Abstract
OBJECTIVE
To evaluate the feasibility of free-living walking training in type 2 diabetic patients and to investigate the effects of interval-walking training versus continuous-walking training upon physical fitness, body composition, and glycemic control.
RESEARCH DESIGN AND METHODS
Subjects with type 2 diabetes were randomized to a control (n = 8), continuous-walking (n = 12), or interval-walking group (n = 12). Training groups were prescribed five sessions per week (60 min/session) and were controlled with an accelerometer and a heart-rate monitor. Continuous walkers performed all training at moderate intensity, whereas interval walkers alternated 3-min repetitions at low and high intensity. Before and after the 4-month intervention, the following variables were measured: VO2max, body composition, and glycemic control (fasting glucose, HbA1c, oral glucose tolerance test, and continuous glucose monitoring [CGM]).
RESULTS
Training adherence was high (89 ± 4%), and training energy expenditure and mean intensity were comparable. VO2max increased 16.1 ± 3.7% in the interval-walking group (P < 0.05), whereas no changes were observed in the continuous-walking or control group. Body mass and adiposity (fat mass and visceral fat) decreased in the interval-walking group only (P < 0.05). Glycemic control (elevated mean CGM glucose levels and increased fasting insulin) worsened in the control group (P < 0.05), whereas mean (P = 0.05) and maximum (P < 0.05) CGM glucose levels decreased in the interval-walking group. The continuous walkers showed no changes in glycemic control.
CONCLUSIONS
Free-living walking training is feasible in type 2 diabetic patients. Continuous walking offsets the deterioration in glycemia seen in the control group, and interval walking is superior to energy expenditure–matched continuous walking for improving physical fitness, body composition, and glycemic control.
The number of patients with type 2 diabetes is rapidly increasing, with an estimated 439 million people diagnosed worldwide by 2030 (1). There are many reasons for this, among which increased life span, increased obesity, and increased urbanization with reduced physical activity levels are seen as key components (1). Physical activity is a first-line treatment in type 2 diabetes, and the effect of physical activity on glycemic control and body composition is well documented (2,3). Most physical activity interventions have used supervised training regimes. When considering the high prevalence of type 2 diabetes, fully supervised training programs are not feasible for primary-care exercise implementation. A need for training methods that can be implemented and sustained in a free-living environment is evident.
Physical fitness level and physical inactivity are strong predictors for all-cause mortality (4), also in subjects with type 2 diabetes (5,6). A training program should therefore increase physical fitness level and physical activity. High-intensity training favors improvements in physical fitness level (7), but more intense training programs are associated with decreased training adherence (8), and the overall impact of exercise intensity in type 2 diabetic patients is still unclear (2).
Walking is feasible for most individuals, and walking training programs have been evaluated extensively in type 2 diabetic patients (9–16). These studies indicate that walking training can be implemented in type 2 diabetic patients, but only minor or no beneficial effects have been shown, potentially indicating that the intensity of normal walking is insufficient. Recently, high-intensity interval training protocols have been evaluated in subjects with metabolic syndrome and type 2 diabetes (17,18). These studies have shown drastic improvements in glycemic control and cardiovascular risk factors, and although the high exercise intensity used may limit the feasibility under free-living and nonsupervised conditions, it highlights the potential of interval training modalities in type 2 diabetic patients.
Interval-walking training (IWT) has been developed as a novel free-living training modality that improves physical fitness and cardiovascular risk factors in older subjects (19,20). IWT is implemented using a triaxial accelerometer training device (JD Mate; Kissei Comtec, Matsumoto, Japan) (21) and consists of repeated cycles of slow and fast walking. These cycles are controlled by the JD Mate, which audibly signals to subjects. IWT has been successfully implemented and sustained in large populations, but it has never been tested in a group with disease.
The aim of this study was therefore to test the feasibility of free-living walking training in patients with type 2 diabetes, using a randomized, controlled design. Furthermore, we compared the efficacy of IWT with energy expenditure–matched continuous-walking training (CWT) with regards to changes in fitness level, body composition, and glycemic control. We hypothesized that walking training could be successfully implemented and that IWT would be superior to CWT with regards to improvements in physical fitness and glycemic control, despite expected equal improvements in body composition.
RESEARCH DESIGN AND METHODS
Subjects
Subjects with type 2 diabetes (22) were recruited by advertisements in newspapers and by contacting local diabetes patient organizations. All volunteers underwent medical screening, including a health status interview, physical exam, blood chemistry analysis, and oral glucose tolerance test (OGTT). Exclusion criteria included the use of exogenous insulin, weight instability (>2 kg/6 months), physical activity (>150 min/week), and evidence of liver, renal, and cardiopulmonary disease and diseases contraindicating physical activity (23).
A priori power calculations indicated that n = 12 in each training group would be sufficient to detect significant changes in glycemic control. Initially, n = 24 subjects were included in the study and randomized to three groups: control (CON; n = 8), CWT (n = 8), and IWT (n = 8). Two subjects dropped out (one CWT subject due to a knee injury and one IWT subject due to new-onset asthmatic symptoms). Subjects in the CON group were offered rerandomization into a training group after the CON period. Five subjects accepted, adding n = 3 to the CWT group and n = 2 to the IWT group. To meet the planned sample size, an additional five subjects were included and randomized to one of the training groups, adding n = 2 to the CWT group and n = 3 to the IWT group. The final study population consisted of n = 27 subjects undergoing the intervention, of whom n = 5 underwent both CON and either CWT or IWT. Therefore, n = 32 pre- and posttrials were included for statistical analysis (CON, n = 8; CWT, n = 12; IWT, n = 12).
