Abstract
OBJECTIVE
To determine whether improvements in glycemic control and diabetes-specific quality of life (QoL) scores reported in research studies for the type 1 diabetes structured education program Dose Adjustment For Normal Eating (DAFNE) are also found when the intervention is delivered within routine U.K. health care.
RESEARCH DESIGN AND METHODS
Before and after evaluation of DAFNE to assess impact on glycemic control and QoL among 262 adults with type 1 diabetes.
RESULTS
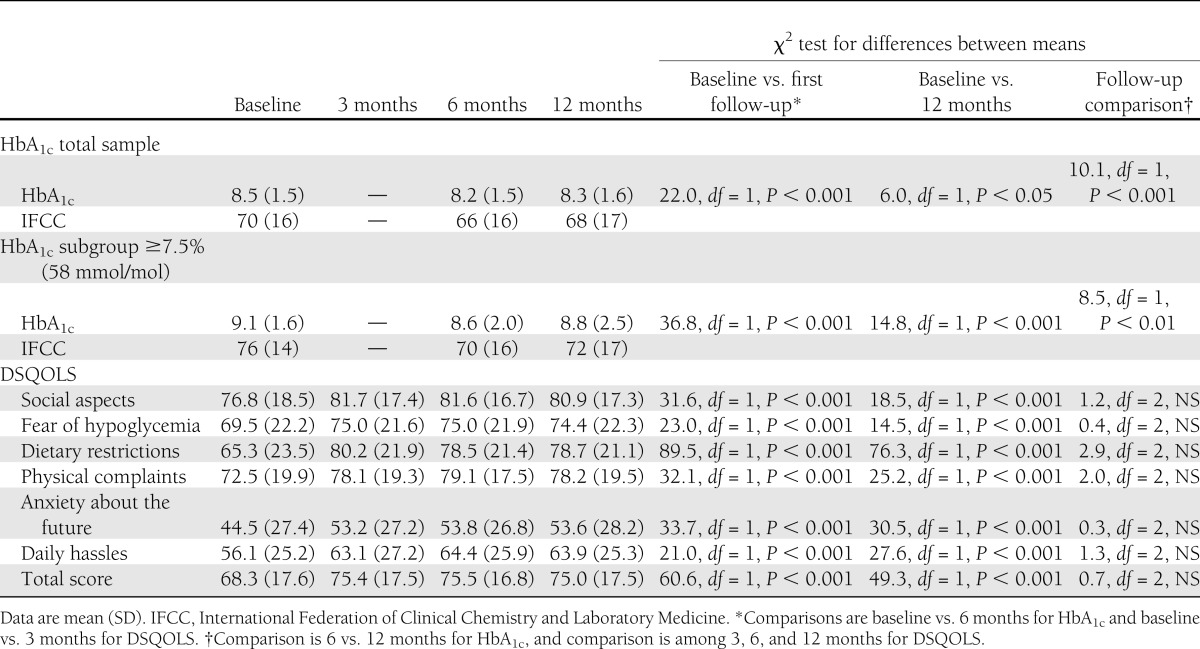
There were significant improvements in HbA1c from baseline to 6 and 12 months (from 9.1 to 8.6 and 8.8%, respectively) in a subgroup with suboptimal control. QoL was significantly improved by 3 months and maintained at both follow-up points.
CONCLUSIONS
Longer-term improved glycemic control and QoL is achievable among adults with type 1 diabetes through delivery of structured education in routine care, albeit with smaller effect sizes than reported in trials.
Self-management training for type 1 diabetes on the model of the Düsseldorf structured teaching and treatment program (STTP) (1) has demonstrated a wide range of positive health and psychological outcomes within randomized controlled trials (RCTs) (1–3), and these effects are maintained wholly or in part in the longer term (4). These programs evolved in response to studies demonstrating benefits of intensified insulin therapy (5,6).
Eligibility criteria used within RCT mean trial populations may be unrepresentative (7). We aimed to determine whether improvements in glycemic control and quality of life (QoL) reported in RCTs of self-management training are found when the program is delivered in routine U.K. health care.
RESEARCH DESIGN AND METHODS
Adults with type 1 diabetes were recruited, consecutively, from 73 courses at 12 U.K. hospitals, representing well-established (n = 8) and new centers (n = 4). The Dose Adjustment For Normal Eating (DAFNE) was selected as an exemplar of an STTP that has achieved widespread adoption by service providers. Efficacy has been demonstrated, obviating the need for a control group (2).
