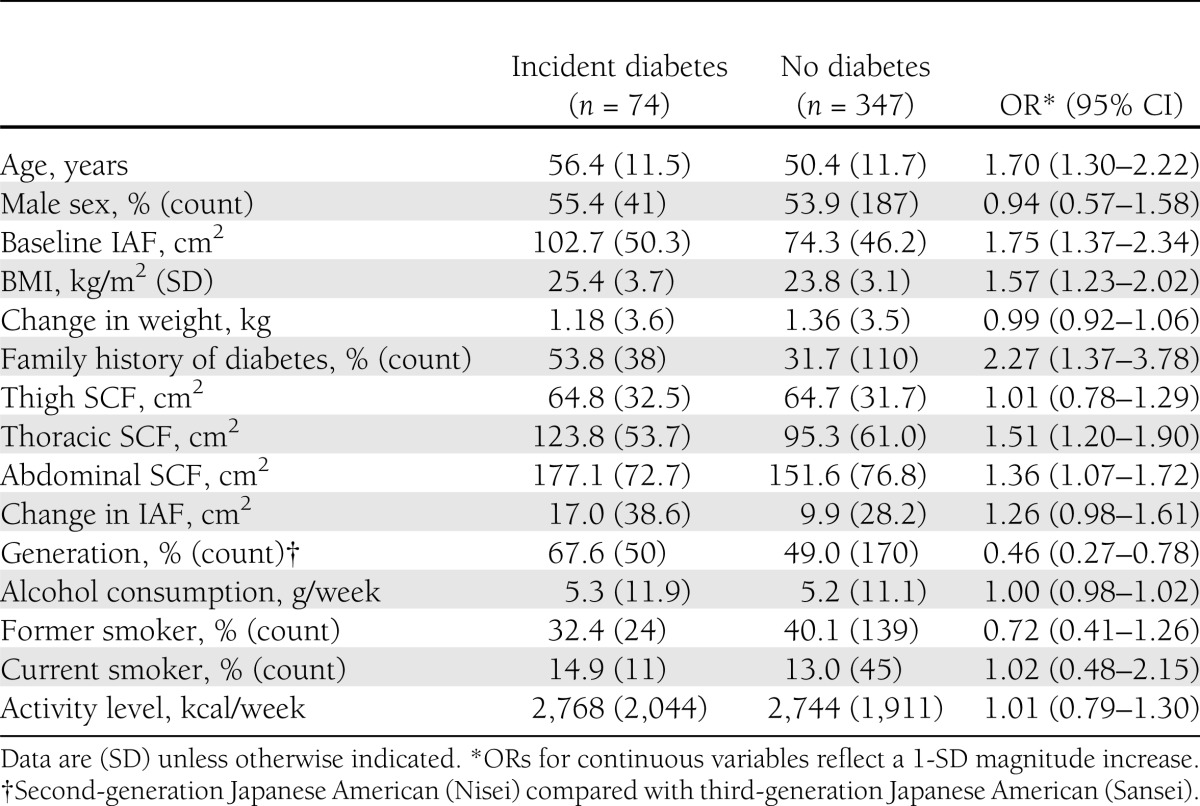
Table 1 shows subject characteristics by the development of diabetes over 10 years of follow-up. The Nisei subjects only (n = 274) were rescreened at 2.5 years after baseline, and the 15 who had developed diabetes were excluded from this analysis, leaving a study population that consisted of 421 subjects. Overall, 54% of the participants were men, with a mean age of 51.4 years (range 34.0–75.1) and a mean BMI of 24.1 kg/m2 (range 16.6–36.9), and 35% had a family history of diabetes. Over the follow-up period, 39 individuals (9.3%) developed diabetes at the 5-year follow-up visit. Seventy-four individuals (17.6%) developed diabetes at 10 years. Table 1 shows subject characteristics by the development of diabetes at either 5 or 10 years of follow-up.
Table 1.