Written informed consent was obtained from all subjects. This study was approved by the ethical committee of the Capital Region of Denmark.
Interventions
All subjects received a JD Mate, which was worn as a pedometer throughout the study. In addition to this, subjects randomized to a training group used the JD Mate’s training function, which, based on triaxial accelerometry, estimates training energy expenditure. Initially, training subjects performed a graded walking VO2 peak test, from which peak energy-expenditure rate was obtained (19,20). The test was repeated monthly to ensure that relative workload was consistent with changes in aerobic fitness. Subjects in the CWT group had the target energy-expenditure rate set for 55% of the peak energy-expenditure rate and were instructed to perform CWT above the target. Subjects in the IWT group had the target energy-expenditure rate set for 70% of the peak energy-expenditure rate and were instructed to perform IWT consisting of cycles of 3 min of fast walking (above the target) and 3 min of slow walking (below the target) according to a protocol described earlier (19,20). The aim was to match CWT and IWT by overall energy expenditure and mean training intensity. All training subjects were prescribed five training sessions per week, 60 min/session for 4 months, and training effort was monitored by data upload every second week. At these sessions, feedback of the training achievements was provided. Furthermore, all training sessions were performed with a heart rate monitor (Polar RS400, Polar, Kempele, Finland) as an additional measurement of training intensity. Subjects in the CON group were instructed to continue their habitual lifestyle for 4 months and had their JD Mate pedometer data uploaded monthly.
Investigations
Prior to pre- and postintervention tests, subjects abstained from their antidiabetic, antihypertensive, and lipid-lowering drugs for 5 days.
On day 1, a triaxial accelerometer (Actiheart; CamNtech, Cambridge, U.K.) (24) was provided to the subjects for continuous monitoring of basal physical activity over the following 6 days. A Minnesota Leisure Time Physical Activity Questionnaire was completed to assess habitual activity habits over the previous 4 months (25). A diet record was started and continued for the following 3 days. A continuous glucose monitoring (CGM) system (Guardian Real-Time; Medtronic, Santa Rosa, CA) was installed by insertion of a glucose sensor (Sof-Sensor; Medtronic) in the abdominal subcutaneous tissue. The CGM system was calibrated three times per day using a point-of-care glucose monitor (Contour Link; Bayer, Zürich, Switzerland). CGM continued for the following 3 days, and data from 2 full days (days 2 and 3) were used for analyses.
On day 4, after an overnight fast (≥8 h), an antecubital venous catheter was placed, and baseline blood samples were collected for determination of plasma glucose, lipids, HbA1c, and serum insulin. A 3-h, 75-g OGTT was then performed with blood collection every 20 min. Blood was collected into tubes containing the following coagulation inhibitors: sodium fluoride tubes for glucose analyses, lithium-heparin tubes for cholesterol and triglyceride analyses, EDTA tubes for HbA1c analyses, and serum tubes (no inhibitors) for insulin analyses. Blood samples for plasma collection were immediately placed on ice and subsequently centrifuged (2,000g, 15 min, 4°C). Samples for serum collection were left at room temperature for 30 min and centrifuged. Samples were stored at −80°C until analysis.
On day 6 or 7, subjects came in for measurement of anthropometrical variables (weight and hip and waist circumference). Body composition was assessed by dual-energy X-ray absorptiometry (Lunar Prodigy Advance; GE Healthcare, Madison, WI). After 10 min of supine resting, blood pressure was measured. Maximal oxygen consumption (VO2max) was measured by indirect calorimetry during an incremental exhaustive treadmill (Technogym Runrace, Gambettola, Italy) walking test. The test consisted of a 5-min warm-up (individually determined moderate walking pace, 0% incline) followed by 2-min stages of increasing inclines (2% per stage) at individually determined brisk walking pace until the following criteria were met: plateauing of heart rate and VO2 with incremental workloads, respiratory exchange ratio >1.1, or volitional exhaustion. VO2max is reported as the mean of the two highest consecutive measurements (10 s per measurement). Finally, a magnetic resonance imaging (MRI) scan (Siemens Magnetom, 3 tesla, Erlangen, Germany) of the abdominal region was performed. After a localization scan, a transversal, multislice, T1-weighted scan was executed with 0.5 cm between slices.
A repeated measure of all tests was conducted 4 months later, after subjects had completed their randomized intervention. Posttraining CGM measurements commenced 48–72 h after the final exercise bout, and fasting blood samples and OGTTs were performed 96–120 h after the last exercise bout.
Analyses
Diet records were analyzed using Dankost Slank version 2.0 (Danish Catering Centre, Herlev, Denmark).
Plasma glucose, cholesterol fractions, and triglycerides were determined by an enzymatic colorimetric assay (P-Modular; Roche, Stockholm, Switzerland), HbA1c by high-performance liquid chromatography (Tosoh G7 Analyzer, San Francisco, CA), and serum insulin by electrochemiluminescence immunoassay (E-Modular; Roche).
MRI scans were analyzed using Mango version 2.5 (Research Imaging Center, University of Texas Health Science Center at San Antonio, San Antonio, Texas). The abdominal region was defined as delimited of the diaphragm muscle proximally and a horizontal level of the upper part of the sacral bone distally. After manual exclusion of subcutaneous and large-vessel fat tissue, a semiautomatic segmentation that included the remaining (visceral) fat tissue was performed. Volume was calculated by voxel summation. All analyses were performed by the same blinded observer.
