Abstract
OBJECTIVE
To compare the pharmacokinetics and glucodynamics of three rapid-acting insulin analogs (aspart, glulisine, and lispro) injected subcutaneously with or without recombinant human hyaluronidase (rHuPH20).
RESEARCH DESIGN AND METHODS
This double-blind six-way crossover euglycemic glucose clamp study was conducted in 14 healthy volunteers. Each analog was injected subcutaneously (0.15 units/kg) with or without rHuPH20.
RESULTS
The commercial formulations had comparable insulin time-exposure and time-action profiles as follows: 50% exposure at 123–131 min and 50% total glucose infused at 183–186 min. With rHuPH20, the analogs had faster yet still comparable profiles: 50% exposure at 71–79 min and 50% glucose infused at 127–140 min. The accelerated absorption with rHuPH20 led to twice the exposure in the first hour and half the exposure beyond 2 h, which resulted in 13- to 25-min faster onset and 40- to 49-min shorter mean duration of insulin action.
CONCLUSIONS
Coinjection of rHuPH20 with rapid-acting analogs accelerated insulin exposure, producing an ultra-rapid time-action profile with a faster onset and shorter duration of insulin action.
Rapid-acting insulin analogs with accelerated insulin absorption and metabolic effect relative to regular human insulin have been introduced over the past 15 years (1–3). These products allow improved control of postprandial hyperglycemia and reduce risk for nocturnal hypoglycemia (4,5). Even these rapid-acting analogs are too slow to allow optimum glycemic response when prandial insulin injection occurs immediately before a meal and a meal delay of 20 min or more is required to optimize postmeal glycemic response (6,7). In response, new ultra-rapid insulin products are undergoing development (8).
Hyaluronidases have a long history of clinical use increasing the dispersion and absorption of subcutaneously administered drugs (9). Recombinant human hyaluronidase (rHuPH20) accelerates the absorption and action of coinjected regular human insulin and the rapid-acting insulin analog lispro (8,10,11). This study evaluated the pharmacokinetic (PK) and glucodynamic properties of three commercially available rapid-acting insulin analogs, lispro, aspart, and glulisine with or without rHuPH20.
RESEARCH DESIGN AND METHODS
This double-blind, six-way crossover study was conducted in 14 healthy fasting volunteers. The active comparators were 100 U/mL insulin lispro injection (Humalog; Lilly), insulin aspart injection (NovoLog; Novo Nordisk), and insulin glulisine injection (Apidra; sanofi-aventis). Investigational drugs were prepared by diluting each to 95 U/mL with rHuPH20 for a final concentration of 5 μg rHuPH20/mL. All study drugs were injected subcutaneously (0.15 units/kg) in the abdomen in random sequence. Eight-hour euglycemic glucose clamps and statistical analyses were conducted as previously described (11,12) and are described in detail (Fig. 1). Mean duration of action, which is the equivalent glucose infusion measure to mean residence time for PK curves, measures the arithmetic mean or first moment of the glucose infusion rate (GIR) as a function of time curve, and like mean residence time is calculated as the area under the first-moment curve (product of time and GIR as a function of time) divided by the area under the GIR curve (total glucose infused).
Figure 1.
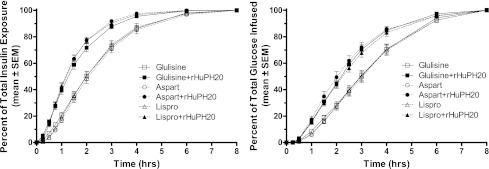
Cumulative exposure and action of insulins glulisine, lispro, and aspart after subcutaneous injection with and without rHuPH20. Blood samples were collected on each dosing day at 30, 20, and 10 min before and 3, 6, 9, 12, 15, 20, 25, 30, 45, 60, 75, 90, 120, 150, 180, 210, 240, 300, 360, 420, and 480 min after injection of each study drug for the measurement of serum insulin samples using a conventional competitive radioimmunoassay (Millipore, St. Charles, MO) using broad-spectrum antiserum (catalog 1013-K; Millipore) that binds each of the analogs and human insulin and was validated for each of the analogs individually. A Biostator was used to clamp blood glucose at 90% fasting level to suppress endogenous insulin production with tight blood glucose control (individual clamp glucose SDs were between 3.5 and 6.8 mg/dL). Results are displayed as a cumulative percent of the total exposure (left panel; see text for total exposure) and total glucose infused (right panel; mean ± SD total glucose infused were 1.7 ± 0.6, 1.7 ± 0.6, 1.8 ± 0.5, 1.7 ± 0.5, 1.9 ± 0.5, and 1.4 ± 0.5 for glulisine, glulisine plus rHuPH20, lispro, lispro plus rHuPH20, aspart, and aspart plus rHuPH20, respectively).
