Abstract
OBJECTIVE
Obesity is associated with alterations in corticolimbic-striatal brain regions involved in food motivation and reward. Stress and the presence of food cues may each motivate eating and engage corticolimibic-striatal neurocircuitry. It is unknown how these factors interact to influence brain responses and whether these interactions are influenced by obesity, insulin levels, and insulin sensitivity. We hypothesized that obese individuals would show greater responses in corticolimbic-striatal neurocircuitry after exposure to stress and food cues and that brain activations would correlate with subjective food craving, insulin levels, and HOMA-IR.
RESEARCH DESIGN AND METHODS
Fasting insulin levels were assessed in obese and lean subjects who were exposed to individualized stress and favorite-food cues during functional MRI.
RESULTS
Obese, but not lean, individuals exhibited increased activation in striatal, insular, and hypothalamic regions during exposure to favorite-food and stress cues. In obese but not lean individuals, food craving, insulin, and HOMA-IR levels correlated positively with neural activity in corticolimbic-striatal brain regions during favorite-food and stress cues. The relationship between insulin resistance and food craving in obese individuals was mediated by activity in motivation-reward regions including the striatum, insula, and thalamus.
CONCLUSIONS
These findings demonstrate that obese, but not lean, individuals exhibit increased corticolimbic-striatal activation in response to favorite-food and stress cues and that these brain responses mediate the relationship between HOMA-IR and food craving. Improving insulin sensitivity and in turn reducing corticolimbic-striatal reactivity to food cues and stress may diminish food craving and affect eating behavior in obesity.
Obesity is a global public health problem predisposing more than 500 million people worldwide (1) to chronic medical conditions such as type 2 diabetes and cardiovascular disease (2). The role of the central nervous system in obesity is currently being explored with the aid of sophisticated neuroimaging techniques that enable investigation of human brain function (3,4). Food cues and stress, two environmental factors that affect eating behaviors (5,6), elicit different behavioral (5,7–11) and neural responses (12–16) in obese compared with lean individuals. These neural alterations include but are not limited to the striatum (17), a structure implicated in reward-motivation processing and stress responsiveness (17), and the insula, which is involved in perceiving and integrating sensations, such as taste (18), within the body (19) in response to food cues (13,15,20) and stressful events (12). It has been suggested that differences in these neural regions in obese individuals (17) may be associated with higher food craving (21) and dysregulated eating behaviors (22), perhaps affecting food choice and consumption (13,20,23). Thus, new obesity interventions may be facilitated by gaining a better understanding of the extent to which other factors associated with obesity (e.g., hormonal and metabolic factors) may relate to neural mechanisms underlying stress and food cue responses and how these differences may affect food-seeking motivations, such as food craving.
Hormonal signals and metabolic factors regulate energy homeostasis through peripheral and central actions (24). In the setting of obesity, alterations in insulin levels and insulin sensitivity frequently occur (25) and may perpetuate maladaptive physiology and behavior (26). It has been suggested that central insulin resistance may be an important factor contributing to altered motivation for food and changes in motivation-reward pathways (27). Indeed, insulin receptors are expressed in brain homeostatic regions, such as the hypothalamus (28), as well as motivation-reward regions linked to food-related behaviors including the ventral tegmental area (VTA) and substantia nigra (SN) (29), two structures that relay signals via dopaminergic neurons to cortical, limbic, and striatal brain regions (30). This view is further supported by studies in both rodents and humans. Neuron-specific insulin receptor knockout mice develop hyperinsulinemia and insulin resistance in conjunction with diet-induced obesity (31). In humans, resting-state network-connectivity strength in the putamen and orbitofrontal cortex (OFC) has been reported to correlate positively with fasting insulin levels and negatively with insulin sensitivity (32), and insulin’s capacity to increase glucose uptake in the ventral striatum and prefrontal cortex was observed to be diminished in insulin-resistant subjects (27). In addition, in response to food pictures, obese individuals with type 2 diabetes exhibited increased activation in the insula, OFC, and striatum compared with individuals without type 2 diabetes (23). Correlations have also been noted between dietary adherence and efficacy measures and activations in the insula and OFC and between emotional eating and activations in the amygdala, caudate, putamen, and nucleus accumbens (23).
It is, however, not known whether differences in insulin levels and insulin sensitivity affect specific human brain responses during exposure to commonly encountered stimuli such as food cues and stressful events and whether such neural responses influence food cravings that may engender eating behaviors. We hypothesized that obese, but not lean, individuals would exhibit increased neural responses in motivation-reward neurocircuits that encompass sensory and somatic integration-interoception (cortical), emotion-memory (limbic), and motivation-reward (striatal) processes during brief guided-imagery exposure to favorite-food, stress, and neutral-relaxing cues; that these neural responses would correlate with food craving as well as insulin levels and insulin resistance (as assessed by homeostasis model assessment of insulin resistance [HOMA-IR]); and that the relationship between insulin resistance and food craving would be mediated by regional brain activations.