Characteristics of study subjects by incident diabetes at 10-year follow-up
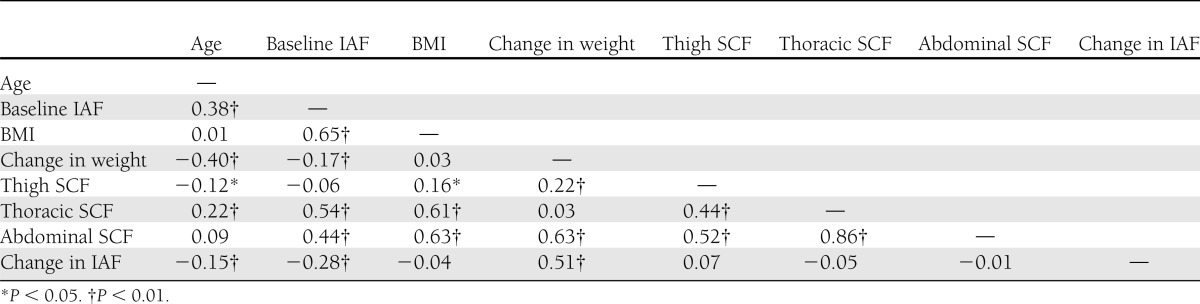
The mean IAF was 79.3 cm2 (range 0.7–242.2) at baseline. Baseline distribution of fat was similar in subjects who did and did not develop diabetes after 10 years of follow-up, although mean CT-measured fat areas were greater among persons who developed diabetes for all depots except thigh SCF (Table 1). The size of SCF depots was highly correlated, but the correlation between visceral and SCF depots was of smaller magnitude (Table 2). The mean change in IAF over 5 years was 11.1 cm2 (range −75.2 to 137.3).
Table 2.
Correlations among continuous variables and in study subjects
Unadjusted odds ratios for the development of diabetes are shown in Table 1. Greater age, baseline IAF, BMI, thoracic SCF, abdominal SCF, presence of a family history of diabetes, and Nisei generation were all significantly associated with incident diabetes in univariate analyses, whereas 5-year change in IAF was not.
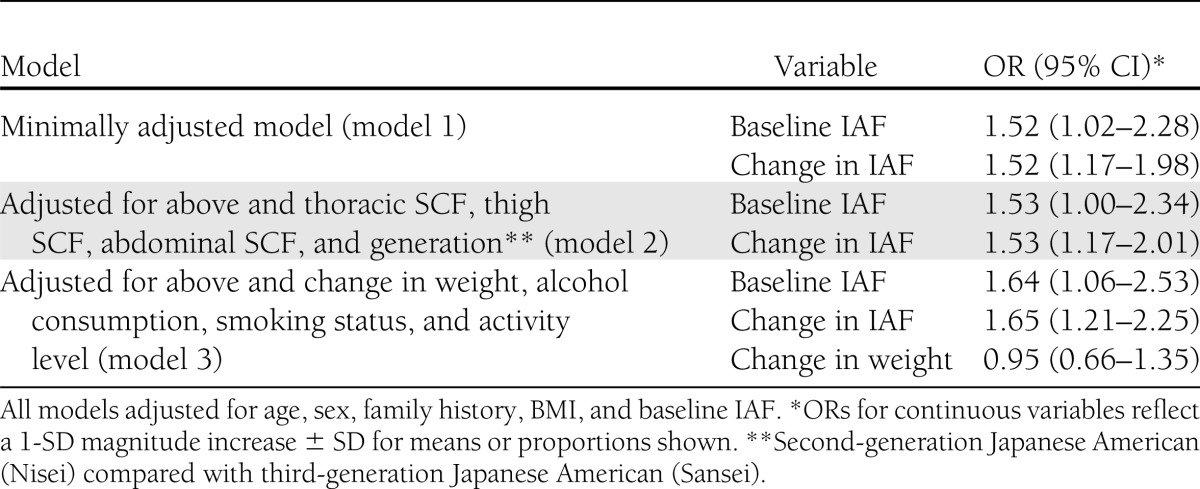
Multiple logistic regression models were fit to estimate the relationship between change in IAF and incident diabetes while adjusting for other independent variables (Table 3). After adjustment for sex and covariates known to be associated with diabetes risk, including age, family history, baseline BMI, and baseline IAF, a significant association between change in IAF and odds of diabetes was observed (Table 3, model 1). This association was preserved after adjustment for SCF depots and generation (Table 3, model 2). When we further adjusted for change in weight from baseline, smoking status, and physical activity level, the association between change in IAF and diabetes incidence remained statistically significant and increased slightly in magnitude (OR 1.65, 95% CI 1.21–2.25). Additionally, we did not see a significant relationship between change in weight from baseline to year 5 and diabetes risk (OR 0.95, 95% CI 0.66–1.35). Results for baseline and change in IAF in relation to diabetes incidence were similar when adjusted for menopausal status (by coding sex into three categories as male, premenopausal female, and postmenopausal female), and when each SCF depot was entered individually into the multivariable models as opposed to entering them simultaneously as shown in Table 3.
