Abstract
OBJECTIVE
The Action to Control Cardiovascular Risk in Diabetes lipid study (ACCORD Lipid), which compared the effects of simvastatin plus fenofibrate (FENO-S) versus simvastatin plus placebo (PL-S) on cardiovascular disease outcomes, measured only fasting triglyceride (TG) levels. We examined the effects of FENO-S on postprandial (PP) lipid and lipoprotein levels in a subgroup of ACCORD Lipid subjects.
RESEARCH DESIGN AND METHODS
We studied 139 subjects (mean age of 61 years, 40% female, and 76% Hispanic or black) in ACCORD Lipid, from a total 529 ACCORD Lipid subjects in the Northeast Clinical Network. PP plasma TG, apolipoprotein (apo)B48, and apoCIII were measured over 10 h after an oral fat load.
RESULTS
The PP TG incremental area under the curve (IAUC) above fasting (median and interquartile range [mg/dL/h]) was 572 (352–907) in the FENO-S group versus 770 (429–1,420) in the PL-S group (P = 0.008). The PP apoB48 IAUC (mean ± SD [μg/mL/h]) was also reduced in the FENO-S versus the PL-S group (23.2 ± 16.3 vs. 35.2 ± 28.6; P = 0.008). Fasting TG levels on the day of study were correlated with PP TG IAUC (r = 0.73 for FENO-S and r = 0.62 for PL-S; each P < 0.001). However, the fibrate effect on PP TG IAUC was a constant percentage across the entire range of fasting TG levels, whereas PP apoB48 IAUC was only reduced when fasting TG levels were increased.
CONCLUSIONS
FENO-S lowered PP TG similarly in all participants compared with PL-S. However, levels of atherogenic apoB48 particles were reduced only in individuals with increased fasting levels of TG. These results may have implications for interpretation of the overall ACCORD Lipid trial, which suggested benefit from FENO-S only in dyslipidemic individuals.
Individuals with type 2 diabetes have a dyslipidemia characterized by high levels of plasma triglycerides (TG) and low levels of HDL cholesterol (HDL-C) (1). The presence of dyslipidemia contributes to the increased rates of atherosclerotic cardiovascular disease (ASCVD) in this group (2). Large meta-analyses have identified fasting plasma TG levels as an independent predictor of future ASCVD, particularly in women (3,4), although a recent report indicated that fasting TG was not predictive for ASCVD after adjusting for HDL and non–HDL-C (5). Various studies have also shown the importance of increased nonfasting or postprandial (PP) TG levels (6,7) as predictors of ASCVD. In these studies, nonfasting TG concentrations remained independent predictors of ASCVD events, even after adjusting for other risk factors, including HDL-C. There is a paucity of studies examining the association between postprandial lipemia (PPL) and cardiovascular events in patients with type 2 diabetes (8), with conflicting results regarding PP TG levels as predictors of CVD (9,10).
Fibrates are indicated to treat fasting hypertriglyceridemia, but their effects on ASCVD are controversial (11). Fibrate monotherapy trials have provided mixed results, although a recent meta-analysis suggested modest but significant benefits, particularly for nonfatal myocardial infarction (12). Various groups have published the beneficial effects of fibrates on top of statins on fasting lipid levels (13–17), but in the lipid arm of the Action to Control Cardiovascular Risk in Diabetes study (ACCORD Lipid), therapy with the combination of fenofibrate and simvastatin did not improve ASCVD outcomes compared with therapy with only simvastatin in the overall cohort of subjects (18). However, a subgroup of participants with dyslipidemia at baseline (defined by upper tertile TG and lower tertile HDL-C) may have benefited from combination treatment (18).
Prior clinical trials comparing the efficacy of fibrate plus statin to statin alone have not examined PP TG. In an attempt to clarify the role of fibrate plus statin on PP TG levels in patients with type 2 diabetes, we conducted a study of PPL in a subset of subjects enrolled in ACCORD Lipid, in which only fasting plasma lipid levels were measured in the overall study cohort. In this ancillary study of ACCORD Lipid, our hypothesis was that combination therapy with fenofibrate (145 mg/day) and simvastatin (FENO-S) would result in lower PPL compared with therapy with placebo and simvastatin (PL-S). We hoped this ancillary study would provide additional insights into the outcomes of the overall ACCORD Lipid trial, in which only fasting lipid levels were measured.
RESEARCH DESIGN AND METHODS
Protocol
Subjects who had been randomized to the ACCORD Lipid study in the Northeast Clinical Network were recruited for a study of PPL; 150 of the entire cohort of 529 individuals participated in the PPL study, which was not a requirement of the overall ACCORD Lipid trial. While recruiting the subjects, we were blind to their randomization within ACCORD Lipid and to any clinical data. In the early stages of ACCORD Lipid, some participants did not, based on the entry LDL-C, receive any simvastatin (18,19); those individuals were excluded from our final analyses (n = 11). Including those non-statin–treated subjects in our analysis did not change our findings. The data presented are, therefore, for 139 subjects who received either FENO-S or PL-S. All participants had been receiving FENO or PL for at least 4 months at the time of their PPL study.