RESEARCH DESIGN AND METHODS
Men and women, between ages 19 and 50 years, with a BMI ≥30.0 kg/m2 (obese group) or 18.5–24.9 kg/m2 (lean group), who were otherwise healthy were recruited via local advertisement. Exclusion criteria included chronic medical conditions, psychiatric disorders (DSM-IV criteria), neurologic injuries or illnesses, taking any prescription medications, IQ <90, overweight (25.0 ≤ BMI ≤ 29.9 kg/m2), inability to read and write in English, pregnancy, and claustrophobia or metal in body incompatible with magnetic resonance imaging (MRI). The study was approved by the Yale Human Investigation Committee. All subjects provided signed informed consent.
Biochemical evaluation
On an assessment day prior to the functional MRI (fMRI) session, blood samples for measurement of fasting plasma insulin and glucose levels were obtained at 8:15 a.m. and stored at −80°C. Glucose (fasting plasma glucose [FPG]) was measured using Delta Scientific glucose reagent (Henry Schein) and insulin using a double-antibody radioimmunoassay (Millipore [previously Linco]). Each sample was processed in duplicate for verification. HOMA-IR was calculated as follows: [glucose (mg/dL) × insulin (μU/mL)]/405. Neuroimaging was conducted within 7 days of laboratory data acquisition.
Imagery script development
Prior to each individual’s fMRI session, guided-imagery scripts for favorite-food cue, stress, and neutral relaxing conditions were developed using previously established methods (33). Personalized scripts were developed because personal events trigger greater physiological reactivity and generate more intense emotional reactions than imagery of standardized nonpersonal situations (34). (See Supplementary Data and Supplementary Table 7 for examples of food included in favorite-food cues and an example of a favorite-food cue script, as well as supplemental materials in Jastreboff et al. [12] for representative stress and neutral-relaxing scripts.)
fMRI session
Participants presented for imaging in the afternoon at 1:00 p.m. or 2:30 p.m. with instructions to have eaten ~2 h prior to the scanning session so that they were neither intensely hungry nor full. We assessed subjective hunger ratings before and after scanning sessions; there was no statistically significant difference between the means of the two groups [t(46) = 1.15, P > 0.1]. Each participant was acclimated in a testing room to the specific aspects of the fMRI study procedures. Subjects were positioned in the MRI scanner and underwent fMRI during a 90-min session. In randomized counter-balanced order, they were exposed to their personalized favorite-food cue, stress, and neutral relaxing imagery conditions. Six fMRI trials (two per condition) were acquired using a block design with each lasting 5.5 min. Each trial included a 1.5-min quiet baseline period followed by a 2.5-min imagery period (including 2 min to imagine their specific story as it was being played to them from a previously made audio recording and 0.5 min of quiet imagery time during which they continued imagining the story while lying in silence) and a 1-min quiet recovery period.
Validation of guided imagery paradigm
To assess subjective responses to stress imagery conditions, anxiety ratings were obtained from subjects before and after each imagery script. To assess anxiety, participants were asked as previously (33) to rate how tense, anxious, and/or jittery they felt using the Likert 10-point scale before and after each fMRI trial. In both the obese and lean subjects, anxiety ratings increased after the stress condition [obese: F(1.96) = 7.11, P < 0.0001; lean: F(1.96) = 6.94, P < 0.0001]. There were no differences in anxiety ratings between the groups at baseline [F(1.48) = 0.13, P = 0.72] or after imagery [F(1.48) = 0.23, P = 0.64]. Additionally, subjective vividness ratings were obtained wherein subjects indicated how well they were able to visualize each of their individual stories while in the scanner. There was no between-group difference in imagery vividness ratings [t(4) = 1.3, P = 0.26].
fMRI acquisition and statistical data analyses
Images were obtained in the Yale Magnetic Resonance Research Center using a 3-Tesla Siemens Trio MRI system equipped with a standard-quadrature head coil, using T2*-sensitive gradient-recalled single-shot echo-planar pulse sequence. See Supplementary Data for further details of fMRI acquisition and analysis. For descriptive statistics, between-group differences in subjective and clinical measures were tested using t test, Fisher exact, and χ2 tests. We used SPSS macro with 10,000 bootstrap to estimate the mediation models (35).
RESULTS
Group demographics and fasting metabolic parameters
Fifty healthy obese and lean volunteers were individually matched based on age (mean 26 years), sex (38% female), race (68% Caucasian), and education (Supplementary Table 1). The obese group (N = 25) had a mean ± SD BMI of 32.6 ± 2.2 kg/m2, and the lean group (N = 25) had a mean BMI of 22.9 ± 1.5 kg/m2. Although no subjects were diagnosed with diabetes, obese and lean subjects differed with regard to insulin resistance as assessed by HOMA-IR [obese group mean 3.8 ± 1.4 and lean group 2.5 ± 1.0, t(41) = −3.42, P = 0.0013] and fasting insulin levels [obese group 16.3 ± 5.8 μU/mL and lean 11.1 ± 3.7 μU/mL, t(33.7) = −3.53, P = 0.0012]. FPG levels did not differ between groups [t(41) = −1.34, P = 0.19] (Supplementary Table 1).
