Abstract
OBJECTIVE
Determine the impact of islet transplantation on carotid intima-media thickness (CIMT), a marker for atherosclerosis, in type 1 diabetes without kidney disease.
RESEARCH DESIGN AND METHODS
Consecutive case series of 15 adults (mean age [SD], 49 years [10 years]; 87% female) with type 1 diabetes for ≥5 years (mean duration [SD], 30 years [12 years]; mean HbA1c [SD], 7.2% [0.9%]), without kidney disease, presenting with severe hypoglycemic unawareness to undergo allogeneic pancreatic islet transplant(s) (one to three each) in a phase 1/2 and 3 clinical trial. Current follow-up ranges from 1 to 5 years (2005–2011). CIMT of the common and internal carotid arteries was measured before and every 12–16 months after the first transplant (two to six CIMTs each) by one ultrasonographer and one blinded reader. CIMT was analyzed as change from baseline to 12- and 50-month follow-up; a combined CIMT score was calculated as the sum of the standardized IMT scores (SD units [SDs]) of both arteries.
RESULTS
All patients achieved insulin independence after one to three transplants. CIMT decreased at 12 months (n = 15) for the common carotid (−0.058 mm; P = 0.006) and combined score (−1.28 SDs; P = 0.004). In those with 50-month follow-up (n = 7), the decrease in the combined score continued from 12 (−1.59 SDs; P = 0.04) to 50 months (−0.77 SDs; P = 0.04). During follow-up, the decreasing slope of change in CIMT was associated with decreasing slopes of change in HbA1c, lipoproteins, and cardiovascular/inflammatory markers.
CONCLUSIONS
Islet transplantation may ameliorate diabetes-related atherosclerosis through improved glycemic control consequent to restoring endogenous insulin secretion, and optimal lipid management posttransplant also contributes.
Mortality from ischemic heart disease in individuals with type 1 diabetes is substantial. Risk estimates for those with type 1 diabetes <60 years of age range from 6- to 9-fold higher for men, and 13- to 15-fold higher for women, compared with the general population (1,2); and there is an exceptionally elevated risk, >40-fold, for women with type 1 diabetes <40 years of age (2). Follow-up of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort demonstrated that intensive glycemic control slows the progression of atherosclerosis, as quantified by carotid intima-media thickness (CIMT) (3,4), with the largest benefit evident during the first 6 of 12 years after intensive treatment (4). Further, intensive glycemic control prevents cardiovascular events in those with type 1 diabetes (5).
Despite known benefits, long-term maintenance of optimal glycemic control is difficult (6). Many patients cannot tolerate intensive insulin therapy and experience debilitating hypoglycemic episodes. One treatment for type 1 diabetes is pancreas transplant, which has been shown not only to improve glycemic control but also to decrease CIMT to levels comparable to those in individuals with type 1 diabetes without kidney disease over 2 years of follow-up (7). However, whole-pancreas transplant represents a difficult and risky surgical procedure (8). Although currently considered an experimental surgery, islet transplantation has emerged as an alternative treatment for patients with type 1 diabetes and debilitating hypoglycemia. This minimally invasive procedure is associated with less procedural-related morbidity than whole-pancreas transplantation and may therefore represent a safer and simpler treatment option than whole-organ transplant to stabilize glucose metabolism and achieve insulin independence while limiting hypoglycemic episodes (9,10). However, adverse events can occur with islet transplantation, including peritoneal bleeding and a decline in kidney function from immunosuppressive drugs (9,11). Fortunately, a recent report indicated that adverse event rates occurring with islet transplantation have steadily improved over the last decade and that mortality is low (12).
Because of the relatively recent development and clinical implementation of this treatment, long-term benefits remain largely unknown. Islet transplantation may represent a treatment that may not only be a safer alternative to whole-pancreas transplantation in achieving insulin independence but also be a way to prevent the considerable morbidity and mortality associated with ischemic heart disease in type 1 diabetes (13). To our knowledge, only one study has explored the effect of islet transplantation on CIMT in type 1 diabetes. Conducted in individuals with end-stage renal disease, several of whom had previous cardiovascular events, this study found that those receiving a kidney-islet transplant had a small, nonsignificant increase in CIMT compared with the kidney-only transplant group, which experienced a significant increase in CIMT over 3 years of follow-up (14).