The study was approved by the Columbia University Medical Center institutional review board, and all subjects signed informed consent. Exclusion criteria were those for the overall study, including a fasting TG >400 mg/dL (18). All subjects completed the study, which was performed on a single day.
The protocol we used has been described previously by our group (20). In brief, subjects were admitted to the Clinical Research Center after fasting for 12 h. Participants were instructed not to ingest any alcoholic beverages for at least 5 days before the study. They had a fasting blood glucose measured by a glucometer and, based on the results, were given some or all of their diabetes medications. This approach was taken because of the relatively low carbohydrate and caloric intake during the 12-h test. Subjects were weighed, and, after fasting bloods were obtained, they ingested a high-fat beverage that provided 1,237 kcal/2-m2 body surface area. The beverage consisted of 75% fat (40% saturated, 20% monounsaturated, and 15% polyunsaturated), 10% protein, and 15% carbohydrate. The high-fat beverage was consumed within 15 min. Sequential blood samples were obtained at 3, 5, 7, and 10 h after ingestion of the beverage. With the exception of the use of the bathroom, participants remained in a semirecumbent position for the entire study.
Laboratory
Fasting levels of total cholesterol, TG, HDL-C, and glucose were measured using standard enzymatic techniques on a Roche Hitachi 912 chemistry analyzer (Roche). LDL-C levels were calculated by the Friedewald method (21). Apolipoprotein (apo)B48 levels were measured by an ELISA kit from BioVendor USA/Canada. This assay does not recognize full-length apoB100 (22). The intra- and interassay coefficients of variation were 2.1 and 12%, respectively. ApoCIII levels were measured by an ELISA Kit from Polymedco. The intra- and interassay coefficients of variation were 3.3 and 3.0%, respectively. HDL apoCIII was measured using the same assay after isolation of HDL from plasma using Mg2+/dextran sulfate (HDL Precipitating Reagent Set; Pointe Scientific, Inc, Detroit, MI); non-HDL apoCIII was determined as the difference between plasma and HDL apoCIII concentrations. Complete blood count, metabolic panel, and HbA1c were measured on fasting samples by the Columbia University Medical Center clinical laboratory.
Statistics
Postprandial IAUC for TG, apoB48, and apoCIII were calculated by the trapezoid method as previously described (20). In brief, with five measurements available, TG IAUC was calculated by the formula: (2.5 × tg3) + (2.5 × tg5) + (2.5 × tg8) + (tg10) − (8.5 × tg0). The IAUCs for apoB48 and apoCIII, with three measurements available, were calculated by the formula: (5 × y5) + (2.5 × y10) − (7.5 × y0). All results are reported as means and SD, except for the day of study fasting TG and apoB48 levels and the IAUCs for TG and non-HDL apoCIII IAUCs, which were not normally distributed and therefore reported as medians and interquartile ranges. All but the non-HDL apoCIII IAUC achieved normal distributions after log transformation and were analyzed by unpaired t tests; the non-HDL apoCIII IAUC was analyzed nonparametrically by the Wilcoxon rank sum test. Error bars in figures are of SEM. The fenofibrate effect on (log-transformed) IAUCs was analyzed by a multiple regression model (Proc Mixed in SAS; SAS Institute, Inc., Cary, NC) with fenofibrate, sex, (log-transformed) day of study fasting TG, and fenofibrate × TG interaction as independent variables. Results are reported after being back-transformed. Because log transformation resulted in normally distributed TG IAUCs, parametric statistical approaches were appropriate. The fenofibrate effect was displayed graphically by a log–log scatter plot of IAUC for TG and a semilog scatter plot of apoB48 IAUC versus day of study fasting TG, with separate regression lines for the placebo and fenofibrate groups. P < 0.05 was considered significant for statistical tests.
RESULTS
This study evaluated PPL in 139 individuals from ACCORD Lipid. Sixty-six subjects were on the FENO-S treatment, and 73 were on the PL-S treatment. Specific medications and doses used for hypertension and type 2 diabetes have been previously published for this cohort (18). Baseline characteristics for each group are presented in Table 1. The mean age of the study participants was 61 ± 7 years, and 40% of the cohort was female; there were no differences in age or sex between the groups. None of the women reported estrogen use, and only one reported use of a progestin. The mean duration of diabetes was 10.3 ± 7.3 years and was the same in both groups. Fifty-two percent of the subjects in this study were in the intensive glycemia arm of the ACCORD trial, and this did not differ between the two groups. A total of 41% of the participants were classified as secondary prevention; this was similar in FENO-S and PL-S. Consistent with the demographics of the Northeast Clinical Network population, the percentages of Hispanics (47%) and blacks (29%) in our cohort were much higher than in the overall ACCORD Lipid study (18). There were no significant differences in sex, age, ethnicity, BMI, or statin dose between the FENO-S and PL-S groups. Furthermore, there were no differences between the groups regarding use of aspirin, diuretics, β-blockers, angiotensin-converting enzyme inhibitor, angiotensin receptor blockers, or calcium channel blockers (data not shown). The PPL studies were performed after at least 4 months on either FENO-S or PL-S treatment; the median time from randomization to the PPL study was 6.9 months (interquartile range 5–11), with no differences between the two groups.