Contrast brain maps: Obese individuals exhibit increased neural responses in corticolimbic-striatal regions
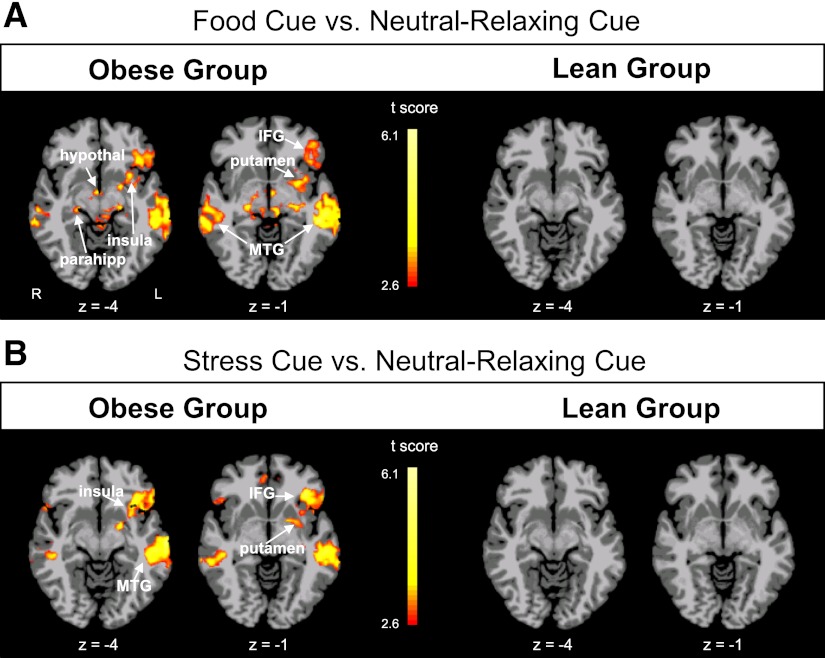
As would be expected, both lean and obese groups showed activation of corticolimbic-striatal regions in response to stress and favorite-food cue conditions and only thalamic and auditory cortical activation during the neutral-relaxing condition (P < 0.01, family-wise error [FWE] corrected (Supplementary Fig. 1). In contrast maps of neural activations of obese versus lean subjects, there was no between-group difference in mean activation in response to the neutral-relaxing condition. Thus, the neutral relaxing condition was used as an active comparison state in between-group contrasts as in prior studies (33). Obese individuals demonstrated increased neural activation to favorite-food cues, relative to the neutral-relaxing condition, in the putamen, insula, thalamus, hypothalamus, parahippocampus, inferior frontal gyrus (IFG), and middle temporal gyrus (MTG), while lean individuals did not demonstrate increased activation in these regions (P < 0.01, FWE corrected) (Fig. 1A). During stress exposure relative to neutral relaxation, again obese but not lean individuals exhibited increased activation in the putamen, insula, IFG, and MTG (P < 0.01, FWE-corrected) (Fig. 1B and Supplementary Table 2). A comparison of obese versus lean subjects during the favorite-food cue condition showed relatively increased activation of striatum (putamen), insula, amygdala, frontal cortex including Broca area, and premotor cortex. In the stress condition, obese versus lean individuals showed greater activation in the insula, superior frontal gyrus, and inferior occipital gyrus (Supplementary Fig. 2).
Figure 1.
Within-group neural response differences in cue condition contrasts. Axial brain slices in the obese and lean groups of neural activation differences observed in contrasts comparing favorite-food cue vs. neutral-relaxing conditions (A) and stress versus neutral-relaxing conditions (B) (threshold of P < 0.01, FWE corrected). Obese individuals show increased activation in the insula, putamen, inferior frontal gyrus (IFG), and middle temporal gyrus (MTG) in both contrasts; lean individuals do not show such activations. The color scale provides t values of the functional activity. Talairach z levels are indicated. hypothal, hypothalamus; L, left; parahipp, parahippocampus; R, right.
Correlation brain maps: Insulin resistance correlates with observed neural responses in obese individuals
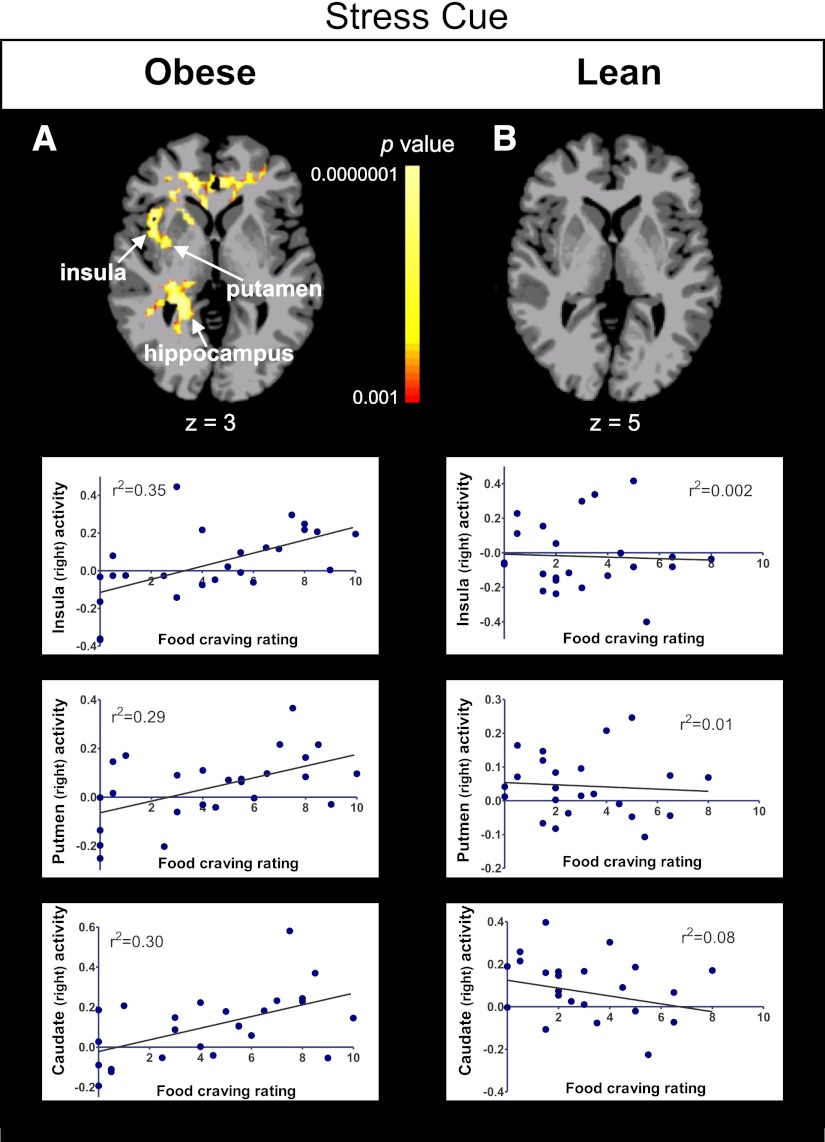
To examine how insulin resistance affects brain activation observed with favorite-food cues and stressful-event cues, we used whole-brain, voxel-based correlation analyses to examine the association of HOMA-IR, fasting insulin, and FPG levels with individual variability in neural responses to these cue conditions. The most robust correlations in the favorite-food cue and stress conditions were seen with HOMA-IR. In the obese but not lean individuals, HOMA-IR values correlated positively with neural activations in corticolimbic-striatal regions in each cue condition. Specifically, positive correlations were found with neural activation in the putamen, insula, thalamus, and hippocampus during the favorite-food cue condition (Fig. 2A and Supplementary Fig. 3A); in the putamen, caudate, insula, amygdala, hippocampus, and parahippocampus during the stress-cue condition (Fig. 2B and Supplementary Fig. 3A); and in the putamen, caudate, insula, thalamus, and anterior and posterior cingulate during the neutral-relaxing condition (Supplementary Fig. 3A and Supplementary Table 3).