Statistical analyses
Results are reported as mean ± SEM. Baseline differences were compared using Fischer exact test for nonparametric variables (sex and medication) and one-way ANOVA for parametric variables. Since baseline systolic blood pressure differed between CWT and IWT, systolic blood pressure was used as a covariate in ANOVA analyses. Furthermore, other potentially confounding variables (sex, age, time since diagnosis, and baseline HbA1c) were also used as covariates. Of these baseline variables, only HbA1c was related to intervention-induced changes in our outcome variables of interest. Two-way (group × time) repeated-measures ANOVA was used to compare pre- to postintervention changes within and between groups. One-way (group) ANOVA of Δ (post − preintervention) values was used to compare intervention-induced differences between groups. For all ANOVAs, Bonferroni post hoc tests were used to examine the difference between means in the event of a significant finding. Training variables were compared using unpaired, two-tailed Student t test. Regression analyses were performed using changes in relative (mL/min/kg) and absolute (mL/min) VO2max and body mass as independent parameters. All analyses were repeated excluding rerandomized subjects, which, although resulting in underpowered comparisons, did not change the pattern of any results. Fischer exact test was performed using SAS version 9.1 (SAS Institute, Cary, North Carolina). All other analyses were performed using Prism version 4 (GraphPad, San Diego, CA) or SPSS version 20 (IBM, Armonk, NY). Statistical significance was accepted when P < 0.05.
RESULTS
Subjects
Table 1 shows the baseline characteristics of the subjects. Besides systolic blood pressure, no baseline differences were found between groups for any of the parameters listed in Table 1. No subjects changed medication during the study. Table 1 also indicates intervention-induced changes in variables of interest, including intervention-induced differences between groups. Table 2 shows the energy expenditure and dietary intake of subjects prior to and during the intervention. No differences were found within or between groups regarding these parameters.
Table 1.
Baseline characteristics and changes in VO2max, body composition, lipids, blood pressure, and glycemic control

Table 2.
Energy intake and expenditure and training data
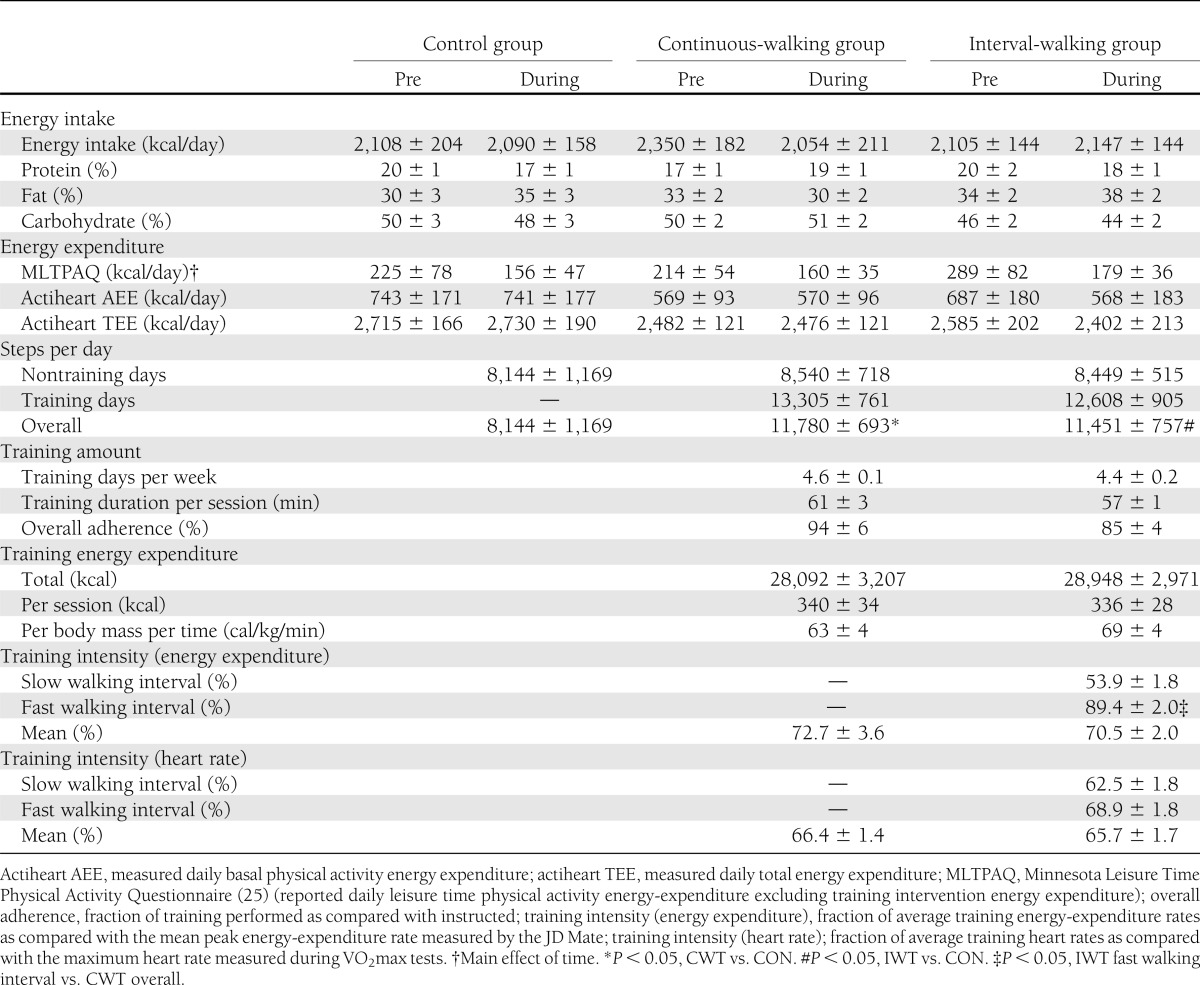
Training data
No differences in the steps per day were found between the three groups on the nontraining days. On training days, steps per day were not different between CWT and IWT, but both training groups walked more daily steps than the CON group when including both training and nontraining days. Subjects in the CWT and IWT groups trained for 59 ± 2 min/day on 4.5 ± 0.1 days/week with a mean adherence (volume of training performed as compared with prescribed) of 89 ± 4%. No differences in mean adherence, mean energy expenditure, or mean training intensity (when comparing percentage of maximum energy expenditure or percentage of maximum heart rate) were found between CWT and IWT groups. Conversely, when comparing mean training intensity of the IWT’s fast intervals with mean CWT training intensity, a significant difference was found for the JD Mate–measured intensity (P < 0.05) (Table 2).