It has not yet been determined whether minimally invasive islet transplantation slows or even reverses the progression of atherosclerosis, as occurs with pancreas transplant, in the absence of kidney disease and previous cardiovascular events. The current study represents the first report to assess the impact of islet transplant on atherosclerosis, as measured by changes in CIMT, in individuals with type 1 diabetes without kidney disease.
RESEARCH DESIGN AND METHODS
Study and participants
This consecutive case series consists of 15 adult patients who underwent allogeneic pancreatic islet transplant(s) as part of an ongoing phase 1/2 and 3 clinical trial (NCT00679042) to achieve insulin independence. The trial has been previously described (11). In brief, patients were eligible for transplant if they were 18–65 years of age, had type 1 diabetes for ≥5 years, and presented with hypoglycemic unawareness despite optimal insulin management efforts. Patients were excluded if one of the following conditions was present: untreated cardiac, kidney (based on creatinine clearance, serum creatinine, and urinary albumin-to-creatinine ratio), or liver disease, hyperlipidemia, history of cancer or stroke, active infection, substance abuse including cigarette smoking, HbA1c >12% or BMI >26, uncontrolled psychiatric disorder, use of corticosteroids or anticoagulants, and pregnancy. The 15 patients are from the University of Illinois at Chicago (UIC) Medical Center and have received a total of 27 islet transplants (one to three transplants each). Current follow-up ranges from 1 to 5 years after first transplant (2005–2011). Study approval was obtained from the Institutional Review Board at UIC, and patients provided written informed consent.
The first four patients received the Edmonton protocol of immunosuppression, including daclizumab (1 mg/kg before transplantation, and 2, 4, 6, and 8 weeks after each islet transplant), sirolimus (0.2 mg/kg loading dose, thereafter 0.1 mg/kg aiming at trough levels of 10–15 mg/mL), and tacrolimus (0.5 mg starting dose, thereafter adjusted to trough levels of 3–6 mg/mL). Sirolimus was stopped and substituted with mycophenolate mofetil when patients presented with side effects such as recurrent mouth sores or the development of macroalbuminuria (urine albumin-to-creatinine ratio >300 mg/g). The remaining 11 patients received the UIC protocol, which included etanercept (50 mg i.v. before and 25 mg s.c. 3, 7, and 10 days after each transplant) and exenatide (5 µg s.c. b.i.d. for 2 weeks and then 10 µg s.c. b.i.d. for 6 months), in addition to the Edmonton protocol. The study protocol followed the American Diabetes Association guidelines for lipid and blood pressure control; addition or adjustment of the statin and antihypertensive dose were permitted because of the side effects of the immunosuppressive therapy. Islet transplant outcomes for the first 10 of the 15 patients, at 15 months post-first transplant, have been reported recently (11). Three patients have withdrawn (one patient at 13 months post-first transplant because of side effects of the immunosuppression therapy, one patient after 19 months because of islet graft loss, and one patient after 22 months because of diagnosis of local breast cancer), and one patient died 19 months after transplant because of sepsis of unknown origin. These four participants had data available from their pretransplant and 12-month posttransplant follow-up exams. The remaining 11 are currently enrolled and continue to be actively followed.
Measurement of CIMT (dependent variable)
CIMT was assessed before and approximately every 12–16 months after the first islet transplant (totaling two to six CIMT assessments over 5 years). The outcomes of interest were change from baseline to 12- and 50-month follow-up after the first transplant. Measurement of CIMT and technician performance have been previously described (15). In brief, carotid arteries were imaged by high-resolution B-mode carotid artery ultrasound using Siemens Acuson Sequoia 512 with a linear-array 7.5-MHz transducer (Phillips Medical Systems NA, Bothell, WA) without contrast. All measurements were performed by a single ultrasonographer at the same center using the same equipment, and assessed by a single reader who was blinded to the study question, patient, and time point of follow-up. For each patient, three measurements were taken on the right and left sides of the near and far walls of the common and internal carotid arteries; the mean of these measurements for the common and internal artery were analyzed. A combined CIMT score, developed by the DCCT/EDIC study (3), was calculated as the sum of the standardized IMT measurements (z scores; SD units [SDs]) of both the common and internal carotid arteries (combined score = common z score + internal z score). CIMT z scores for the common and internal arteries were calculated as (patient value – “population” mean)/“population” SD, where the age- and sex-specific CIMT “population” mean and SD in those with type 1 diabetes were taken from published DCCT/EDIC data (16).