Table 1.
Baseline characteristics and day of study lipid and apolipoprotein levels
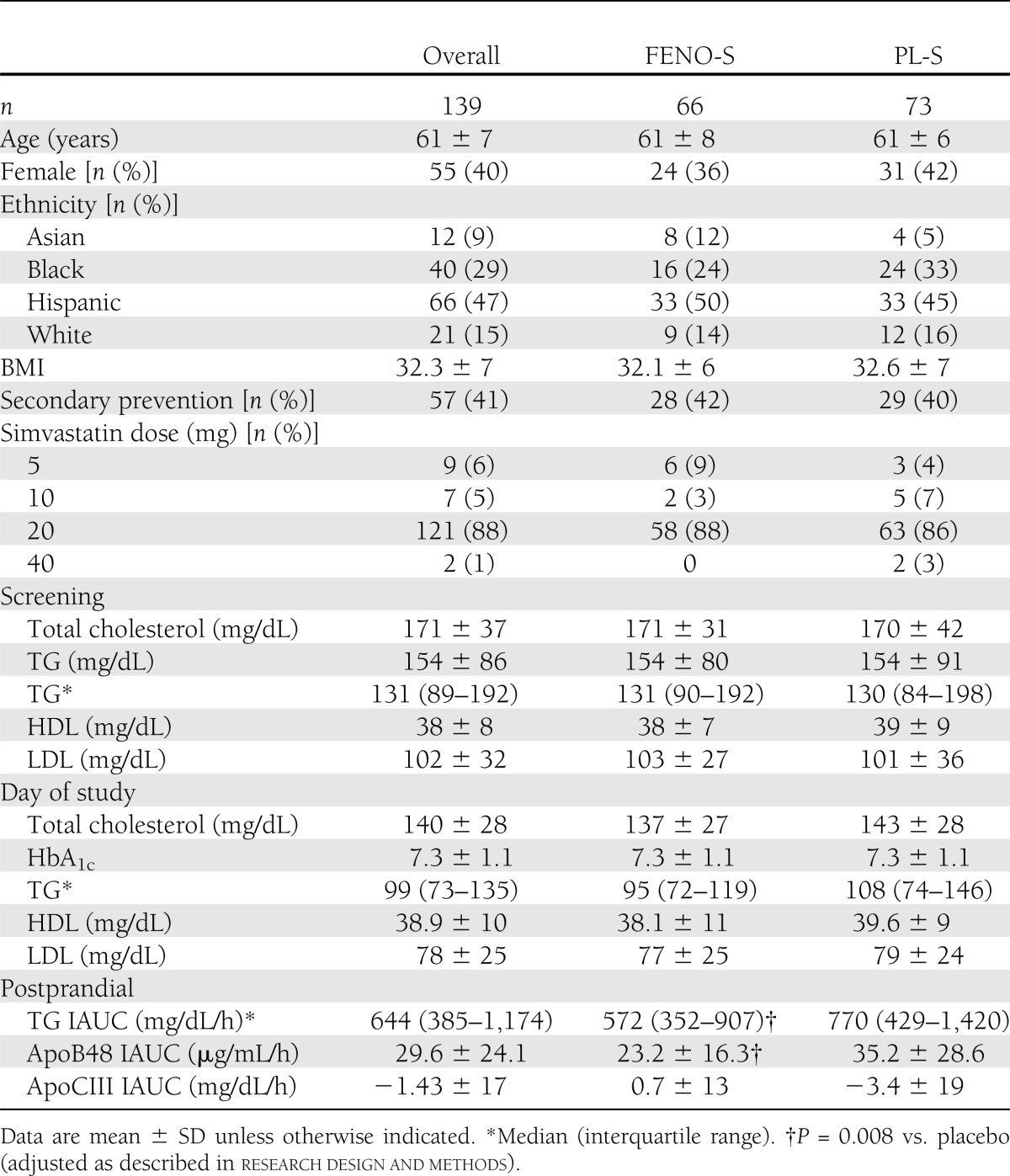
Screening lipid levels (Table 1) were obtained when subjects were first enrolled into ACCORD Lipid; at that time, ∼64% were on a lipid-altering medication, with >90% of those individuals receiving a statin. There were no differences in screening plasma lipids between FENO-S and PL-S. However, the median TG for our 139 subjects was 133 mg/dL, whereas it was 162 mg/dL for the entire 5,518 subjects in ACCORD Lipid. We believe this difference was due to the larger proportion of black participants in our cohort (29%) compared with the overall ACCORD Lipid study population (15%). Blacks have significantly lower plasma TG levels than other racial groups (23).