VO2max
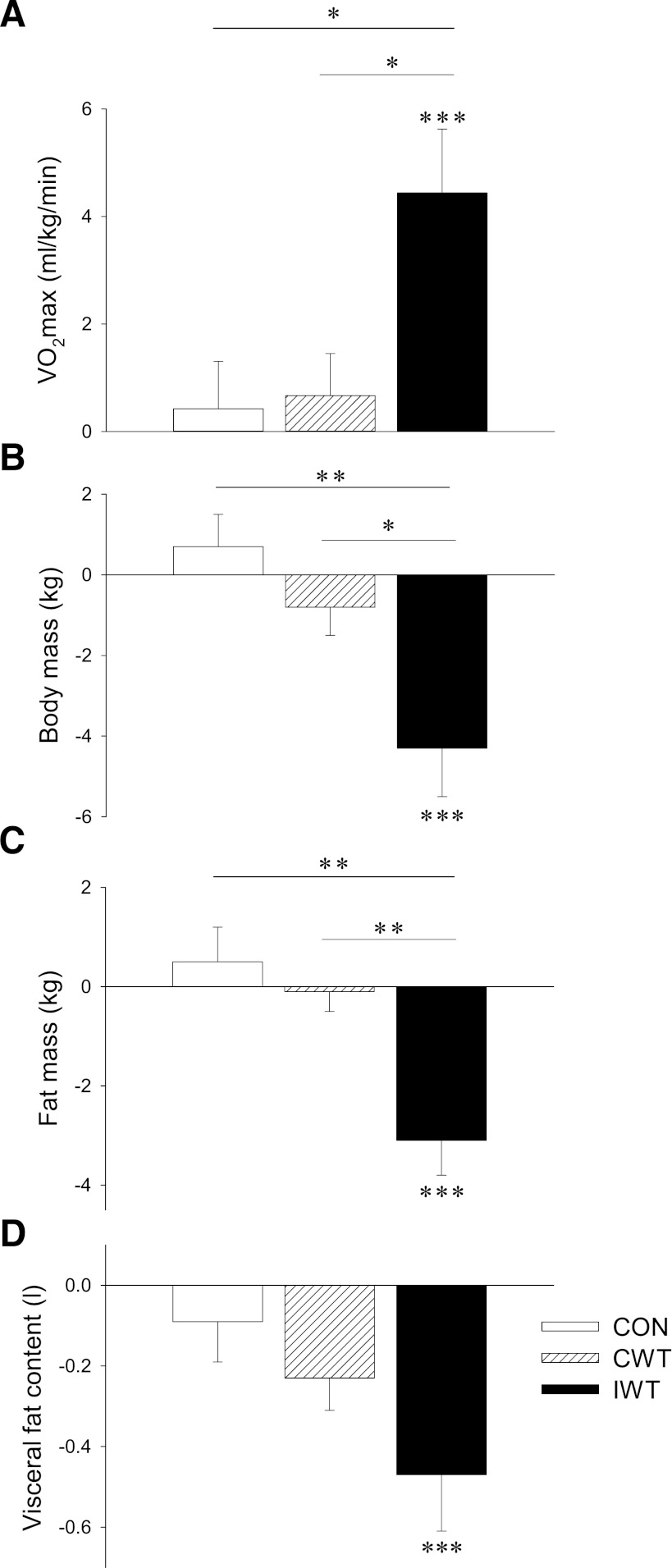
IWT subjects improved their relative VO2max by 4.4 ± 1.2 mL/kg/min (16.1 ± 3.7%, P < 0.001) and their absolute VO2max by 249 ± 85 mL/min (10.9 ± 3.2%, P < 0.01). No changes were found in the CON or CWT groups (Table 1 and Fig. 1A).
Figure 1.
Subjects with type 2 diabetes were randomized to a CON (white bars), CWT (striated bars), or IWT group (black bars). Aerobic fitness (VO2max) (A), body mass (B), whole-body fat mass (dual-energy X-ray absorptiometry) (C), and abdominal visceral adiposity (MRI) (D) were measured at baseline and after 4 months. Data are presented as mean Δ values (post − preintervention values) ± SEM. Statistical differences were analyzed by two-way repeated-measures ANOVA when comparing pre to post changes within groups (indicated by ***P < 0.001), and one-way ANOVA of Δ values when comparing differences between groups (indicated by a connecting line between bars; *P < 0.05 and **P < 0.01).