Clinical measurements (independent variables)
Patient characteristics included age and sex. At baseline and each follow-up exam, diabetes- and cardiovascular-related factors were measured using the same standardized protocols. Body composition was assessed using BMI (weight [kg]/height2 [m2]), and abdominal adipose tissue distribution (visceral, subcutaneous, and total) was measured with a 150 electron beam tomography scanner (Imatron, San Francisco, CA). Blood pressure was measured after patients were seated for 5 min. Data on insulin independence (yes/no), antihypertensive and statin medication use (yes/no), and immunosuppressive regimen (sirolimus/tacrolimus vs. mycophenolate mofetil/tacrolimus) were collected. An extensive lipid and cardiovascular and inflammatory marker profile was performed (Clinical Reference Laboratory, Lenexa, KS), which included the following: total cholesterol, lipoproteins (HDL, LDL, and VLDL), triglycerides, free fatty acids, high-sensitivity C-reactive protein, apolipoprotein B, apolipoprotein A-1, fibrinogen, intercellular adhesion molecule-1, monocyte chemotactic protein-1 (MCP-1), matrix metallopeptidase-9, plasminogen activator inhibitor-1 (PAI-1) antigen and activity, vascular cell adhesion molecule-1 (VCAM-1), and tissue plasminogen activator. HbA1c and urine albumin-to-creatinine ratio were measured at the UIC Pathology Laboratories (Chicago, IL) by high-performance liquid chromatography and the Beckman LX20 standard chemistry method, respectively. Urine and serum creatinine were used to calculate creatinine clearance; serum creatinine was used to estimate glomerular filtration rate (eGFR) with the modification of diet in renal diseases equation (17).
Statistical analysis
Analyses were performed in SAS (version 9.2; SAS Institute, Cary, NC). The Sign test was used to compare paired medians for nonnormally distributed clinical characteristics. The McNemar test was used to compare paired proportions. Paired Student t tests were used to compare normally distributed clinical characteristics and baseline CIMT levels with 12- and 50-month follow-up CIMT levels for the common and internal arteries and the combined score. Correlation analyses explored cross-sectional associations of CIMT with diabetes- and cardiovascular-related factors pretransplant, and at 12- and 50-month follow-up. These statistical tests were considered significant at P < 0.05.
Whether the slope of the change in CIMT levels during follow-up (e.g., a decline in common artery IMT) was associated with the slope of change in levels of diabetes- and cardiovascular-related factors during the same follow-up period (e.g., a decline in HbA1c) was determined using unadjusted and multivariable mixed-effects linear regression models of repeated measures. Empirical SEs were calculated, and the autoregressive variance matrix was specified for the correlation of the repeated measures. Variables not normally distributed, including internal carotid artery CIMT, were log transformed. The multivariable models estimating slope of change in common, internal, and combined CIMT score were built by first entering all independent variables with P < 0.15 from the unadjusted regressions and then using a stepwise approach to remove the nonsignificant covariates. Therefore, only those covariates that were significantly associated with change in CIMT levels during follow-up at P < 0.01 (to minimize type 1 error from the multiple factors analyzed) were left in the final models. Interactions between the significant covariates were tested in each model, and interactions that were statistically significant at P < 0.01 remained in the final models. The association between change in CIMT level and change in HbA1c during follow-up was also explored for confounding and mediation by other covariates (e.g., insulin independence, antihypertensive use, and immunosuppressive regimen); the magnitude of the HbA1c regression coefficients did not change by >10% when other covariates were entered. Therefore, nonsignificant covariates did not remain in the final models as none were found to be confounders or mediators of the CIMT/HbA1c association. Adjusting for islet transplant protocol (Edmonton/UIC) did not substantially change the regression coefficients. Sensitivity analyses were conducted by excluding the two males (and the four patients who had resumed a small dose of insulin at the end of their follow-up), and the results did not appreciably change.