Table 3.
Odds of incident diabetes at 10-year follow-up
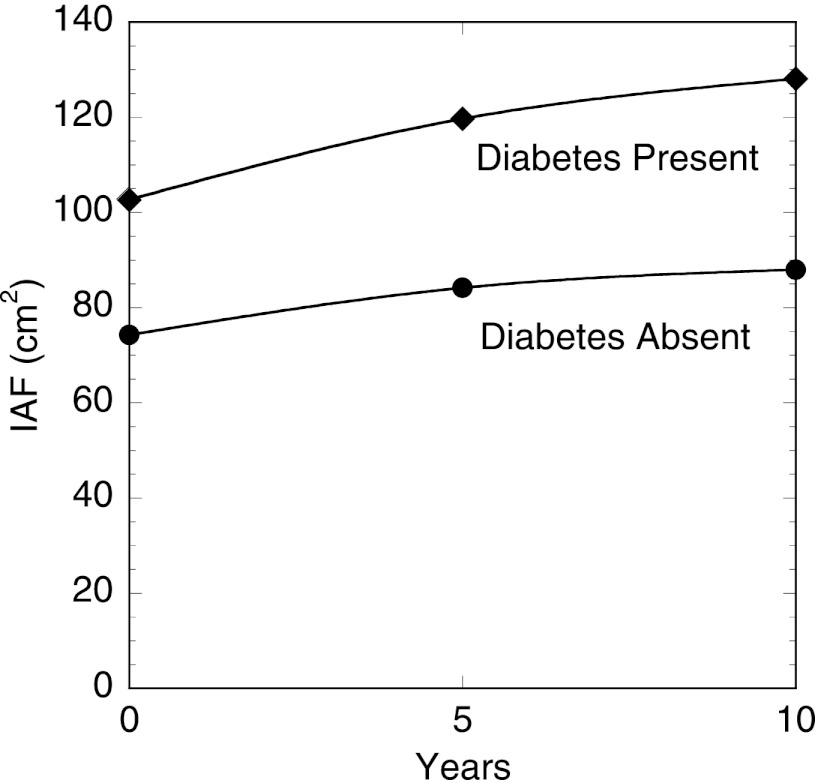
In our final model, a 1-SD increase in IAF change over 5 years was associated with a 1.65-fold increase in the odds of diabetes at 10 years (OR 1.65, 95% CI 1.21–2.25). The values of IAF over time for persons who did and did not develop diabetes are shown graphically in Fig. 1. There was no evidence of co-linearity in the multivariable models as judged by the variance inflation factor. We tested for the presence of first-order interactions between age, sex, and family history of diabetes and both baseline IAF and change in IAF and found significant interactions between baseline IAF and both age and family history. The association between baseline IAF and diabetes odds diminished with both greater age (P = 0.006) and in the presence of a family history of diabetes (P = 0.014). There were no significant interactions between sex and baseline IAF (P value for interaction = 0.789) or between sex and change in IAF (P value for interaction = 0.191).