Body composition
Subjects in the IWT group lost 4.3 ± 1.2 kg of body weight (P < 0.001) (Table 1 and Fig. 1B). No significant change in lean body mass was observed, whereas body fat mass decreased by 3.1 ± 0.7 kg (P < 0.001) (Table 1 and Fig. 1C). The fat mass lost was accompanied by a decrease in the waist-to-hip ratio (P < 0.01) (Table 1). Additionally, abdominal visceral fat was decreased in the IWT group (0.54 ± 0.15 L, P < 0.001) (Table 1 and Fig. 1D). No changes in any body compositional parameters were found in the CON or CWT groups (Table 1 and Fig. 1B–D).
Lipids
A decrease in LDL cholesterol of 0.4 ± 0.2 mmol/L was found in the IWT group (P < 0.05) (Table 1). Total cholesterol increased 0.5 ± 0.2 mmol/L in the CON group (P < 0.05) (Table 1). No changes were found for HDL cholesterol or triglycerides in any of the groups (Table 1).
Blood pressure
No changes were found in systolic or diastolic blood pressure in any of the groups (Table 1).
Glycemic control
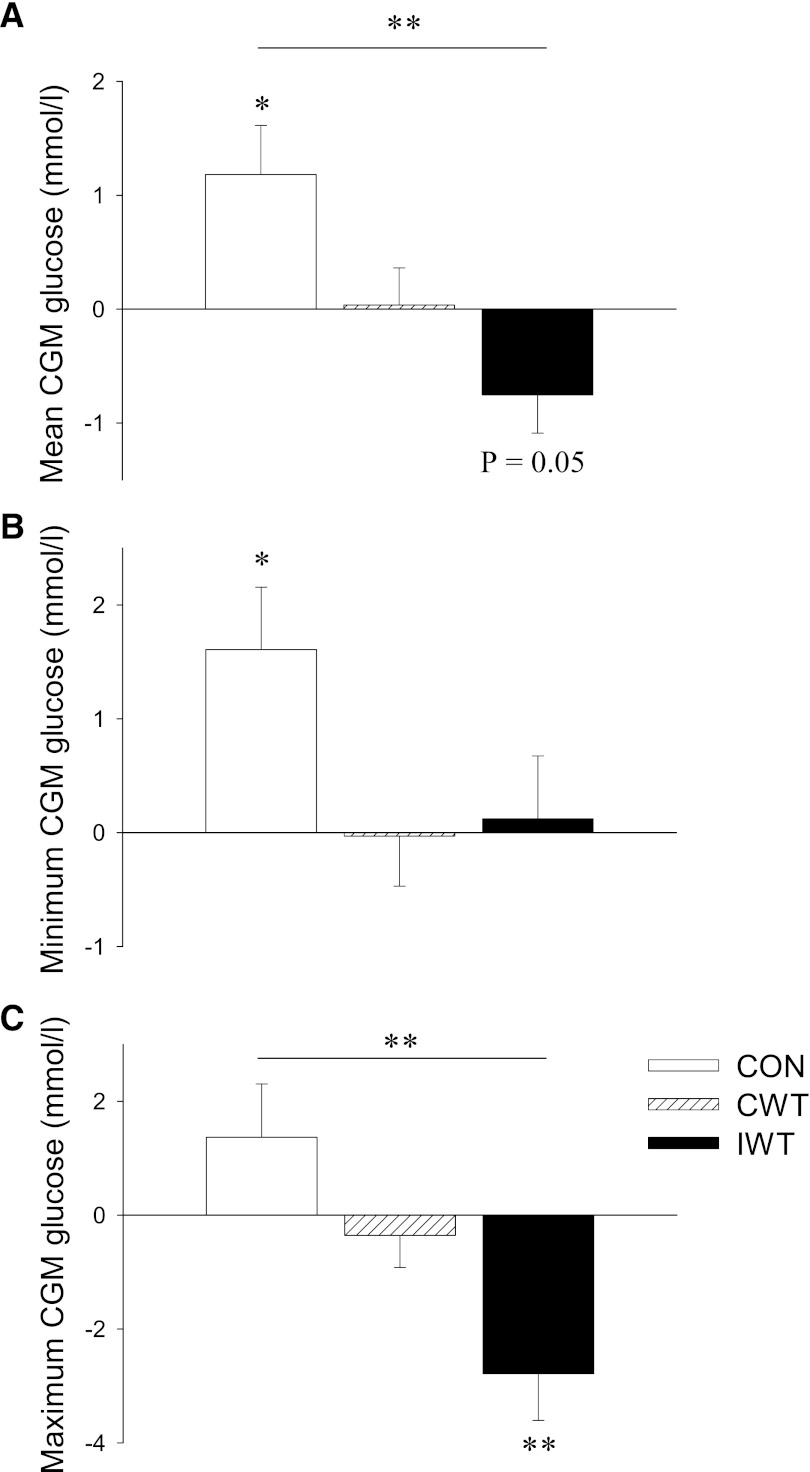
HbA1c, fasting glucose, 2-h OGTT glucose, maximum OGTT glucose, and area under the OGTT glucose curve showed a trend to increase in the CON group, while fasting insulin significantly increased (P < 0.05) (Table 1). In the CON group, there was also an increase in the mean (P < 0.05) (Fig. 2A) and minimum (P < 0.05) (Fig. 2B) CGM glucose concentrations. Mean (P = 0.05) (Fig. 2A) and maximum (P < 0.01) (Fig. 2C) CGM glucose concentrations decreased in the IWT group.