RESULTS
All 15 patients achieved insulin independence after one to three transplants. At the end of their respective follow-up in the current analysis, 11 patients remained insulin free; 3 of the patients on insulin therapy at the end of their follow-up had large declines in their average dose compared with pretransplant (37.5 to 10, 33 to 6, and 25.5 to 1.5 units/day), and 1 patient was on 20 units/day when withdrawn from the trial because of islet graft loss, as previously discussed. During follow-up, there were no severe hypoglycemic events.
Mean age (SD) and diabetes duration (SD) were 49 years (10 years) and 30 years (12 years), respectively; 13 patients were female (Table 1). HbA1c decreased from 7.2% before transplant to 5.9% 12 months posttransplant (P < 0.001). On the basis of clinical trial exclusion criteria, no patient was classified as having kidney disease at baseline, and no patient presented with urine albumin-to-creatinine ratio >300 mg/g at 12- and 50-month follow-up. However, two patients had an eGFR <60 mL/min/1.73 m2 (44 and 53 mL/min/1.73 m2) at baseline. There was an increase in urine albumin-to-creatinine ratio (7 vs. 26 mg/g; P = 0.04; to convert to mg/mmol, multiply by 0.113) between baseline and 12 months; eGFR did not significantly change during follow-up.
Table 1.
Demographic and clinical characteristics of islet transplant recipients
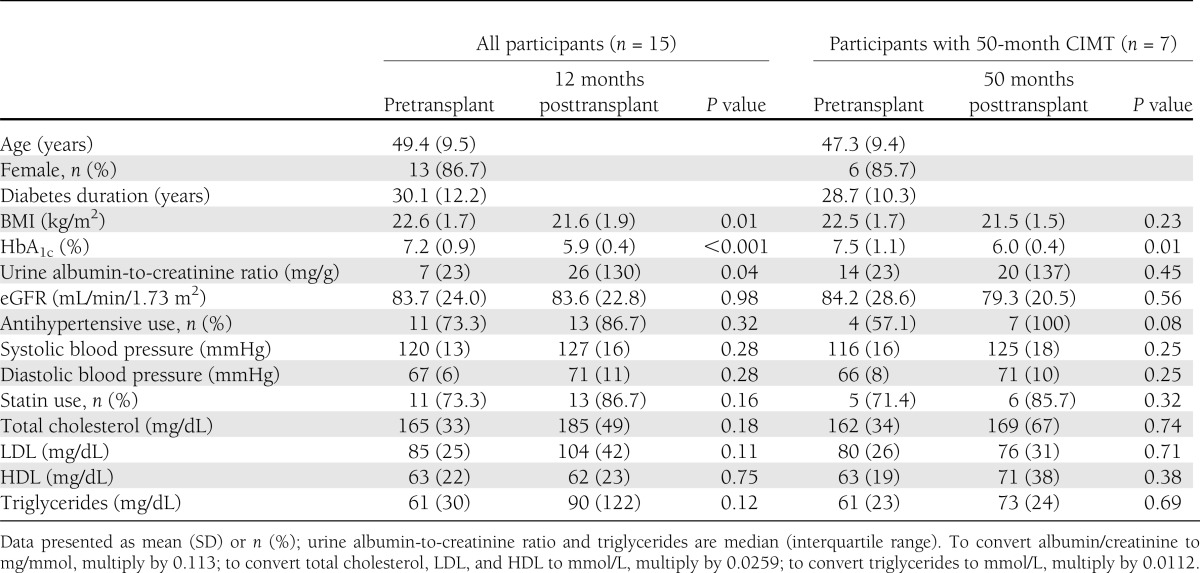
Patients experienced a small decline in BMI (22.6 vs. 21.6 kg/m2; P = 0.01) between baseline and 12 months. Blood pressure and lipids were well controlled with nonsignificant changes in both parameters during follow-up; 12 patients were on either antihypertensive or statin medication at baseline (of which 10 were treated with both, 1 with only statins, and 1 with only antihypertensives). The 11 patients on statin therapy at baseline were using atorvastatin, pravastatin, rosuvastatin, or simvastatin, with an average dose of 20 mg/day (range, 10–40 mg/day). At their last follow-up visit, 11 patients were using a similar brand of statins or ezetimibe, with an average dose of 26 mg/day (range, 10–80 mg/day).