Figure 2.
Whole-brain, voxel-based correlation analyses with HOMA-IR. Axial brain slices and corresponding scatterplots show correlations between neural activation (β weights) in the obese group during the favorite-food cue condition with HOMA-IR (A) and the stress condition with HOMA-IR (B). The lean group did not exhibit correlations between neural activations with these regions. Maps are thresholded at P < 0.05 (FWE corrected). The color scale provides P values. Talairach z levels are indicated. β Weight values are depicted on the y-axis. IFG, inferior frontal gyrus; L, left; R, right. (A high-quality color representation of this figure is available in the online issue.)
Not surprisingly, fasting insulin levels in obese, but not lean, individuals correlated positively in regions similar to those correlated with HOMA-IR. Additionally, positive correlations with insulin levels were found in the stress condition with ventral striatal and amygdalar activation, and a positive correlation was seen in the neutral-relaxing condition with ventral striatal activation (Supplementary Fig. 3B). Additionally, FPG levels in obese individuals correlated positively with activations during the favorite-food cue condition in the putamen and thalamus and during the neutral-relaxing condition in the putamen, caudate, insula, thalamus, and anterior and posterior cingulate (Supplementary Fig. 3C and Supplementary Table 3).
Food craving increases after favorite-food cues and stress cues
To assess subjective responses, food craving ratings were obtained from subjects before and after each imagery trial on a scale ranging from 0 to 10. There were no differences in baseline food craving ratings prior to each imagery trial between the obese and lean groups [F(1.46) = 0.09, P = 0.76]. When food cravings were compared after imagery conditions, there was a significant condition effect [F(1.92) = 34.68, P = 0.0001] (favorite-food cue, obese 6.1 ± 2.9, lean 5.8 ± 2.7; stress cue, obese 4.4 ± 3.2, lean 3.1 ± 2.2; and neutral-relaxing cue, obese 3.9 ± 3.4, lean 3.4 ± 2.4) but no group main effect [F(1.46) = 0.99, P = 0.32] or group-by-condition interaction effect [F(1.92) = 1.34, P = 0.27)]. There were increases in food craving ratings after the favorite-food cue versus neutral-relaxing conditions [t(92) = 7.33, P < 0.0001] and after the favorite-food cue versus stress conditions [t(92) = 7.09, P < 0.0001] and no significant difference after stress versus neutral-relaxing conditions [t(92) = 0.25, P = 0.81].
Correlation brain maps: Subjective food craving responses to the favorite-food cue and stress conditions correlate positively with activations in corticolimbic-striatal regions in obese individuals
To investigate the link between neural responses and food craving, we examined the association of each individual’s self-reported food-craving ratings with neural responses to the favorite-food cue and stress conditions. In obese but not lean individuals, food craving in response to the favorite-food cue and stress conditions correlated positively with activations in multiple corticolimbic-striatal regions (Fig. 3, Supplementary Fig. 4, and Supplementary Table 4).
Figure 3.