The day of study fasting lipid levels were obtained just before consumption of the fat load at the time of the PPL studies. Although doses of simvastatin were modified during the first few years of ACCORD Lipid (18,19), ∼90% of the participants were taking either 20 or 40 mg/day on the day of the PPL study. The median fasting TG level for the entire PPL cohort on the day of study was 102 mg/dL; there was a trend toward a lower fasting median TG (100 mg/dL) in the FENO-S group compared with the PL-S group (108 mg/dL). There was the expected difference between the two groups for reduction in TG from screening to day of study fasting TG levels (P = 0.02). Fasting HDL-C and LDL-C levels were the same in the two groups on the day of study.
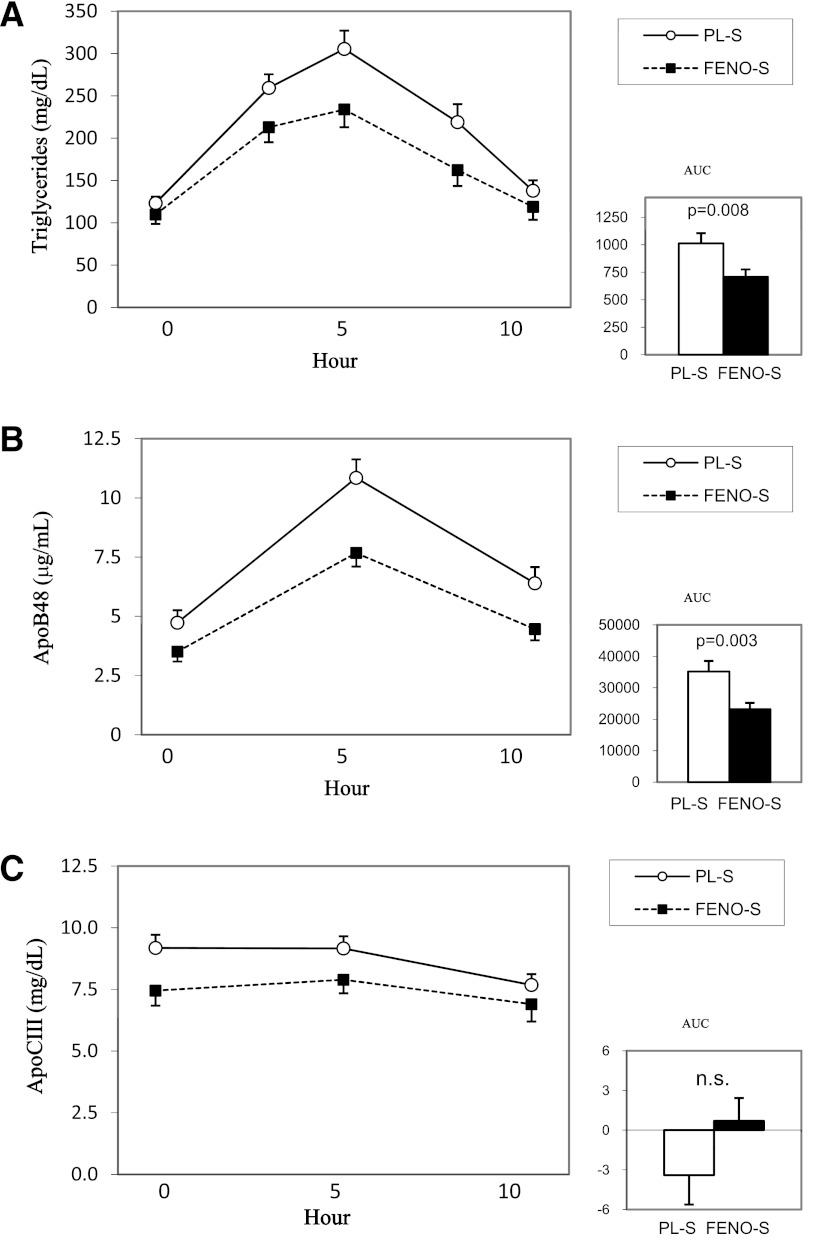
Our hypothesis for this study was that FENO-S would result in significantly lower PP lipid and lipoprotein levels compared with PL-S. We performed three measures of PPL: the IAUC for total plasma TG, the IAUC for plasma apoB48, and the IAUC for plasma apoCIII. The unadjusted data are presented in Table 1. However, as described in research design and methods, the fenofibrate effect on (log-transformed) IAUCs was analyzed by multiple regression with fenofibrate, sex, (log-transformed) day of study fasting TG, and fenofibrate × TG interaction as independent variables. Including age, BMI, HbA1c, duration of diabetes, race, or glycemic treatment group in the model had no significant effects on the results. The FENO-S group had a significant reduction in TG IAUC (median and interquartile range) compared with the PL-S group: 572 (352–907) vs. 770 (429–1,420) mg/dL/h (unadjusted P = 0.008; adjusted P = 0.008). The plasma apoB48 IAUC was also reduced in the FENO-S versus the PL-S group: 23.2 ± 16.3 vs. 35.2 ± 28.6 μg/mL/h (unadjusted P = 0.003; adjusted P = 0.008). Although plasma apoCIII concentrations were reduced 10–20% in FENO-S compared with PL-S at each of the three time points across the 10-h study, plasma apoCIII IAUCs were not different between FENO-S and PL-S. These data are presented graphically in Fig. 1. We were able to measure non-HDL apoCIII IAUCs in 30 FENO-S subjects and 29 PL-S participants; the levels of non-HDL apoCIII were ∼20% lower in FENO-S compared with PL-S throughout the 10-h study (data not shown). The median and interquartile range for the IAUC (mg/dL/h) was 10.1 (2–30) for FENO-S and 4.7 (−1 to 27) for PL-S (P = 0.47).