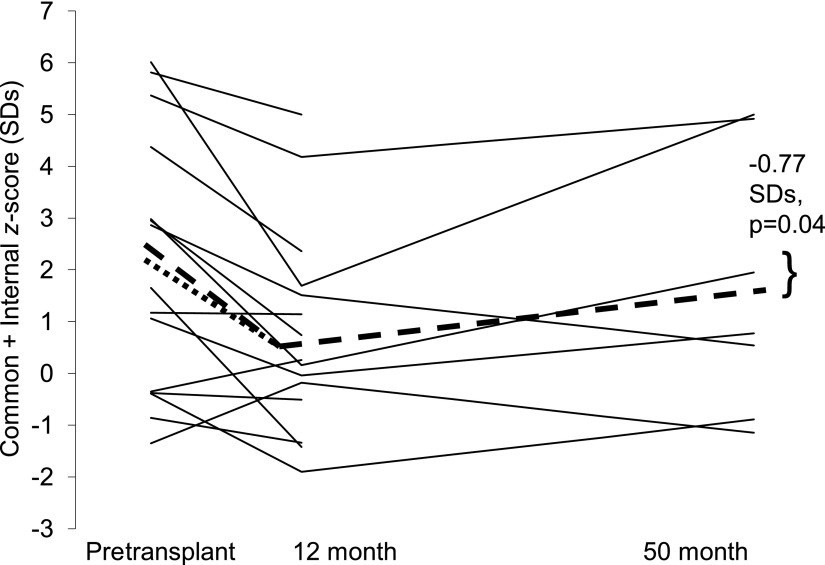
There was a significant decrease in CIMT at 12 months (n = 15) for the common carotid (−0.058 mm; P = 0.006) and combined score (−1.28 SDs; P = 0.004) (Table 2 and Fig. 1). The power to detect the significant changes in CIMT (two-sided, paired Student t test with α = 0.05) was >85%. There was a trend toward a decrease at 12 months for internal CIMT (−0.047 mm; P = 0.10). For those with a 50-month follow-up (n = 7), there was a slightly larger reduction in CIMT at 12 months for the three CIMT measures, which was statistically significant for the combined score (−1.59 SDs; P = 0.04). At 50 months posttransplant, there was a continued reduction in CIMT, but of smaller magnitude, which was significant for the combined score (−0.77 SDs; P = 0.04) (Fig. 1) and marginally significant for the internal artery (−0.037 mm; P = 0.06). The power to detect the significant changes in the combined score was >55%. Power for the nonsignificant changes ranged from 11 to 50%. Taken together, those with a 50-month follow-up demonstrated a significant reduction in CIMT 12 months posttransplant, with subsequent progression of CIMT. Common carotid IMT progressed from 12 (0.739 mm) (Table 2) to 50 months (0.775 mm) at an average rate of 0.011 mm/year ([0.775 − 0.739 mm]/38 months × 12).
Table 2.
Absolute values and change in CIMT pretransplant to 12 and 50 months posttransplant
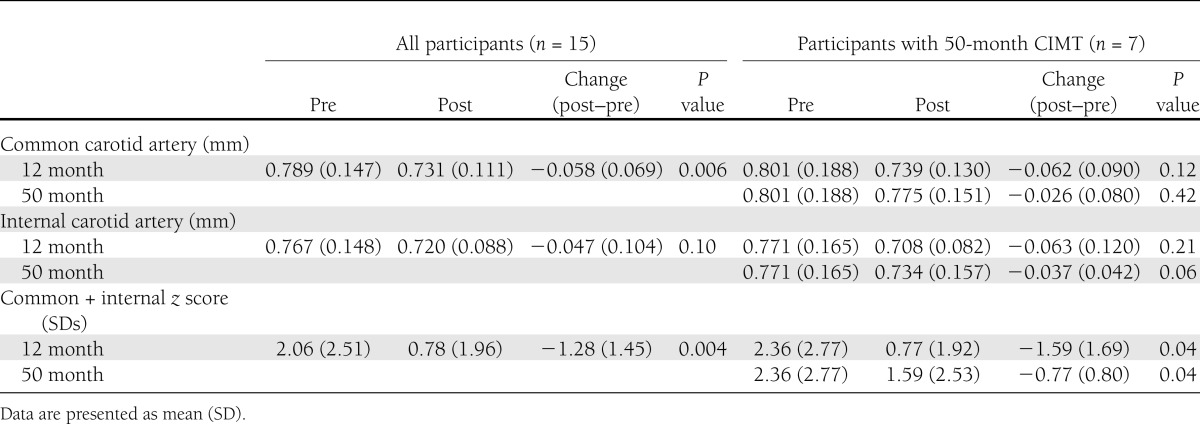
Figure 1.