Whole-brain, voxel-based correlation analyses with food craving. Axial brain slices showing correlations between food-craving ratings and neural activation in the stress condition in the obese (A) and lean (B) groups (thresholded at P < 0.05, FWE corrected). Representative scatterplots are presented from key relevant brain regions within each group. The color scale provides P values. Talairach coordinate z levels are indicated. β Weight values are depicted on the y-axis. (A high-quality color representation of this figure is available in the online issue.)
Brain regions correlating with both food craving and insulin resistance: mediation effects
Finally, we assessed whether insulin resistance was correlated with food craving in each condition and whether these relationships were mediated by neural responses. HOMA-IR levels correlated with food-craving ratings during favorite-food cue exposure in obese subjects (r2 = 0.20; P = 0.04) but not lean individuals (r2 = 0.006; P = 0.75) (Fig. 4A). HOMA-IR levels did not correlate with food craving in the stress (obese: r2 = 0.12, P = 0.12; lean: r2 = 0.003, P = 0.82) or neutral-relaxing (obese: r2 = 0.04, P = 0.38; lean: r2 = 0.004, P = 0.80) conditions.
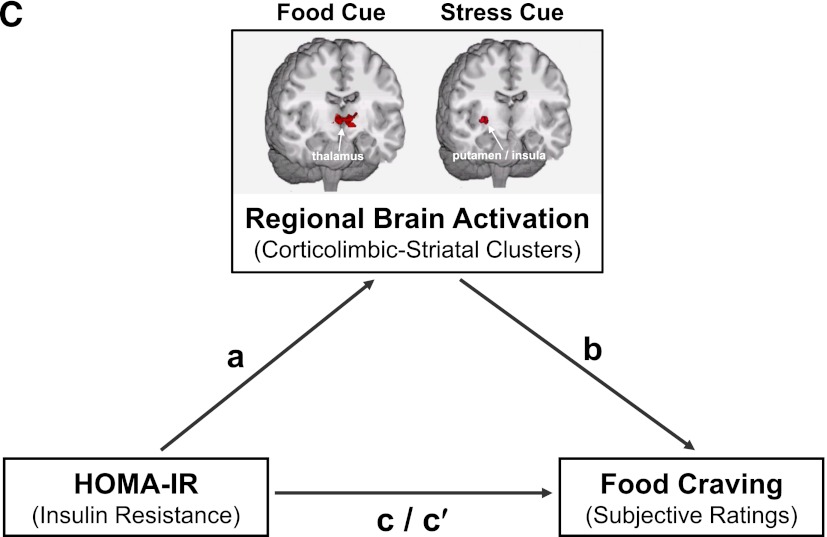
Figure 4.
Mediation model: Overlapping brain regions mediate the effect observed between HOMA-IR and food craving in obese individuals. A: Correlation between HOMA-IR levels and food craving ratings in the obese and lean groups. B: Overlapping regions of neural activation. C: Diagram representation of the mediation model of HOMA-IR, regional brain activation, and food craving in obese individuals. Overlap brain maps for each condition were generated from individual maps during the three conditions that were thresholded at P < 0.05, FWE corrected, and that examined within each condition the correlations between 1) brain activation and HOMA-IR values and 2) brain activation and subjective food craving values. A cluster size of 108 was subsequently applied the overlap maps. Talairach z levels are indicated. Subjective food craving correlation brain maps overlapped with HOMA-IR correlation brain maps in the obese group in the three conditions (favorite-food cue, stress cue, and neutral-relaxing cue).
To examine whether insulin resistance modulated food craving via neural responses, we first assessed the specific overlap in regions that were common in their neural associations to insulin resistance and to food craving. In obese subjects, activity in the thalamus and VTA/SN correlated with both insulin resistance and food craving in the favorite-food cue condition (Fig. 4B and Supplementary Table 5). Similar patterns were observed for the putamen and insula in the stress condition and the thalamus, caudate, putamen, and insula in the neutral-relaxing condition (Fig. 4B and Supplementary Table 5). We found no such overlapping regions in the lean subjects.
Next, we examined whether the relationships between HOMA-IR and food craving were mediated by the overlapping regional brain activations that correlated both with HOMA-IR and with food craving (Fig. 4C). Statistical mediation analyses can be used to examine the relationship between two variables and determine the extent to which a third, potentially intervening, variable may be responsible for the observed relationship (35). Stated another way, we examined whether the observed neural activations in corticolimbic-striatal brain regions were statistically mediating the relationship between HOMA-IR and food craving in obese participants. As indicated by significant indirect effect (a × b path) values (Supplementary Table 6), the relationship between HOMA-IR and food craving was mediated by neural responses in the thalamus, brainstem (including the VTA/SN), and cerebellum in the favorite-food cue condition and in the putamen and insula in the stress cue condition.
CONCLUSIONS
We observed striking corticolimbic-striatal activations in obese, but not lean, individuals in response to favorite-food cue and stress compared with neutral-relaxing conditions. Neural responses in these regions during food cue exposure are consistent with previous studies (12,13,15,36). The more pronounced neural responses seen in obese subjects in brain regions implicated in reward-motivation, emotion-memory, taste processing, and interoception, correlated with HOMA-IR, a measurement of insulin resistance, as well as hyperinsulinemia. Furthermore, these neural responses statistically mediated the relationship between insulin resistance and food craving in obese persons, suggesting that in obese people, insulin resistance may directly or indirectly impact neural pathways driving desires to consume favorite, and often highly caloric, foods.