Figure 1.
Postprandial incremental excursions for plasma TG, apoB48, and apoCIII. After fasting 12 h, participants had 0-h blood samples taken and then ingested a high-fat beverage containing 1,237 kcal/2 m2 body surface area from 75% fat (40% saturated, 20% monounsaturated, 15% polyunsaturated), 10% protein, and 15% carbohydrate. Sequential blood samples were obtained at 3, 5, 7, and 10 h after ingestion of the beverage. IAUC was calculated as described in research design and methods. The FENO-S group had a 30% reduction in plasma TG IAUC (A) and a 34% reduction in plasma apoB48 (B) compared with the PL-S group (statistical significance reported without adjustments). The IAUC for plasma apoCIII (C) was not different between the two groups.
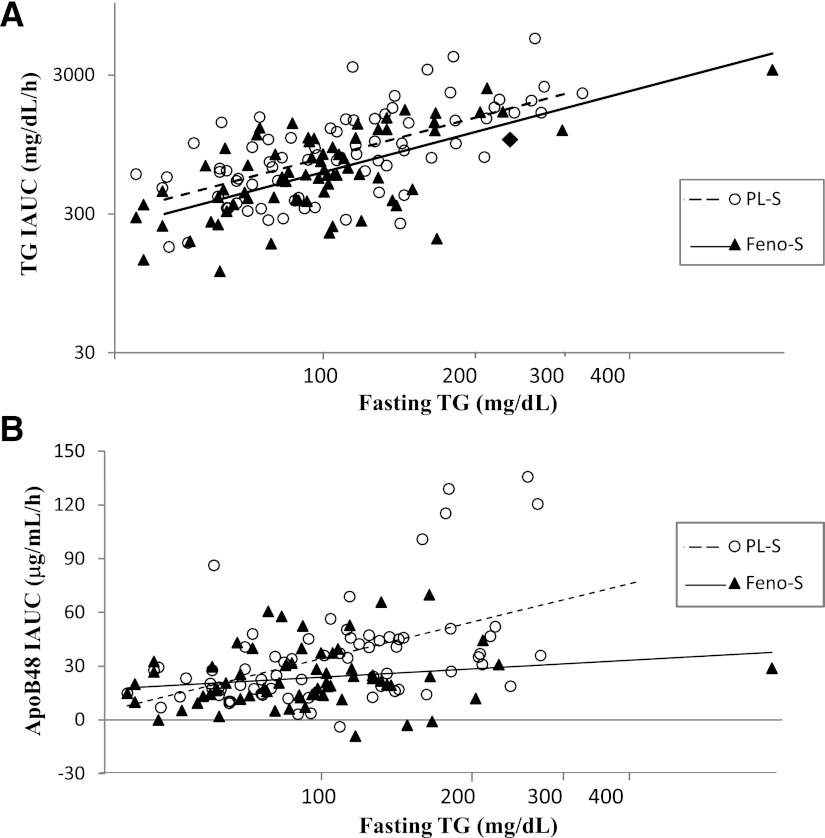
As a result of the ethnic distribution of our PPL cohort, we enrolled participants with a much wider range of TG levels than present in the overall ACCORD Lipid cohort. This allowed us to examine whether the effect of FENO-S on PP TG was independent of fasting TG, even at low fasting levels. The necessity of this analysis derives from prior demonstrations of very strong correlations between fasting TG and PP TG IAUC (24,25).Indeed, in our two groups, these correlations were r = 0.73 in FENO-S and r = 0.62 in PL-S; P < 0.001 for both. Therefore, we created a model to account for the effect of both day of study fasting TG and treatment on PP IAUCs. We found that even at the same day of study fasting TG levels, subjects on fenofibrate had lower PP TG IAUCs (Table 2). In addition, the percent-lowering effect of fenofibrate was the same at all TG levels, as demonstrated by the parallel regression lines in Fig. 2A. In contrast, there was a strong interaction between fasting TG on the day of study and the PP response of apoB48 to fenofibrate; the overall effect of FENO-S to reduce the apoB48 IAUC was due completely to a marked reduction in apoB48 IAUC in individuals with higher fasting TG levels (Table 2 and Fig. 2B). The differences in apoB48 IAUCs across the range of day of study fasting TG levels are shown in Table 2.
Table 2.