RESULTS
Fourteen subjects were enrolled and completed all six dosing visits. The subjects had a mean ± SD age of 34 ± 9 years and BMI 24.7 ± 1.5 kg/m2. Eight subjects were male. Ten were white, two were black, one was Asian, and one was Pacific Islander. Adverse events in this study were generally mild, primarily procedure related, and occurred at a similar low frequency regardless of the treatment. All injections were well tolerated, and no events were considered causally related to study drug exposure.
Total exposure was comparable with or without rHuPH20. Geometric mean ratios (90% CI) were 106% (96–118%), 112 (101–124%), and 101 (91–112%) for glulisine, lispro, and aspart, respectively, here and in subsequent listings. None of the differences was statistically significant, and because the 90% CIs for each analog with rHuPH20 were within the 80–125% range of control, the total exposure with or without rHuPH20 is considered bioequivalent (13).
Insulin exposure was accelerated for all three analogs with rHuPH20 (Fig. 1). Time to 50% exposure (mean ± SD) was decreased from approximately 2 h for the analogs alone to approximately 75 min when injected with rHuPH20 (124 ± 27 to 79 ± 19, 123 ± 28 to 71 ± 13, and 131 ± 30 to 73 ± 15 min; all P < 0.0001). The peak exposure (Cmax) was greater and earlier (tmax) when each analog was injected with rHuPH20 (P < 0.0001 unless noted); geometric mean Cmax ratios were 158, 210, and 187%, and tmax was reduced from 80 to 41, from 68 to 41 (P = 0.0032), and from 86 to 44 min. Early (first-hour) exposure was doubled with rHuPH20 (geometric ratios: 208, 291, and 266%) and halved beyond 2 h (58, 53, and 41%; all P < 0.0001).
The faster insulin PK led to an accelerated time-action profile for each analog when injected with rHuPH20 (Fig. 1). The approximately 45-min acceleration of insulin exposure was mirrored with a corresponding acceleration of time to 50% glucose infused from approximately 3 h when injected alone to just more than 2 h with rHuPH20 (183 ± 34 to 135 ± 28, 186 ± 38 to 140 ± 28, and 186 ± 34 to 127 ± 32 min; all P < 0.0001). The accelerated time-action profiles with rHuPH20 had a 13- to 25-min faster onset of action (early T50% GIRmax), 47 ± 19 to 34 ± 15 min (P = 0.0092), 51 ± 21 to 35 ± 8 min (P = 0.0022), and 58 ± 16 to 33 ± 10 min (P < 0.0001), and approximately 45-min shorter mean duration of action: 189 ± 33 to 148 ± 29, 194 ± 35 to 154 ± 28, and 194 ± 32 to 145 ± 29 min (all P < 0.0001).
The commercial rapid-acting analog products have generally comparable rapid PK and time-action profiles, although compared with the others glulisine, they did have a slightly faster onset (early t50%) of exposure (21 min for glulisine compared with 31 min [P = 0.0007] for lispro and 32 min [P = 0.0002] for aspart). rHuPH20 coinjection accelerated the absorption of each of the three analogs, producing a faster-in faster-out time-exposure profile, and a faster onset and shorter mean duration of insulin action. With rHuPH20, the now ultra-rapid PK and time-action profiles were also generally comparable for the three analogs. There were no meaningful differences between lispro plus rHuPH20 and aspart plus rHuPH20 for any PK or time-action parameter. Glulisine plus rHuPH20 had a similar profile, although it was somewhat broader, with both slightly greater early and slightly greater late insulin exposure; early t50% was 10 min for glulisine plus rHuPH20, faster than aspart plus rHuPH20 (18 min; P = 0.005) and lispro plus rHuPH20 (19 min; P = 0.002), and late t50% was 119 min for glulisine plus rHuPH20, slower than lispro plus rHuPH20 (90 min; P = 0.0034), and trending slower than aspart plus rHuPH20 (103 min; P = 0.10).
CONCLUSIONS
The acceleration of insulin PK and time-action profiles by coinjection with rHuPH20 observed in this study confirms the results of previous studies with regular human insulin and lispro (8,10,11) and extends these findings to insulins aspart and glulisine. The ultra-rapid profiles described here could have benefits improving control of postprandial glycemic excursions, and administration of lispro plus rHuPH20 has been shown to reduce postprandial hyperglycemia relative to lispro alone dosing immediately before liquid meals in patients with type 1 and type 2 diabetes (14,15). Studies are currently underway to compare the utility of ultrafast aspart plus rHuPH20 and lispro plus rHuPH20 drug products with commercial Humalog as part of intensive basal-bolus insulin therapy in the treatment of type 1 and type 2 diabetes (NCT01194245 and NCT01194258, clinicaltrials.gov).