Our findings are consistent with, and expand upon, previous work showing that insulin acts as a central nervous system regulatory signal of food intake and body weight (37,38). Consistent with data implicating the hypothalamus and dopaminergic reward pathways in obesity and insulin actions (28–30), 1) obese individuals demonstrated increased activation in corticolimbic-striatal regions including the striatum (both the putamen and caudate), insula, and thalamus and 2) the magnitude of insulin resistance, as assessed by HOMA-IR, positively correlated with the activation of the striatum and insula in response to both favorite-food cue and stress conditions in obese individuals. These data are supported by earlier work showing that alterations in insulin sensitivity in the VTA modify downstream responses of projections to the striatum (39); insulin-stimulated glucose metabolism in the ventral striatum is diminished in insulin-resistant subjects (27); and insular and hippocampal activation in response to food cues is directly related to hyperinsulinemia (40). Considered together, these observations may have important clinical implications for food-related behaviors and suggest that insulin resistance may impair insulin’s ability to suppress promotivational pathways, thereby accentuating stress- and food cue–related neural responses selectively in obese individuals.
Subjective, self-reported food craving ratings, which are dependent on individual perceptions, were not found to be statistically significantly different in obese and lean individuals. Additionally, obese and lean subjects identified remarkably similar favorite foods for their individualized favorite-food cues (Supplementary Table 7), with the majority of the foods being high in fat and caloric content. Thus, the differences observed do not involve differences in the foods desired but, rather, how this information is processed and interpreted and likely what consummatory behaviors subsequently result after real-life exposure to favorite-food cues. It is noteworthy, however, that HOMA-IR levels in obese, but not lean, individuals correlated with favorite-food cue–related food-craving ratings. In keeping with this observation, when we examined which brain region activations correlated with both HOMA-IR and food-craving ratings, we found overlapping brain regions in obese but not lean individuals. These regions included not only the VTA and SN but also the striatum, insula, and thalamus, which respectively contribute to reward-motivation processing and stress responsiveness (17), flavor and interoceptive signaling (18,19), and the relay of peripheral sensory information to the cortex (41). These data suggest that insulin resistance, and/or the consequences of insulin resistance, may magnify or sensitize responses in neural circuits that affect food craving for highly desirable foods and ultimately influence further weight gain. The significant relationship between insulin and HOMA-IR levels with food craving and brain activations seen in obese, but not lean, individuals may be related to a lack of variability in insulin levels in the lean individuals and/or other factors contributing importantly to food craving.
Data support associations between high uncontrollable stress, chronic stress, high BMI, and weight gain (5,7). Stress influences eating behaviors (5,10), increasing frequency of consumption of fast food (42), snacks (43), and calorie-dense and highly palatable foods (44), and stress has been associated with increased weight gain (7). In our study, during stress exposure food-craving ratings in obese, but not lean, individuals correlated positively with activation in the caudate, hippocampus, insula, and putamen. These different relationships suggest that stress-related food cravings are driven by distinct neural correlates in obese individuals and raise the possibility that this difference may increase the risk of consuming desired, highly palatable foods during times of stress in obese individuals. These findings are consistent with data suggesting that stress-driven eating is exacerbated in obese women (45), whereas stress-driven eating appears to have an inconsistent effect on food consumption in lean individuals (46). After exposure to psychological stress, satiated overweight people have greater craving for desserts and snacks and higher caloric intake compared with lean individuals under identical conditions (10). Compared with individuals with lower BMIs, those with higher BMIs demonstrate stronger associations between psychological stress and future weight gain (7). Taken together, these studies and our findings suggest that obese individuals may be more vulnerable to stress and stress-related food consumption and subsequent weight gain. Since both favorite-food cue– and stress cue–induced food cravings correlated with corticolimbic-striatal neural activation, it would be relevant in future studies to simulate real-life high-stress situations to examine neural circuitry function when obese people are exposed simultaneously to acute life stressors and favorite-food cues.
Finally, it is noteworthy that obese individuals with evidence of insulin resistance exhibited alterations in food craving even in a relaxed state. Corticolimbic-striatal activations observed in obese individuals during the neutral-relaxing condition correlated with subjective food craving. HOMA-IR levels in obese individuals also correlated with neural responses during the neutral-relaxing condition, suggesting that a chronic insulin-resistant state is associated with a persistent activation in corticolimbic-striatal brain regions even during non–food cue and nonstress conditions (e.g., during resting or relaxed states) in obese individuals, and this relationship may sustain food craving and promote eating behavior during noncued or baseline states.