TG and apoB48 IAUC at the 25th, 50th, and 75th percentiles for day of study fasting TG level
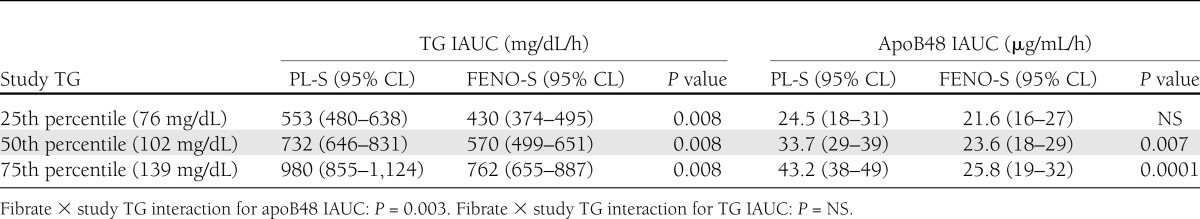
Figure 2.
Relationships between fasting plasma TG and the postprandial incremental excursions of plasma TG and apoB48. The fenofibrate effect on (log-transformed) IAUCs was analyzed by multiple regression with fenofibrate, sex, (log-transformed) day of study fasting TG, and fenofibrate × TG interaction as independent variables. For TG IAUC, the effect of fenofibrate was similar across the full range of day of study fasting TG (the solid and dashed regression lines are parallel) (A). The r value for the correlation between fasting TG and TG IAUC was 0.61 (P < 0.0001) for each group. In contrast, there was a significant interaction between day of study fasting TG levels and the effect of fenofibrate on the IAUC for apoB48 (the solid and dashed regression lines are not parallel) (B), whereby only participants with TG levels at and above the median had significant reductions in apoB48 IAUC (P = 0.003 for interaction; Table 2). Fasting TG and apoB48 IAUC were significantly correlated in the PL-S (r = 0.49; P < 0.001), whereas there was no significant relationship between fasting TG and apoB48 IAUC in the FENO-S group (r = 0.20; P = 0.11).
CONCLUSIONS
Significant controversy exists as to the role of fibrates in the treatment of dyslipidemia to prevent ASCVD (11). The overall result of ACCORD Lipid, in which only fasting levels of plasma lipids were measured, was negative. However, a prespecified subgroup analysis of ACCORD Lipid suggested that when TG was >200 mg/dL and HDL-C <35 mg/dL, fenofibrate reduced the primary end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke (18). Post hoc subgroup analyses conducted for several fibrate monotherapy trials, including the Helsinki Heart Study, which had a positive overall result (26), support the potential benefit of this class of drugs in individuals with significant baseline dyslipidemia (18,27). Recent observations that nonfasting or PP TG levels may be better predictors of CVD risk (6,7) suggested that examination of the effects of fenofibrate on PPL might provide new insights regarding the outcomes in ACCORD Lipid.
Although there have been numerous studies of the effects of combination therapy with fibrates and statins on fasting plasma lipids (13–17,28) and the effects of fibrate monotherapy on PP TG levels after a standardized fat challenge (29,30), there have been no studies comparing PP TG levels with fibrate plus statin to statin alone. Additionally, participants in studies of the effects of fibrates on PPL have usually had hypertriglyceridemia (29) or were Caucasian (30), and few studies focused on people with type 2 diabetes (31). We compared the effects of fenofibrate plus statin versus statin alone in a heterogeneous population of both men and women with type 2 diabetes. Our recruitment strategy resulted in enrollment of subjects with a much wider range of TG levels than reported in prior clinical studies. This allowed us to address both the question of whether fenofibrate therapy would reduce PP lipid and lipoprotein levels in patients treated aggressively with statins and whether those reductions would be independent of the fasting levels on the day of study.
Our main finding was the significant differences in the PPL responses between the FENO-S group and the PL-S group. This is not a trivial finding, because several studies have demonstrated significant lowering of PP TG with statins alone, and one study indicated that statins were as potent as gemfibrozil in this regard (32). Consistent with prior literature (24,25), we found a strong correlation between fasting TG and TG IAUC. However, using a model that accounted for fasting TG levels on the day of study, we still observed a significant effect of fenofibrate on the PP responses of TG and apoB48. Furthermore, the FENO-S group had lower PP TG IAUCs across the entire range of fasting TG levels; although this was somewhat surprising, considering the relationship between day of study fasting TG and PPTG excursions, abnormalities of PPTG in patients with type 2 diabetes and normal fasting TG concentrations have been reported (33). In contrast, the effect of fenofibrate on the PP response of apoB48 was significantly modified by day of study fasting levels of TG (P = 0.003 for interaction). The dissociation between the PP responses of TG and apoB48 across the range of fasting TG levels is compatible with a dual model in which: 1) fenofibrate increases the removal of TG from chylomicrons by stimulating lipoprotein lipase synthesis in adipose tissue (34) and suppressing synthesis and secretion of apoCIII from the liver (35,36), and 2) better lipolysis facilitates the clearance of remnant intestinal TG-rich lipoproteins only if the levels of TG-rich lipoproteins are significantly increased at baseline, as they typically are in people with type 2 diabetes (1). Indeed, we found that both plasma apoCIII and, more importantly, in a subset of subjects, non-HDL apoCIII concentrations were reduced throughout the day-long study in FENO-S compared with PL-S, consistent with better lipolysis in the FENO-S group (37).