Figure 1.
IAF area measured by CT at the level of the umbilicus, at baseline and at 5- and 10-year follow-up examinations among Japanese Americans without diabetes at baseline by whether diabetes developed during the 10-year follow-up period.
CONCLUSIONS
These data demonstrate that accumulation of visceral fat over 5 years is independently associated with greater risk of incident type 2 diabetes in Japanese Americans. The association of central adiposity with the presence of metabolic disorders is well established, but this longitudinal analysis is the first, to our knowledge, to demonstrate the role of accumulation of abdominal fat over time in the development of diabetes. These findings thus provide a novel argument in support of the hypothesis that visceral fat may be causally linked to diabetes risk.
The results of our analysis help provide additional information about the association between an increase in body fat, and in particular visceral fat, and near-term diabetes risk. We did not find an association between IAF change over 5 years and cumulative diabetes incidence over 10 years on univariate analysis, but a significant association emerged for change in IAF after adjustment for age and other covariates, including other CT measures of adipose depots as well as overall adiposity assessed with BMI. Although the associations between greater adiposity and specifically visceral adiposity and diabetes risk are well established, there is the potential that change in visceral fat may not appear to be associated with diabetes risk because of the strong association between increase in this depot and younger age, as demonstrated previously by our group (18) and in the Insulin Resistance Atherosclerosis Study (19).
The association between baseline IAF and diabetes odds diminished with greater age and in the presence of a positive family history of diabetes. The stronger association between IAF and diabetes risk at younger ages is consistent with previously published findings (20,21). It may be explained by genetic factors related to β-cell dysfunction or by the association between age and declining β-cell function, in which case the effect of IAF might be expected to be reduced. We are unaware of a possible biological explanation for the interaction between family history and IAF. No interaction was observed by sex, or between change in IAF and either sex, age, or diabetes family history.
Historically, several hypotheses have been advanced to explain the relationship between visceral fat accumulation and diabetes risk, including deposition of free fatty acids mobilized from visceral fat into the liver’s portal circulation and the presence of adipocyte-associated inflammatory markers (22). Levels of cytokines and visceral fat have been linked to type 2 diabetes and atherosclerosis in cross-sectional analysis (23). Interestingly, surgical removal of both visceral (24) and subcutaneous (25) fat depots has not been associated with improvement in insulin sensitivity, and some recent evidence suggests that the negative metabolic changes associated with fat in the abdomen may be associated specifically with intrahepatic triglyceride content (26,27).
Our study has some potential limitations. Although we did adjust for known covariates, the potential for confounding by unmeasured factors exists given the observational design. Diabetes status was determined with a single measurement at baseline and again at 5- and 10-year follow-up. Variability in the performance of these tests over time may have introduced error, although the error is probably random and any bias created would have been toward the null value, resulting in reporting of underestimates of associations. Although the definition of diabetes status by a single glucose measurement is not adequate for clinical diagnosis of diabetes, the World Health Organization has consistently supported using a single measurement of hyperglycemia to classify diabetes status for epidemiologic purposes (28). Although HbA1c levels were available for this population, they were not used for classification of diabetes because of the significant overlap in A1C distribution between normal subjects and individuals classified as having diabetes based on the oral glucose tolerance test and fasting plasma glucose among Japanese (29). The reason for this phenomenon is unknown, but may be due to higher rates of isolated elevated postprandial glucose levels in Asian subjects (30).
Because we did not measure islet cell antibody status of participants, we cannot exclude the possibility that some individuals may have had type 1 diabetes. However, the incidence of type 1 diabetes in individuals of Japanese ancestry is among the very lowest in the world, at less than 2.5 per 100,000 in children and probably just slightly higher in older individuals (31). The rarity of the outcome and the fact that the youngest age at enrollment in our study was 34 years makes it very likely that all subjects with incident diabetes in this analysis had type 2 diabetes. Body composition measurements were derived from a single CT-scan slice. However, a high correlation has been demonstrated between a single CT slice and direct measurement of visceral fat volume, which limits the potential for bias (32).