Combined score (SDs) before and 12 and 50 months after islet transplant. The dotted line represents the mean change in the score between baseline and 12-month follow-up (n = 15). The dashed line represents the mean change in the score between baseline and 12- and 50-month follow-up (n = 7).
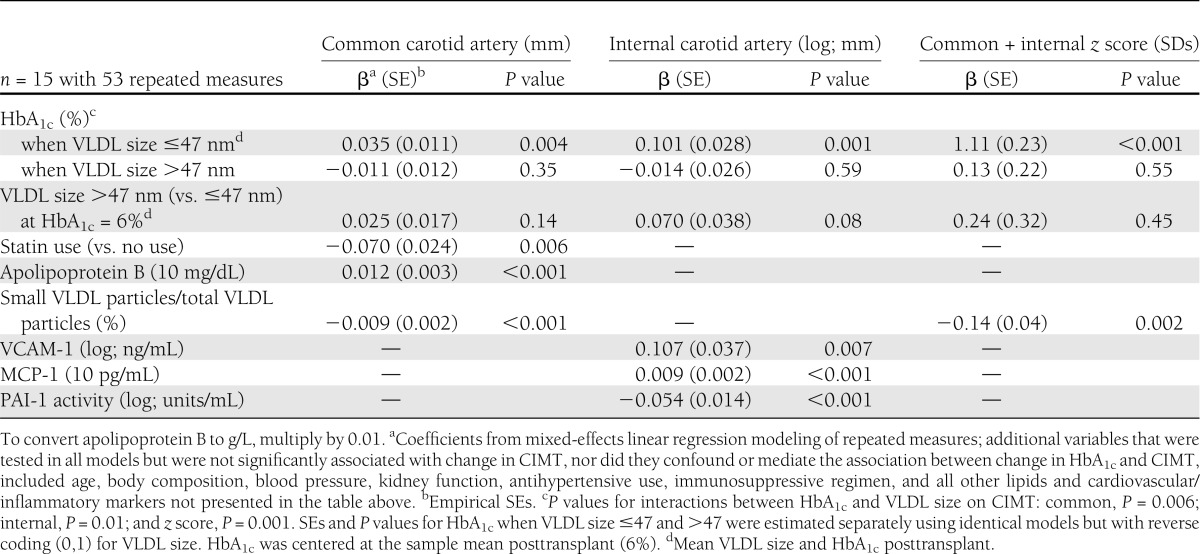
Factors associated with the slope of change in CIMT during follow-up are presented in Table 3. For the common and internal artery and combined CIMT score, the decreasing slope in CIMT was associated with a decreasing slope of change in HbA1c, but this was limited to individuals with smaller VLDL particle size. For those individuals with larger VLDL particle size, the slope in CIMT was not related to the slope in glycemic control. The decreasing slope of change in CIMT during follow-up was also associated with decreasing slopes in apolipoprotein B, VCAM-1, and MCP-1 and increasing slopes in the proportion of the number of small VLDL particles relative to the total number of VLDL particles and PAI-1 activity. Statin use posttransplant was associated with a decreasing slope of change in common carotid IMT. CIMT was not associated with age, BMI, blood pressure, kidney function (creatinine clearance, albumin-to-creatinine ratio, and eGFR), insulin independence, antihypertensive use, or immunosuppressive regimen in repeated-measures modeling or cross-sectional analyses, nor did these factors confound or mediate the association between the slope of change in CIMT and HbA1c.
Table 3.