DAFNE comprises a 5-day course with booster session 6 weeks later, delivered to groups of up to eight by two trained diabetes educators. It promotes flexible, intensive, insulin therapy, separating basal from prandial rapid-acting insulin, and a flexible, varied diet with no forbidden foods. Dose adjustment depends on preprandial and bedtime blood glucose values. DAFNE is based on social learning theory (8). The main topics are carbohydrate counting and dose adjustment, along with managing hypoglycemia and illness. Eligibility criteria for course enrolment were applied as follows: 1) type 1 diabetes for at least 6 months, 2) >17 years of age, 3) absence of end-stage complications (e.g., renal failure), and 4) multiple daily injections
HbA1c data were collected from routine records up to 8 weeks before and 6 and 12 months after the course. Participants completed the Diabetes-Specific Quality of Life Scale (DSQOLS) (9), before course enrollment and 3, 6, and 12 months after completion. Follow-up coincided with points at which HbA1c was routinely determined in outpatients. The 3-month follow-up was included to allow sufficient time to observe short-term changes in QoL. The DSQOLS was designed to evaluate the Düsseldorf STTP, on which DAFNE is based. Psychometric validation of the scale in English used data from this study and two others (10,11) and will be reported elsewhere. The DSQOLS includes 57 items that form six subscales summed to gain a total score. Higher scores indicate better QoL.
RESULTS
Of 474 patients approached, 262 (55%) participated. Basic data, maintaining anonymity, permitted a comparison between participants (n = 262) and those who declined or were could not be contacted (n = 254). Nonparticipants had slightly higher baseline HbA1c (8.8 ± 1.6% vs. 8.5 ± 1.5% or 73 ± 18 mmol/mol vs. 69 ± 16 mmol/mol; P = 0.02).
DSQOLS response rate was 74% at each time point. HbA1c data were available for 78% of participants at 6 months and 93% at 12 months. There was no sex imbalance. Average age was 40 ± 14 years (range, 17–73 years); mean duration of diabetes was 18 ± 13 years (range, 6 months–55 years); 89% were white British; 44% had a degree; 49% were in professional or managerial occupations; 61% were in full-time employment; and 68% were in a significant relationship. Mean baseline HbA1c was 8.5 ± 1.5% (70 ± 16 mmol/mol), ranging from 5.4–14.2% (36–132 mmol/mol). A quarter (n = 65) had an HbA1c level <7.5% (<58 mmol/mol), which was regarded as acceptable because further improvement would increase the risk of severe hypoglycemia. This subgroup was excluded from an analysis of people with suboptimal HbA1c.
Linear mixed models were run with direct maximum likelihood to account for missing data. For HbA1c in the total group, there was significant improvement from baseline to 6 months (P < 0.001) (Table 1), which was maintained at 12 months (P < 0.001), although there was a slight deterioration from 6 to 12 months (P < 0.05). In the subgroup with an HbA1c level ≥7.5%, there was a clinically and statistically significant improvement in HbA1c from baseline to both follow-up points (6 months, P < 0.0001; 12 months, P < 0.01), which also showed a slight deterioration from 6 to 12 months (P < 0.001). Each DSQOLS subscale and total score showed significant improvements by 3 months, all of which were maintained at 6 and 12 months in the total sample (Table 1).
Table 1.
HbA1c results and DSQOLS scores across four time points
CONCLUSIONS
Among adults with type 1 diabetes undergoing skills-based training in routine U.K. health care, there was a clinically relevant improvement in HbA1c in the total sample, which was, unsurprisingly, larger in those with suboptimal control (HbA1c ≥ 7.5%) before the course. This was accompanied by significant improvement in QoL, fully maintained at 1-year follow-up, as demonstrated in previous RCTs (4) and reported from audit data (12,13). Statistically, the improvement in glycemic control was only slightly reduced by 12 months, mirroring the DAFNE RCT, the Epidemiology of Diabetes Interventions and Complications (EDIC) study, the EURODIAB cohort, and the DAFNE Database Study (2,5,6,14).
Study strengths include the observational design and relatively large numbers. Limitations include the high proportion of well-educated subjects and the lack of a control group, although the current findings reflect similar results to the DAFNE and STTP RCTs. Limited data were available for nonrespondents who had a higher baseline HbA1c.