In the main ACCORD Lipid study, subjects with dyslipidemia, defined as a baseline TG in the upper tertile and an HDL-C in the lower tertile, appeared to have benefited from fenofibrate treatment. That finding, together with a greater fenofibrate-mediated reduction in fasting TG in the dyslipidemic subgroup compared with all others on fenofibrate, raised the possibility that greater fenofibrate-mediated reductions in PP TG in the dyslipidemic group might also have occurred, with a potentially beneficial effect on CVD outcomes. Extrapolating our results to the entire ACCORD cohort is, we admit, very speculative, but would support a more nuanced scheme. Thus, although our present results suggest that fenofibrate did not have a greater relative effect on PP TG in the dyslipidemic subgroup, it had a greater relative benefit on the levels of intestinally derived remnant lipoproteins in that subgroup. Because TG-rich remnants are atherogenic, any benefits of fenofibrate treatment on ASCVD outcomes might be seen only in subjects with the highest levels of TG-rich remnant lipoproteins at baseline.
Limitations of our study include enrollment of a subset of ACCORD Lipid subjects that differed by racial mix compared with the overall study cohort. However, this was also a strength that allowed us to study individuals with a wide range of fasting TG levels on the day of study. Another weakness is the relatively small number of subjects in terms of any ability to extrapolate our results to the ASCVD outcomes in the overall ACCORD Lipid study.
Acknowledgments
This work was supported by NIH R01-HL-69190, NIH HL-HC-95184, NIH T32-HL-07343, NIH M01-RR-00645, and NIH UL1-RR-024156. A portion of the work was supported by a grant from the Residual Risk Reduction Initiative Foundation.
ACCORD Lipid received fenofibrate from Abbott Laboratories and simvastatin from Merck Pharmaceuticals. H.N.G. has been a consultant for Abbott Laboratories and Merck Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
G.R.-S. carried out the studies, collected the data, wrote the initial version of the manuscript, and participated in manuscript completion. C.I.N. assisted G.R.-S. in conduct of the studies and participated in development of the manuscript. L.L. maintained masking of the participants and provided statin medication, adherence, and demographic data on participants. W.K. participated in study design, protocol, development, and manuscript preparation. R.R. participated in study design, conducted statistical analyses, and participated in manuscript development. S.H. conducted statistical analyses and participated in manuscript development. H.N.G. participated in all aspects of the study and supervised manuscript development. H.N.G.is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in poster form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
Footnotes
Clinical trial reg. no. NCT00000620, clinicaltrials.gov.
References
- 1.Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am 2006;35:491–510, vii–viii [DOI] [PubMed] [Google Scholar]
- 2.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993;16:434–444 [DOI] [PubMed] [Google Scholar]
- 3.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 1996;3:213–219 [PubMed] [Google Scholar]
- 4.Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007;115:450–458 [DOI] [PubMed] [Google Scholar]
- 5.Di Angelantonio E, Sarwar N, Perry P, et al. Emerging Risk Factors Collaboration Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007;298:309–316 [DOI] [PubMed] [Google Scholar]
- 7.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299–308 [DOI] [PubMed] [Google Scholar]
- 8.Enkhmaa B, Ozturk Z, Anuurad E, Berglund L. Postprandial lipoproteins and cardiovascular disease risk in diabetes mellitus. Curr Diab Rep 2010;10:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carstensen M, Thomsen C, Gotzsche O, Holst JJ, Schrezenmeir J, Hermansen K. Differential postprandial lipoprotein responses in type 2 diabetic men with and without clinical evidence of a former myocardial infarction. Rev Diabet Stud 2004;1:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes-Soffer G, Holleran S, Karmally W, et al. Measures of postprandial lipoproteins are not associated with coronary artery disease in patients with type 2 diabetes mellitus. J Lipid Res 2009;50:1901–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldfine AB, Kaul S, Hiatt WR. Fibrates in the treatment of dyslipidemias—time for a reassessment. N Engl J Med 2011;365:481–484 [DOI] [PubMed] [Google Scholar]
- 12.Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010;375:1875–1884 [DOI] [PubMed] [Google Scholar]
- 13.Roth EM, McKenney JM, Kelly MT, et al. Efficacy and safety of rosuvastatin and fenofibric acid combination therapy versus simvastatin monotherapy in patients with hypercholesterolemia and hypertriglyceridemia: a randomized, double-blind study. Am J Cardiovasc Drugs 2010;10:175–186 [DOI] [PubMed] [Google Scholar]