Figure 2.
Subjects with type 2 diabetes were randomized to a CON (white bars), CWT (striated bars), or IWT group (black bars). Intervention-induced changes in glycemic control were assessed by examining post − preintervention changes in the following variables: mean 48-h CGM glucose (A), minimum 48-h CGM glucose (B), and maximum 48-h CGM glucose (C). Data are presented as mean Δ values (post − preintervention values) ± SEM. Statistical differences were analyzed by two-way repeated-measures ANOVA when comparing pre to post changes within groups (indicated by *P < 0.05 and **P < 0.01), and one-way ANOVA of Δ values when comparing differences between groups (indicated by a connecting line between bars; **P < 0.01).
CONCLUSIONS
The main findings of this study are that walking exercise can be implemented as a free-living training method in type 2 diabetic patients, and that IWT is superior to energy expenditure–matched CWT with regards to improvements in physical fitness, body composition, and glycemic control, under the conditions used in the current study. Our study design included careful and successful matching of the training energy expenditure and mean intensity of the training groups, despite the free-living nature of the intervention. This justifies comparisons between IWT and CWT.
Despite the free-living nature and the large amount of intended training in the intervention (5 h/week), we experienced high adherence and low dropout rates. Other studies investigating walking training programs in type 2 diabetic patients have, in general, reported lower adherence rates (∼60%) to the training (9,11,13,14). Morikawa et al. (20) previously described excellent adherence to IWT in a very large population. They speculated that the individualization of training intensity and the regular feedback and instructions given to subjects played a role in the high adherence (20). Furthermore, the use of a device (the JD Mate) has proven important for maintaining adherence to training interventions (15).
As hypothesized, we found a difference in the physical fitness level changes between the training groups, with an increase in VO2max in the IWT group and no increase in the CWT group. Furthermore, subjects in the CWT group did not show any significant improvements in body composition or glycemic control. Type 2 diabetic patients’ self-paced walking speed is low, and potentially too low to improve health-related outcome (16,26), and previous studies using CWT interventions report only modest improvements in health-related parameters (9–11,13,15). Thus, it is likely that walking speed in our CWT group was close to the subjects’ normal walking speed, possibly explaining the apparent lack of effect.
Since overall energy expenditure was matched between the training groups, and no evidence of different dietary intake within or between any of the groups was found, we expected that body composition would change equally (27,28). We found that IWT led to a greater reduction in body weight, fat mass, and abdominal visceral adiposity than CWT. Irving et al. (29) described similar findings, with a greater loss of fat mass, including visceral fat mass, after higher-intensity training. Our results indicate that fluctuations in training intensity may have greater influence on body composition than average training intensity. Postexercise O2 consumption has been shown to correlate exponentially with training intensity (30). Although there are no published studies examining this concept in our study design, we speculate that postexercise O2 consumption was greater in the IWT group, leading to a greater overall energy expenditure during the intervention and thereby inducing greater weight loss than CWT. Further studies, where food intake is strictly controlled and standardized, are necessary to prove this speculation.
Over the 4-month period, the glycemic control of CON group subjects deteriorated. This deterioration was prevented in subjects who undertook CWT. Subjects who undertook IWT showed significant improvements in some parameters of glycemic control (Table 1 and Fig. 2). The fact that IWT reduced hyperglycemic episodes without leading to hypoglycemic episodes is also a very important finding. Glycemic excursions cause oxidative stress and cardiovascular complications in subjects with type 2 diabetes (31), and glycemic spikes are related to atherosclerosis in type 2 diabetic patients (32). Manders et al. (33) showed that a single low-intensity exercise bout was able to reduce free-living hyperglycemic episodes measured by CGM the 24 h after the exercise, but to our knowledge, no studies have previously described a long-term exercise intervention’s ability to reduce free-living hyperglycemia. Recently, however, Mikus et al. (34) showed that 7 days of aerobic exercise with strict dietary control reduces postprandial glucose and glycemic variability in patients with type 2 diabetes. Our results are in line with these previous findings, and extend them to a longer-term training intervention with free-living dietary intake. Whether or not this reduction in hyperglycemic episodes after training improves cardiovascular outcome in subjects with type 2 diabetes is unknown.
We did not find significant changes in most of the “classical” glycemic control variables (e.g., fasting glucose and HbA1c), whereas significant changes in CGM were apparent. This divergence may be explained by several points. First, fasting glucose is primarily dependent on endogenous glucose production (35). Whether or not endogenous glucose production is altered in response to training is controversial (36,37), but at least fasting glucose seems unaffected by training (38). Second, we experienced large variability in HbA1c changes in the IWT group (mean Δ values ± SEM, −0.09 ± 0.17%). If a single subject with rapidly progressing (3 years since diagnosis), severe disease (fasting glucose = 16.3 mmol/L; HbA1c = 8.2%), who experienced serious deterioration in classical glycemic control variables after the training intervention (fasting glucose = 18.3 mmol/L; HbA1c = 9.8%), was removed from the statistical analysis, significant improvements in HbA1c were encountered in the IWT group (ΔHbA1c = −0.25 ± 0.08%, P < 0.05). This may indicate a potential interaction between the stage of disease and the response to exercise training. Indeed, Dela et al. (39) previously showed that type 2 diabetic patients with poor glycemic control had poor responsiveness to exercise training. In support of this, regression analyses of our data indicate that in type 2 diabetic patients, a higher baseline HbA1c is associated with a smaller training-induced reduction in HbA1c (baseline HbA1c vs. ΔHbA1c in training groups; r = 0.54, P < 0.01), evidence that exercise responsiveness may be influenced by the underlying state of glycemic control.