The 20% loss to follow-up could have introduced bias if such loss were related both to diabetes odds and to change in IAF. Nevertheless, this rate is low for a prospective study of this type conducted over a prolonged period of time. Although the exclusively Japanese-American cohort limits the ability to generalize, it also decreases the chance of confounding because of genetic admixture. Finally, this analysis could not exclude all incident cases of diabetes that developed in the period before repeat IAF measurement at 5 years. It is possible that the development of diabetes preceded change in IAF in some of these subjects.
This observational study does not provide direct implications for diabetes prevention, but it does suggest opportunities for interventional studies. In the Diabetes Prevention Program, loss of visceral adiposity was associated with lower risk of diabetes in post hoc analyses (8). In middle-aged and older populations, weight loss has been shown to come from central adipose depots early (33). Because of these phenomena, future research targeting and quantifying central weight loss may help provide important information pertinent to diabetes prevention efforts. In summary, this analysis adds support for a causal role of IAF in diabetes risk, although additional research will be needed to confirm its value and exclude a chance association.
Acknowledgments
This work was supported by National Institutes of Health grants DK-31170, HL-49293, and DK-02654 and by facilities and services provided by the Diabetes Research Center (DK-17047), Clinical Nutrition Research Unit (DK-35816), and the General Clinical Research Center (RR-00037) at the University of Washington. VA Puget Sound Health Care System provides support for the involvement of E.J.B. and S.E.K. in this research.
No potential conflicts of interest relevant to this article were reported.
P.L.W. researched the data and wrote the manuscript. E.J.B., D.L.L., and M.J.M. collected and researched the data, contributed to the discussion, and edited the manuscript. S.E.K. reviewed and edited the manuscript. W.Y.F. collected and researched the data, contributed to the discussion, and edited the manuscript. E.J.B. and P.L.W. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful to the King County Japanese-American community for support and cooperation.
References
- 1.Cassano PA, Rosner B, Vokonas PS, Weiss ST. Obesity and body fat distribution in relation to the incidence of non-insulin-dependent diabetes mellitus. A prospective cohort study of men in the normative aging study. Am J Epidemiol 1992;136:1474–1486 [DOI] [PubMed] [Google Scholar]
- 2.Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol 1997;145:614–619 [DOI] [PubMed] [Google Scholar]
- 3.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 2000;23:465–471 [DOI] [PubMed] [Google Scholar]
- 4.Pontiroli AE, Galli L. Duration of obesity is a risk factor for non-insulin-dependent diabetes mellitus, not for arterial hypertension or for hyperlipidaemia. Acta Diabetol 1998;35:130–136 [DOI] [PubMed] [Google Scholar]
- 5.de Koning L, Hu FB. Commentary: obesity-years—a new metric to measure health effects of obesity. Int J Epidemiol 2011;40:996–997 [DOI] [PubMed] [Google Scholar]
- 6.Li M, Campbell S, McDermott RA. Six year weight change and type 2 diabetes among Australian Indigenous adults. Diabetes Res Clin Pract 2010;88:203–208 [DOI] [PubMed] [Google Scholar]
- 7.Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol 2004;159:1150–1159 [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto WY, Jablonski KA, Bray GA, et al. Diabetes Prevention Program Research Group Body size and shape changes and the risk of diabetes in the diabetes prevention program. Diabetes 2007;56:1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao D, Asberry PJ, Shofer JB, et al. Improvement of BMI, body composition, and body fat distribution with lifestyle modification in Japanese Americans with impaired glucose tolerance. Diabetes Care 2002;25:1504–1510 [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto WY, Leonetti DL, Kinyoun JL, et al. Prevalence of diabetes mellitus and impaired glucose tolerance among second-generation Japanese-American men. Diabetes 1987;36:721–729 [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto WY, Bergstrom RW, Boyko EJ, et al. Diabetes and diabetes risk factors in second- and third-generation Japanese Americans in Seattle, Washington. Diabetes Res Clin Pract 1994;24(Suppl):S43–S52 [DOI] [PubMed] [Google Scholar]