The 0.5% reduction in HbA1c by 6 months in the group with suboptimal baseline HbA1c is clinically significant, reflecting a small effect size (15). The initial improvement in QoL at 3 months was equivalent to just under a medium effect size (15). The maintenance of effects from baseline to 12 months suggests that improvements in HbA1c and QoL were attributable to the DAFNE intervention. The reduction in the magnitude of change relative to the original DAFNE RCT (2) may reflect the higher proportion of participants with baseline HbA1c values closer to target. DAFNE audit data demonstrate significant improvements in frequency of severe hypoglycemia and hypoglycemia awareness (14). These benefits are only seen by not restricting DAFNE to people with suboptimal HbA1c.
Although it seems that the effects on glycemic control attenuate somewhat with time, it is possible to achieve sustainable improvements in HbA1c and QoL among adults with type 1 diabetes during routine delivery of structured education, This is encouraging given the rollout of DAFNE both nationally and internationally.
Acknowledgments
This article presents independent research commissioned by the NIHR under its Program Grants for Applied Research scheme (RP-PG-0606-1184).
No potential conflicts of interest relevant to this article were reported.
D.C. researched data, contributed to the discussion, and wrote, reviewed, and edited the manuscript. M.C. researched data. R.B., J.L., D.R., S.H., M.C., and J.S. contributed to discussion and reviewed and edited the manuscript.
D.C. is the guarantor of this work and as such, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank all the participants who gave up their time to take part in this study.
Footnotes
The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
References
- 1.Mühlhauser I, Jörgens V, Berger M, et al. Bicentric evaluation of a teaching and treatment programme for type 1 (insulin-dependent) diabetic patients: improvement of metabolic control and other measures of diabetes care for up to 22 months. Diabetologia 1983;25:470–476 [DOI] [PubMed] [Google Scholar]
- 2.DAFNE Study Group Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plank J, Köhler G, Rakovac I, et al. Long-term evaluation of a structured outpatient education programme for intensified insulin therapy in patients with Type 1 diabetes: a 12-year follow-up. Diabetologia 2004;47:1370–1375 [DOI] [PubMed] [Google Scholar]
- 4.Speight J, Amiel SA, Bradley C, et al. Long-term biomedical and psychosocial outcomes following DAFNE (Dose Adjustment For Normal Eating) structured education to promote intensive insulin therapy in adults with sub-optimally controlled Type 1 diabetes. Diabetes Res Clin Pract 2010;89:22–29 [DOI] [PubMed] [Google Scholar]
- 5.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Microvascular and acute complications in IDDM patients. The EURODIAB IDDM Complications Study. Diabetologia 1994;37:278–285 [DOI] [PubMed] [Google Scholar]
- 7.Armstrong BK, White E, Saracci R. Principles of Exposure Measurement in Epidemiology. Oxford, U.K., Oxford University Press, 1992 [Google Scholar]
- 8.Oliver L, Thompson G. The DAFNE Collaborative. Experiences of developing and delivering an evidenced based quality assured programme for people with Type 1 diabetes. Pract Diabetes Int 2009;26:371–377 [Google Scholar]
- 9.Bott U, Mühlhauser I, Overmann H, Berger M. Validation of a diabetes-specific quality-of-life scale for patients with type 1 diabetes. Diabetes Care 1998;21:757–769 [DOI] [PubMed] [Google Scholar]
- 10.Dinneen SF, O’Hara MC, Byrne M, et al.; Irish DAFNE Study Group. The Irish DAFNE Study Protocol: a cluster randomised trial of group versus individual follow-up after structured education for Type 1 diabetes. Trials 2009;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansell P, Chater T, Cooke D, et al. A research database for structured diabetes education (DAFNE) (Abstract). Diabet Med 2011;28(Suppl. 1):27 [Google Scholar]
- 12.McIntyre HD, Knight BA, Harvey DM, Noud MN, Hagger VL, Gilshenan KS. Dose adjustment for normal eating (DAFNE) - an audit of outcomes in Australia. Med J Aust 2010;192:637–640 [DOI] [PubMed] [Google Scholar]
- 13.Sämann A, Mühlhauser I, Bender R, Kloos Ch, Müller UA. Glycaemic control and severe hypoglycaemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospective implementation study. Diabetologia 2005;48:1965–1970 [DOI] [PubMed] [Google Scholar]
- 14.Hopkins D, Lawrence I, Mansell P, et al. Improved biomedical and psychological outcomes 1 year after structured education in flexible insulin therapy for people with type 1 diabetes mellitus: the U.K. DAFNE experience. Diabetes Care 2012;35:1638–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Mahwah, New Jersey, Lawrence Erlbaum, 1988 [Google Scholar]