The cross-sectional nature of this study precludes assessment of causality. Longitudinal studies would enable assessment of whether obesity results in increased responsivity to food cues and stress in motivation-reward brain regions or whether neural differences and their associations with insulin resistance are initially present. The measurement of insulin resistance using HOMA-IR lacks the precision afforded by the euglycemic clamp technique, although it is closely related to peripheral insulin responsiveness and widely used in research and clinical practice (47). Insulin and glucose levels were drawn in the morning to enable assessment of insulin sensitivity using fasting blood samples for HOMA-IR calculation; the fMRI imaging procedures were conducted in the afternoon so that subjects would be neither intensely hungry nor full. In future studies, taking blood measurements immediately before, during, and after MRI might provide useful information, although there may be potential complications (e.g., possible influences of phlebotomy on stress response systems). Fasting blood samples were not obtained on the day of the fMRI session; thus, a temporal relationship between metabolic parameters and neural responses cannot be made and potential between-group differences in the stability of HOMA-IR measures in obese and lean individuals could possibly influence correlations observed in the current study. Notably, though, HOMA-IR measures have been shown to have relatively low intra- and interindividual variability in nondiabetic obese (48) and overweight (49) individuals, and steady-state plasma insulin and glucose have been found to be stable in healthy subjects at a 4-year interval (50). Additionally, the coefficients of variation for HOMA are between 7.8 and 11.7% (47). Despite these study limitations, our data provide the first evidence that insulin resistance directly or indirectly plays an important role in neural activations associated with both favorite-food cues and stress and that such neural responses modulate food craving in obese individuals. Whether central insulin resistance is a primary event or the change in brain responses occurs secondary to chronic exposure to systemic hyperinsulinemia and in turn downregulation of central nervous system insulin receptors remains uncertain; nevertheless, these results have potential important therapeutic implications.
With the substantial increase in the prevalence of obesity over the last three decades, these findings have considerable clinical implications for the treatment of metabolic dysfunction and prevention of type 2 diabetes. The current findings indicate that insulin resistance in obesity relates to neural mechanisms that regulate food-related motivational states or behaviors, such as food craving or the desire to obtain and eat food. These findings suggest that individuals with this altered metabolic phenotype may be at risk for continued or persistent weight gain. Moreover, as many of the neural regions involved are subcortical, we speculate that diminished conscious control over resulting food-related behaviors may arise in such obese individuals, resulting in the further perpetuation of obesity and insulin resistance.
We conclude that exposure to favorite-food cue and stressful-event scenarios promotes activation of brain motivation-reward regions as well as food craving in insulin-resistant obese individuals. It is intriguing to speculate that insulin resistance may occur centrally in obesity and contribute to dysregulated motivations to consume food that may in turn predispose individuals to overeat, producing a viscous cycle driving weight gain. Thus, investigating central effects and behavioral ramifications of medications that alter insulin resistance may provide insight into novel treatments to attenuate craving for calorie-dense, highly palatable foods.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health T32 DK07058, Diabetes Mellitus and Disorders of Metabolism; T32 DK063703-07, Training in Pediatric Endocrinology and Diabetes Research; Diabetes and Endocrinology Research Center P30DK045735; and R37-DK20495 and the NIH Roadmap for Medical Research Common Fund grants RL1AA017539, UL1-DE019586, UL1-RR024139, and PL1-DA024859.
No potential conflicts of interest relevant to this article were reported.
A.M.J. conducted data analysis, contributed to the interpretation of data, and wrote the manuscript. R.S. was responsible for the study design, funding, and data collection; contributed to the interpretation of data; and wrote the manuscript. C.L. conducted data analysis. D.M.S. contributed to the interpretation of data. R.S.S. contributed to the interpretation of data and wrote the manuscript. M.N.P. was responsible for the study design, funding, and data collection; contributed to the interpretation of data; and wrote the manuscript. M.N.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1112/-/DC1.
References
- 1.World Health Organization Obesity and Overweight Fact Sheet [article online], 2011. Accessed 15 July 2012
- 2.Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity Among Adults in the United States–No Statistically Significant Chance Since 2003–2004. NCHS Data Brief, 2007, p. 1–8 [PubMed] [Google Scholar]
- 3.Berthoud HR. Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity (Silver Spring) 2006;14(Suppl. 5):197S–200S [DOI] [PubMed] [Google Scholar]
- 4.Tataranni PA, DelParigi A. Functional neuroimaging: a new generation of human brain studies in obesity research. Obes Rev 2003;4:229–238 [DOI] [PubMed] [Google Scholar]
- 5.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav 2007;91:449–458 [DOI] [PubMed] [Google Scholar]
- 6.Lowe MR, van Steenburgh J, Ochner C, Coletta M. Neural correlates of individual differences related to appetite. Physiol Behav 2009;97:561–571 [DOI] [PubMed] [Google Scholar]
- 7.Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial stress and change in weight among US adults. Am J Epidemiol 2009;170:181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellanos EH, Charboneau E, Dietrich MS, et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes (Lond) 2009;33:1063–1073 [DOI] [PubMed] [Google Scholar]