Acknowledgments
This study was sponsored by Halozyme Therapeutics, San Diego, California.
D.B.M. and D.E.V. are employees with equity interest in Halozyme Therapeutics, the sponsor of this study, and E.A.L. consulted for Halozyme. L.M. and M.H. are employees with equity interest in Profil Institute for Clinical Research. No other potential conflicts of interest relevant to this article were reported.
L.M., D.B.M., and M.H. conducted the study. D.B.M. and D.E.V. conceived the study. L.M., D.B.M., M.H., E.A.L., and D.E.V. designed the study, analyzed and interpreted the results, and contributed to the review and revision of the study. D.E.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented as an oral presentation at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010 and in poster form at the 49th European Association for the Study of Diabetes, Stockholm, Sweden, 20–24 September 2010.
The authors are grateful to Lutz Heinemann (Profil) and Beatrix Schramm (Halozyme) for careful and thoughtful review of the manuscript and suggestions that have improved it.
Footnotes
Clinical trial reg. no. NCT00979875, clinicaltrials.gov.
References
- 1.Holleman F, Hoekstra JB. Insulin lispro. N Engl J Med 1997;337:176–183 [DOI] [PubMed] [Google Scholar]
- 2.Lindholm A, Jacobsen LV. Clinical pharmacokinetics and pharmacodynamics of insulin aspart. Clin Pharmacokinet 2001;40:641–659 [DOI] [PubMed] [Google Scholar]
- 3.Becker RH, Frick AD. Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin Pharmacokinet 2008;47:7–20 [DOI] [PubMed] [Google Scholar]
- 4.Hirsch IB. Insulin analogues. N Engl J Med 2005;352:174–183 [DOI] [PubMed] [Google Scholar]
- 5.Plank J, Siebenhofer A, Berghold A, et al. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch Intern Med 2005;165:1337–1344 [DOI] [PubMed] [Google Scholar]
- 6.Howey DC, Bowsher RR, Brunelle RL, et al. [Lys(B28), Pro(B29)]-human insulin: effect of injection time on postprandial glycemia. Clin Pharmacol Ther 1995;58:459–469 [DOI] [PubMed] [Google Scholar]
- 7.Rassam AG, Zeise TM, Burge MR, Schade DS. Optimal administration of lispro insulin in hyperglycemic type 1 diabetes. Diabetes Care 1999;22:133–136 [DOI] [PubMed] [Google Scholar]
- 8.Muchmore DB, Vaughn DE. Review of the mechanism of action and clinical efficacy of recombinant human hyaluronidase coadministration with current prandial insulin formulations. J Diabetes Sci Tech 2010;4:419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv 2007;4:427–440 [DOI] [PubMed] [Google Scholar]
- 10.Vaughn DE, Yocum RC, Muchmore DB, et al. Accelerated pharmacokinetics and glucodynamics of prandial insulins injected with recombinant human hyaluronidase. Diabetes Technol Ther 2009;11:345–352 [DOI] [PubMed] [Google Scholar]
- 11.Morrow L, Muchmore DB, Ludington EA, Vaughn DE, Hompesch M. Reduction in intrasubject variability in the pharmacokinetic response to insulin after subcutaneous co-administration with recombinant human hyaluronidase in healthy volunteers. Diabetes Technol Ther 2011;13:1039–1045 [DOI] [PubMed] [Google Scholar]
- 12.Heinemann L, Anderson JH., Jr Measurement of insulin absorption and insulin action. Diabetes Technol Ther 2004;6:698–718 [DOI] [PubMed] [Google Scholar]
- 13.US FDA Guidance for Industry. Bioavailability and bioequivalence studies for orally administered drug products–general considerations. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070124.pdf Accessed 26 April 2012
- 14.Hompesch M, Muchmore DB, Morrow L, Vaughn DE. Accelerated insulin pharmacokinetics and improved postprandial glycemic control in patients with type 1 diabetes after coadministration of prandial insulins with hyaluronidase. Diabetes Care 2011;34:666–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hompesch M, Muchmore DB, Morrow L, Ludington EA, Vaughn DE. Improved postprandial glycemic control in patients with type 2 diabetes from subcutaneous injection of insulin lispro with hyaluronidase. Diabetes Technol Ther 2012;14:218–224 [DOI] [PubMed] [Google Scholar]