Factors significantly associated with the slope of change in CIMT pretransplant through 50 months posttransplant
CONCLUSIONS
The current prospective study demonstrated a significant decrease in CIMT after islet transplantation in individuals with type 1 diabetes. In the first year after transplant, common carotid artery IMT decreased by ∼0.060 mm. A slightly smaller decrease in CIMT was found in the first prospective study to look at the effect of pancreas transplant; 1.8 years after transplant, common artery IMT had significantly decreased by 0.045 mm (7). Minimally invasive islet transplantation therefore appears to reverse the progression of atherosclerosis within the first few years after transplant, similar to pancreas transplant. Between 12 and 50 months after islet transplant, our results showed a progression of common artery IMT, on average 0.011 mm/year. However, at 50 months posttransplant, the combined CIMT score continued to be significantly reduced compared with pretransplant levels.
Previous intervention studies aimed at achieving superior glycemic control have slowed the progression of CIMT in individuals with diabetes, but actual regression of CIMT is rare (18). For example, the CHICAGO trial demonstrated that antidiabetic medications stabilize (pioglitazone, −0.001 mm over 72 weeks) or slow the progression (glimepiride, 0.012 mm over 72 weeks) of common carotid IMT in type 2 diabetes (15). For type 1 diabetes, the DCCT/EDIC demonstrated 6 years after the end of the trial that common carotid IMT progressed at a significantly slower rate of 0.006 mm/year in the intensive treatment group, vs. 0.008 mm/year in the conventional treatment group (3). In the one previous study to look at the effect of islet transplantation on CIMT, conducted in individuals with end-stage renal disease, there was a nonsignificant increase in CIMT (0.020 mm/year) in those receiving a kidney-islet transplant compared with a significant increase in CIMT (0.033 mm/year) in the kidney-only transplant group over 3 years of follow-up (14). For comparison, the mean progression of CIMT in healthy individuals without diabetes is 0.005 mm/year (19). The average rate of common carotid IMT progression seen in the current study during follow-up (0.011 mm/year), after the initial large decrease 1 year posttransplant, was larger than the progression in individuals with type 1 diabetes without transplant over 6 years after conventional therapy (0.008 mm/year) (3) and twice that seen in healthy individuals (0.005 mm/year) (19). However, it was half of that seen in patients with end-stage renal disease with a kidney-islet transplant (0.020 mm/year) (14). Therefore, although there is a significant decrease in CIMT initially after islet transplant, the substantial progression in CIMT after transplantation may increase CIMT back to pretransplant levels such that it may no longer remain significantly reduced beyond 50 months posttransplant.
Greater CIMT is associated with an increased risk of coronary heart disease (20,21). In terms of clinical significance, for individuals without diabetes, a 0.100-mm increase in common carotid IMT is associated with an 11% increase in the risk of acute myocardial infarction (20). The Multi-Ethnic Study of Atherosclerosis demonstrated a 20% increase in coronary heart disease for a one SD increase (0.190 mm) in common carotid artery IMT (21). In the DCCT/EDIC, a 0.002 mm/year slower progression in common artery CIMT in the intensive versus conventional treatment group (3) paralleled a 57% reduction in nonfatal myocardial infarction, stroke, or death from cardiovascular disease in the intensive versus conventional group (5). Therefore, the initial reduction in common carotid IMT of 0.060 mm in the first year after islet transplant, with continued reduction of ∼0.030 mm after 50 months, may have a clinical impact on the risk of ischemic heart disease in those with type 1 diabetes in the first years after transplant. However, the progression in CIMT of 0.011 mm/year after transplant may limit the clinical impact on long-term cardiovascular outcomes. During follow-up after islet transplant in the current study, no patient experienced a myocardial infarction or stroke; two patients required cardiac procedures (the first patient, with the second highest combined CIMT score during follow-up, required two stents placed in the left anterior descending artery 1 year after the first and only transplant; the second patient, with the lowest combined CIMT score during follow-up, required balloon angioplasty of the posterior descending artery between the second and third transplant). Long-term follow-up is underway to document any additional cardiovascular events.