- 9.Coelho JS, Jansen A, Roefs A, Nederkoorn C. Eating behavior in response to food-cue exposure: examining the cue-reactivity and counteractive-control models. Psychol Addict Behav 2009;23:131–139 [DOI] [PubMed] [Google Scholar]
- 10.Lemmens SG, Rutters F, Born JM, Westerterp-Plantenga MS. Stress augments food ‘wanting’ and energy intake in visceral overweight subjects in the absence of hunger. Physiol Behav 2011;103:157–163 [DOI] [PubMed] [Google Scholar]
- 11.Tetley A, Brunstrom J, Griffiths P. Individual differences in food-cue reactivity. The role of BMI and everyday portion-size selections. Appetite 2009;52:614–620 [DOI] [PubMed] [Google Scholar]
- 12.Jastreboff AM, Potenza MN, Lacadie C, Hong KA, Sherwin RS, Sinha R. Body mass index, metabolic factors, and striatal activation during stressful and neutral-relaxing states: an FMRI study. Neuropsychopharmacology 2011;36:627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin LE, Holsen LM, Chambers RJ, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 2010;18:254–260 [DOI] [PubMed] [Google Scholar]
- 14.Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007;37:410–421 [DOI] [PubMed] [Google Scholar]
- 15.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 2008;117:924–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008;41:636–647 [DOI] [PubMed] [Google Scholar]
- 17.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci 2008;363:3191–3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Small DM. Flavor is in the brain. Physiol Behav. 17 April 2012 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology 2006;131:1925–1942 [DOI] [PubMed] [Google Scholar]
- 20.Karhunen LJ, Lappalainen RI, Vanninen EJ, Kuikka JT, Uusitupa MI. Regional cerebral blood flow during food exposure in obese and normal-weight women. Brain 1997;120:1675–1684 [DOI] [PubMed] [Google Scholar]
- 21.Pepino MY, Finkbeiner S, Mennella JA. Similarities in food cravings and mood states between obese women and women who smoke tobacco. Obesity (Silver Spring) 2009;17:1158–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 2011;15:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chechlacz M, Rotshtein P, Klamer S, et al. Diabetes dietary management alters responses to food pictures in brain regions associated with motivation and emotion: a functional magnetic resonance imaging study. Diabetologia 2009;52:524–533 [DOI] [PubMed] [Google Scholar]
- 24.Sharkey KA. From fat to full: peripheral and central mechanisms controlling food intake and energy balance: view from the chair. Obesity (Silver Spring) 2006;14(Suppl. 5):239S–241S [DOI] [PubMed] [Google Scholar]
- 25.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 26.Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci 2007;30:367–398 [DOI] [PubMed] [Google Scholar]
- 27.Anthony K, Reed LJ, Dunn JT, et al. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes 2006;55:2986–2992 [DOI] [PubMed] [Google Scholar]
- 28.Schwartz MW. Biomedicine. Staying slim with insulin in mind. Science 2000;289:2066–2067 [DOI] [PubMed] [Google Scholar]
- 29.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 2003;964:107–115 [DOI] [PubMed] [Google Scholar]
- 30.Redgrave P, Coizet V. Brainstem interactions with the basal ganglia. Parkinsonism Relat Disord 2007;13(Suppl. 3):S301–S305 [DOI] [PubMed] [Google Scholar]
- 31.Brüning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science 2000;289:2122–2125 [DOI] [PubMed] [Google Scholar]
- 32.Kullmann S, Heni M, Veit R, et al. The obese brain: Association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp 2012;33:1052–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol 2009;14:84–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann NY Acad Sci 2008;1141:105–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 2008;40:879–891 [DOI] [PubMed] [Google Scholar]
- 36.Davids S, Lauffer H, Thoms K, et al. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J Obes (Lond) 2010;34:94–104 [DOI] [PubMed] [Google Scholar]
- 37.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev 1992;13:387–414 [DOI] [PubMed] [Google Scholar]
- 38.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 1979;282:503–505 [DOI] [PubMed] [Google Scholar]
- 39.Sandoval D, Cota D, Seeley RJ. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol 2008;70:513–535 [DOI] [PubMed] [Google Scholar]
- 40.Wallner-Liebmann S, Koschutnig K, Reishofer G, et al. Insulin and hippocampus activation in response to images of high-calorie food in normal weight and obese adolescents. Obesity (Silver Spring) 2010;18:1552–1557 [DOI] [PubMed] [Google Scholar]
- 41.Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol 2007;17:417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steptoe A, Lipsey Z, Wardle J. Stress, hassles and variations in alcohol consumption, food choice and physical exercise: a diary study. Br J Health Psychol 1998;3:51–63 [Google Scholar]
- 43.Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol Behav 1999;66:511–515 [DOI] [PubMed] [Google Scholar]
- 44.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 2001;26:37–49 [DOI] [PubMed] [Google Scholar]
- 45.Laitinen J, Ek E, Sovio U. Stress-related eating and drinking behavior and body mass index and predictors of this behavior. Prev Med 2002;34:29–39 [DOI] [PubMed] [Google Scholar]
- 46.Greeno CG, Wing RR. Stress-induced eating. Psychol Bull 1994;115:444–464 [DOI] [PubMed] [Google Scholar]
- 47.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 48.Jayagopal V, Kilpatrick ES, Jennings PE, Hepburn DA, Atkin SL. Biological variation of homeostasis model assessment-derived insulin resistance in type 2 diabetes. Diabetes Care 2002;25:2022–2025 [DOI] [PubMed] [Google Scholar]
- 49.Jayagopal V, Kilpatrick ES, Holding S, Jennings PE, Atkin SL. The biological variation of insulin resistance in polycystic ovarian syndrome. J Clin Endocrinol Metab 2002;87:1560–1562 [DOI] [PubMed] [Google Scholar]
- 50.Facchini F, Humphreys MH, Jeppesen J, Reaven GM. Measurements of insulin-mediated glucose disposal are stable over time. J Clin Endocrinol Metab 1999;84:1567–1569 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.