Even though divergence between changes in OGTT- and CGM-derived parameters has recently been described (34), we did expect that changes in 2-h and maximum OGTT glucose would be in line with changes in mean CGM glucose. As a result, we were surprised that these OGTT-derived parameters did not improve in the IWT group, especially when considering the drop in maximum CGM glucose values after the intervention. A possible explanation for this discrepancy would be that dietary intake changed pre to post in the IWT group, which, however, was not the case (Table 2). Another explanation could be that the time between the last training session and blood sampling was longer than the duration over which improved glycemic control after exercise persisted (40). The last training day of the subjects was at least 48 h before beginning the CGM period and at least 96 h before the blood sampling. Although this explanation is speculative, it highlights the importance of regular physical activity upon sustained improvements in glycemic control.
A limitation of this study is that we experienced a difference in body compositional changes between the training groups. Therefore, we cannot conclude whether the improved VO2max on its own leads to better glycemic control in the IWT group or if this is solely a result of the improved body composition. Regression analyses, where Δ values (poststudy − prestudy) of either body mass or relative VO2max (mL/kg/min) were used as the independent variable, indicate that changes in VO2max and body mass are both correlated with changes in most of the investigated parameters of glycemic control (data not shown). When absolute changes in VO2max (L/min) were used as the independent variable, relationships persisted between changes in VO2max and changes in mean 48-h CGM glucose (r = −0.47, P < 0.05) and maximum 48-h CGM glucose (r = −0.53, P < 0.01). Although these are not causative relationships, they indicate that improvements in VO2max per se are related to changes in glycemic control independent of weight loss. In addition to this, the improvement in body composition seen in the IWT group remains an important clinical finding.
A further limitation of the study is that postintervention parameters were measured at least 48 h after and up to 8 days after the last training bout (day 7). This duration indeed may result in detraining effects. However, this would elicit equal underestimation of the beneficial effects in both training groups. Furthermore, our design enables us to evaluate the training-induced effects on glycemic control, independent of the acute effects of exercise.
In summary, we have shown that free-living walking training can be implemented in a population with type 2 diabetes. Furthermore, we have shown that IWT and CWT counteract deteriorations in glycemic control and that IWT is superior to CWT with regards to improvements in physical fitness, body composition, and glycemic control. IWT may therefore be a good option when considering which type of training type 2 diabetic patients should be offered in primary care.
Acknowledgments
This study was primarily funded by DD2 (The Danish Centre for Strategic Research in Type 2 Diabetes) supported by the Danish Agency for Science (grants 09-067009 and 09-075724 to B.K.P.). The project partners are listed on the project website, www.DD2.nu. This study was further supported by grants from the AP Møller Foundation (K.K.), Trygfonden (B.K.P.), and the European Foundation for the Study of Diabetes (T.P.J.S.). The Centre of Inflammation and Metabolism (CIM) is supported by a grant from the Danish National Research Foundation (02-512-55). CIM is part of the UNIK Project: Food, Fitness & Pharma for Health and Disease, supported by the Danish Ministry of Science, Technology, and Innovation.
No potential conflicts of interest relevant to this article were reported.
K.K. and T.P.J.S. designed the study, obtained the funding, researched and analyzed data, wrote the manuscript, contributed to the discussion, reviewed and edited the manuscript, and approved the final version. K.W. and S.H.K. researched and analyzed data, contributed to the discussion, reviewed and edited the manuscript, and approved the final version. J.S.N. designed the study, contributed to the discussion, reviewed and edited the manuscript, and approved the final version. C.T. contributed to the discussion, reviewed and edited the manuscript, and approved the final version. B.K.P. designed the study, obtained the funding, contributed to the discussion, reviewed and edited the manuscript, and approved the final version. T.P.J.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Ruth Rovsing, Hanne Villumsen, Tine B. Krüger, Louise Lehrskov-Schmidt, Ida Christine Hansen, Rikke M. Dahl, Dean Tuladhar, and Thomas S. Grøndahl (University of Copenhagen) for their technical assistance.