- 14.Athyros VG, Papageorgiou AA, Athyrou VV, Demitriadis DS, Kontopoulos AG. Atorvastatin and micronized fenofibrate alone and in combination in type 2 diabetes with combined hyperlipidemia. Diabetes Care 2002;25:1198–1202 [DOI] [PubMed] [Google Scholar]
- 15.Derosa G, Maffioli P, Salvadeo SA, et al. Fenofibrate, simvastatin and their combination in the management of dyslipidaemia in type 2 diabetic patients. Curr Med Res Opin 2009;25:1973–1983 [DOI] [PubMed] [Google Scholar]
- 16.Goldberg AC, Bittner V, Pepine CJ, et al. Efficacy of fenofibric acid plus statins on multiple lipid parameters and its safety in women with mixed dyslipidemia. Am J Cardiol 2011;107:898–905 [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Vega GL, Yuan Z, Battisti WP, Brady WE, Palmisano J. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial). Am J Cardiol 2005;95:462–468 [DOI] [PubMed] [Google Scholar]
- 18.Ginsberg HN, Elam MB, Lovato LC, et al. ACCORD Study Group Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginsberg HN, Bonds DE, Lovato LC, et al. ACCORD Study Group Evolution of the lipid trial protocol of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99(12A):56i–67i [DOI] [PubMed] [Google Scholar]
- 20.Ginsberg HN, Jones J, Blaner WS, et al. Association of postprandial triglyceride and retinyl palmitate responses with newly diagnosed exercise-induced myocardial ischemia in middle-aged men and women. Arterioscler Thromb Vasc Biol 1995;15:1829–1838 [DOI] [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 22.Kinoshita M, Kojima M, Matsushima T, Teramoto T. Determination of apolipoprotein B-48 in serum by a sandwich ELISA. Clin Chim Acta 2005;351:115–120 [DOI] [PubMed] [Google Scholar]
- 23.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr 2009;155:e7–e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syvänne M, Talmud PJ, Humphries SE, et al. Determinants of postprandial lipemia in men with coronary artery disease and low levels of HDL cholesterol. J Lipid Res 1997;38:1463–1472 [PubMed] [Google Scholar]
- 25.Cohn JS, McNamara JR, Cohn SD, Ordovas JM, Schaefer EJ. Plasma apolipoprotein changes in the triglyceride-rich lipoprotein fraction of human subjects fed a fat-rich meal. J Lipid Res 1988;29:925–936 [PubMed] [Google Scholar]
- 26.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 1987;317:1237–1245 [DOI] [PubMed] [Google Scholar]
- 27.Elam M, Lovato LC, Ginsberg H. Role of fibrates in cardiovascular disease prevention, the ACCORD-Lipid perspective. Curr Opin Lipidol 2011;22:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyes-Soffer G, Rondon-Clavo C, Ginsberg HN. Combination therapy with statin and fibrate in patients with dyslipidemia associated with insulin resistance, metabolic syndrome and type 2 diabetes mellitus. Expert Opin Pharmacother 2011;12:1429–1438 [DOI] [PubMed] [Google Scholar]
- 29.Rosenson RS, Wolff DA, Huskin AL, Helenowski IB, Rademaker AW. Fenofibrate therapy ameliorates fasting and postprandial lipoproteinemia, oxidative stress, and the inflammatory response in subjects with hypertriglyceridemia and the metabolic syndrome. Diabetes Care 2007;30:1945–1951 [DOI] [PubMed] [Google Scholar]
- 30.Lai CQ, Arnett DK, Corella D, et al. Fenofibrate effect on triglyceride and postprandial response of apolipoprotein A5 variants: the GOLDN study. Arterioscler Thromb Vasc Biol 2007;27:1417–1425 [DOI] [PubMed] [Google Scholar]
- 31.Skoczyńska A, Kreczyńska B, Poreba R. Postprandial lipemia in diabetic men during hypolipemic therapy. Pol Arch Med Wewn 2009;119:461–468 [PubMed] [Google Scholar]
- 32.McLauglin T, Abbasi F, Lamendola C, Leary E, Reaven GM. Comparison in patients with type 2 diabetes of fibric acid versus hepatic hydroxymethyl glutaryl-coenzyme a redutase inhibitor treatment of combined dyslipidemia. Metabolism 2002;51:1355–1359 [DOI] [PubMed] [Google Scholar]
- 33.Rivellese AA, De Natale C, Di Marino L, et al. Exogenous and endogenous postprandial lipid abnormalities in type 2 diabetic patients with optimal blood glucose control and optimal fasting triglyceride levels. J Clin Endocrinol Metab 2004;89:2153–2159 [DOI] [PubMed] [Google Scholar]
- 34.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, et al. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 1996;15:5336–5348 [PMC free article] [PubMed] [Google Scholar]
- 35.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart J-C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998;98:2088–2093 [DOI] [PubMed] [Google Scholar]
- 36.Malmendier CL, Lontie JF, Delcroix C, Dubois DY, Magot T, De Roy L. Apolipoproteins C-II and C-III metabolism in hypertriglyceridemic patients. Effect of a drastic triglyceride reduction by combined diet restriction and fenofibrate administration. Atherosclerosis 1989;77:139–149 [DOI] [PubMed] [Google Scholar]
- 37.Ginsberg HN, Le NA, Goldberg IJ, et al. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest 1986;78:1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]