- 12.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 13.Lee IM, Paffenbarger RS, Jr, Hsieh CC. Time trends in physical activity among college alumni, 1962-1988. Am J Epidemiol 1992;135:915–925 [DOI] [PubMed] [Google Scholar]
- 14.Shuman WP, Morris LL, Leonetti DL, et al. Abnormal body fat distribution detected by computed tomography in diabetic men. Invest Radiol 1986;21:483–487 [DOI] [PubMed] [Google Scholar]
- 15.Li H, Isomaa B, Taskinen MR, Groop L, Tuomi T. Consequences of a family history of type 1 and type 2 diabetes on the phenotype of patients with type 2 diabetes. Diabetes Care 2000;23:589–594 [DOI] [PubMed] [Google Scholar]
- 16.Narayan KM, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care 2007;30:1562–1566 [DOI] [PubMed] [Google Scholar]
- 17.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–1890 [DOI] [PubMed] [Google Scholar]
- 18.Lee CG, Fujimoto WY, Brunzell JD, et al. Intra-abdominal fat accumulation is greatest at younger ages in Japanese-American adults. Diabetes Res Clin Pract 2010;89:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hairston KG, Scherzinger A, Foy C, et al. Five-year change in visceral adipose tissue quantity in a minority cohort: the Insulin Resistance Atherosclerosis Study (IRAS) family study. Diabetes Care 2009;32:1553–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeely MJ, Boyko EJ, Shofer JB, Newell-Morris L, Leonetti DL, Fujimoto WY. Standard definitions of overweight and central adiposity for determining diabetes risk in Japanese Americans. Am J Clin Nutr 2001;74:101–107 [DOI] [PubMed] [Google Scholar]
- 21.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 1999;282:1523–1529 [DOI] [PubMed] [Google Scholar]
- 22.Bergman RN, Kim SP, Catalano KJ, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14(Suppl 1):16S–19S [DOI] [PubMed] [Google Scholar]
- 23.Indulekha K, Anjana RM, Surendar J, Mohan V. Association of visceral and subcutaneous fat with glucose intolerance, insulin resistance, adipocytokines and inflammatory markers in Asian Indians (CURES-113). Clin Biochem 2011;44:281–287 [DOI] [PubMed] [Google Scholar]
- 24.Fabbrini E, Tamboli RA, Magkos F, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 2010;139:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 2004;350:2549–2557 [DOI] [PubMed] [Google Scholar]
- 26.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 2009;106:15430–15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magkos F, Fabbrini E, Mohammed BS, Patterson BW, Klein S. Increased whole-body adiposity without a concomitant increase in liver fat is not associated with augmented metabolic dysfunction. Obesity (Silver Spring) 2010;18:1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15(7):539–553. [DOI] [PubMed]
- 29.Kuzuya T, Nakagawa S, Satoh J, et al. Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract 2002;55:65–85 [DOI] [PubMed] [Google Scholar]
- 30.Dong XL, Liu Y, Sun Y, et al. Comparison of HbA1c and OGTT criteria to diagnose diabetes among Chinese. Exp Clin Endocrinol Diabetes 2011;119:366–369 [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki E, Matsuura N, Eguchi K. Type 1 diabetes in Japan. Diabetologia 2006;49:828–836 [DOI] [PubMed] [Google Scholar]
- 32.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 2000;278:E941–E948 [DOI] [PubMed] [Google Scholar]
- 33.Colman E, Katzel LI, Rogus E, Coon P, Muller D, Goldberg AP. Weight loss reduces abdominal fat and improves insulin action in middle-aged and older men with impaired glucose tolerance. Metabolism 1995;44:1502–1508 [DOI] [PubMed] [Google Scholar]