Footnotes
Clinical trial reg. no. NCT01234155, clinicaltrials.gov.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14 [DOI] [PubMed] [Google Scholar]
- 2.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care 2006;29:2518–2527 [DOI] [PubMed] [Google Scholar]
- 3.Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA 2001;286:1218–1227 [DOI] [PubMed] [Google Scholar]
- 4.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801 [DOI] [PubMed] [Google Scholar]
- 5.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 2000;132:605–611 [DOI] [PubMed] [Google Scholar]
- 6.Church TS, Cheng YJ, Earnest CP, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004;27:83–88 [DOI] [PubMed] [Google Scholar]
- 7.Boulé NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in type 2 diabetes mellitus. Diabetologia 2003;46:1071–1081 [DOI] [PubMed] [Google Scholar]
- 8.Perri MG, Anton SD, Durning PE, et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol 2002;21:452–458 [PubMed] [Google Scholar]
- 9.Praet SF, van Rooij ES, Wijtvliet A, et al. Brisk walking compared with an individualised medical fitness programme for patients with type 2 diabetes: a randomised controlled trial. Diabetologia 2008;51:736–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morton RD, West DJ, Stephens JW, Bain SC, Bracken RM. Heart rate prescribed walking training improves cardiorespiratory fitness but not glycaemic control in people with type 2 diabetes. J Sports Sci 2010;28:93–99 [DOI] [PubMed] [Google Scholar]
- 11.Gram B, Christensen R, Christiansen C, Gram J. Effects of nordic walking and exercise in type 2 diabetes mellitus: a randomized controlled trial. Clin J Sport Med 2010;20:355–361 [DOI] [PubMed] [Google Scholar]
- 12.Walker KZ, Piers LS, Putt RS, Jones JA, O’Dea K. Effects of regular walking on cardiovascular risk factors and body composition in normoglycemic women and women with type 2 diabetes. Diabetes Care 1999;22:555–561 [DOI] [PubMed] [Google Scholar]
- 13.Fritz T, Wändell P, Aberg H, Engfeldt P. Walking for exercise—does three times per week influence risk factors in type 2 diabetes? Diabetes Res Clin Pract 2006;71:21–27 [DOI] [PubMed] [Google Scholar]
- 14.Negri C, Bacchi E, Morgante S, et al. Supervised walking groups to increase physical activity in type 2 diabetic patients. Diabetes Care 2010;33:2333–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araiza P, Hewes H, Gashetewa C, Vella CA, Burge MR. Efficacy of a pedometer-based physical activity program on parameters of diabetes control in type 2 diabetes mellitus. Metabolism 2006;55:1382–1387 [DOI] [PubMed] [Google Scholar]
- 16.Tudor-Locke C, Bell RC, Myers AM, et al. Controlled outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type II diabetes. Int J Obes Relat Metab Disord 2004;28:113–119 [DOI] [PubMed] [Google Scholar]
- 17.Tjønna AE, Lee SJ, Rognmo O, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation 2008;118:346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little JP, Gillen JB, Percival ME, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol 2011;111:1554–1560 [DOI] [PubMed] [Google Scholar]
- 19.Nemoto K, Gen-no H, Masuki S, Okazaki K, Nose H. Effects of high-intensity interval walking training on physical fitness and blood pressure in middle-aged and older people. Mayo Clin Proc 2007;82:803–811 [DOI] [PubMed] [Google Scholar]
- 20.Morikawa M, Okazaki K, Masuki S, et al. Physical fitness and indices of lifestyle-related diseases before and after interval walking training in middle-aged and older males and females. Br J Sports Med 2011;45:216–224 [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki T, Gen-No H, Kamijo Y, Okazaki K, Masuki S, Nose H. A new device to estimate VO2 during incline walking by accelerometry and barometry. Med Sci Sports Exerc 2009;41:2213–2219 [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35(Suppl. 1):S64–S71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 2006;16(Suppl. 1):3–63 [DOI] [PubMed] [Google Scholar]
- 24.Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr 2005;59:561–570 [DOI] [PubMed] [Google Scholar]
- 25.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–755 [DOI] [PubMed] [Google Scholar]
- 26.Johnson ST, Tudor-Locke C, McCargar LJ, Bell RC. Measuring habitual walking speed of people with type 2 diabetes: are they meeting recommendations? Diabetes Care 2005;28:1503–1504 [DOI] [PubMed] [Google Scholar]
- 27.Ballor DL, McCarthy JP, Wilterdink EJ. Exercise intensity does not affect the composition of diet- and exercise-induced body mass loss. Am J Clin Nutr 1990;51:142–146 [DOI] [PubMed] [Google Scholar]
- 28.Slentz CA, Aiken LB, Houmard JA, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol 2005;99:1613–1618 [DOI] [PubMed] [Google Scholar]
- 29.Irving BA, Davis CK, Brock DW, et al. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc 2008;40:1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bahr R, Sejersted OM. Effect of intensity of exercise on excess postexercise O2 consumption. Metabolism 1991;40:836–841 [DOI] [PubMed] [Google Scholar]
- 31.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008;57:1349–1354 [DOI] [PubMed] [Google Scholar]
- 32.Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 2000;23:1830–1834 [DOI] [PubMed] [Google Scholar]
- 33.Manders RJ, Van Dijk JW, van Loon LJ. Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med Sci Sports Exerc 2010;42:219–225 [DOI] [PubMed] [Google Scholar]
- 34.Mikus CR, Oberlin DJ, Libla J, Boyle LJ, Thyfault JP. Glycaemic control is improved by 7 days of aerobic exercise training in patients with type 2 diabetes. Diabetologia 2012;55:1417–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faerch K, Borch-Johnsen K, Holst JJ, Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia 2009;52:1714–1723 [DOI] [PubMed] [Google Scholar]
- 36.Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2009;297:E151–E156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winnick JJ, Sherman WM, Habash DL, et al. Short-term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole-body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. J Clin Endocrinol Metab 2008;93:771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Healy GN, Dunstan DW, Shaw JE, Zimmet PZ, Owen N. Beneficial associations of physical activity with 2-h but not fasting blood glucose in Australian adults: the AusDiab study. Diabetes Care 2006;29:2598–2604 [DOI] [PubMed] [Google Scholar]
- 39.Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance beta-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab 2004;287:E1024–E1031 [DOI] [PubMed] [Google Scholar]
- 40.Schneider SH, Amorosa LF, Khachadurian AK, Ruderman NB. Studies on the mechanism of improved glucose control during regular exercise in type 2 (non-insulin-dependent) diabetes. Diabetologia 1984;26:355–360 [DOI] [PubMed] [Google Scholar]