The decreasing trend in CIMT during follow-up was associated with improvements in HbA1c, particularly in those individuals with small VLDL particle size, a factor strongly affected by enhanced insulin sensitivity (22). It is well documented that euglycemia can contribute to stabilization of endothelial function and proliferation (23), and indeed, lower mean HbA1c largely explained the slower progression of CIMT in the intensive glycemic control group in the DCCT/EDIC (4). Our results expand upon the DCCT/EDIC data by demonstrating that the superior level of glycemic control that can be achieved with islet transplant compared with intensive insulin management may have contributed not only to a slower progression of CIMT but also significant improvements in CIMT, specifically for the insulin-sensitive patients. Therefore, the reduction in CIMT during the first year after transplant may be explained by the significant reduction in HbA1c consequent to restoring endogenous insulin secretion through transplant (11), and subsequent progression of CIMT may be explained by declining islet graft function and glycemic control after the first year (9). The twofold rate of progression in CIMT compared with healthy individuals (19) may also be associated with chronically higher HbA1c levels compared with those without diabetes.
The decreasing trend in common CIMT was also associated with statin use and improvements in lipids, specifically declining apolipoprotein B and increasing concentrations of the small (vs. large) VLDL particles. This is consistent with previous research demonstrating that statin therapy can promote regression of atherosclerotic plaques (24) and CIMT (25). Improvements in internal CIMT were also associated with decreasing trends in VCAM-1 and MCP-1 and an increasing trend in PAI-1 activity, consistent with decreased inflammation and atherogenesis. CIMT was not associated with kidney function, periods of insulin independence, or medications such as antihypertensives and immunosuppressive regimen posttransplant.
A strength of the current study is that it is prospective, with up to 50 months of follow-up, in which each individual was his/her own control, measured before and after the intervention. The lack of a concurrent control group of similar patients without transplant to study 12-month change in CIMT may be considered a weakness, but such a concurrent control group was not feasible in this study; the average time on the islet transplant waiting list at the UIC Medical Center was only 4.4 months, and only one patient was on the list for >1 year. Additionally, the U.S. Food and Drug Administration has stated that historical control data such as the DCCT/EDIC are sufficient as concurrent control groups in islet transplantation trials are not practical because of the following: the unwillingness of patients to be control subjects; the potentially high control dropout rate that may occur, even if control subjects are able to be recruited, because of the open-label nature of the trial; the limitations of the comparative information that would result from the inability to blind patients and investigators; and the inability to power a trial to detect treatment-related effects given the limited availability of islets and the high costs of each patient (26,27). An additional strength of the study is that it did not enroll individuals with kidney disease or previous cardiovascular events, as defined by the clinical trial exclusion criteria, two potentially confounding factors. However, there were decreases in kidney function for some patients after islet transplant, a concerning and not uncommon side effect of immunosuppressive medications in islet transplantation (9).
The current study was limited to a case series of 15 individuals, with half of the cohort followed for the full 5 years. To increase statistical power, the analyses evaluating predictors of CIMT used repeated measures. These results are suggestive of potentially important changes for those with type 1 diabetes and will need validation in a larger cohort of patients. It would also be informative to determine whether regression of CIMT and/or the slowing of other cardiovascular outcomes occur in a xenotransplant setting in light of the recent successes in xenotransplantation (28). As periods of insulin independence have been found to increase with potent induction immunotherapy (29), longer-term follow-up will also be needed in other cohorts to see whether improvements in CIMT could potentially be sustained for >50 months as insulin independence becomes more durable; although insulin independence was not significantly associated with CIMT in the current study.
In conclusion, minimally invasive islet transplantation leads to insulin independence and may also slow the progression of atherosclerosis caused by type 1 diabetes. The underlying mechanism is likely related to improved glycemic control consequent to restoring endogenous insulin secretion through the islet transplant, and optimal lipid management posttransplant also contributes.
Acknowledgments
This study was supported by funding from the College of Medicine at UIC, the Christopher Foundation, the Efroymson Foundation, the Dr. Scholl Foundation, the Washington Square Health Foundation, the Chicago Diabetes Project, and the National Center for Advancing Translational Sciences (National Institutes of Health Grant UL1TR000050). J.O. was supported by the National Center for Research Resources (National Institutes of Health islet cell resource Grant U42-RR-023245).
Wyeth provided free sirolimus for the duration of the study. No other potential conflicts of interest relevant to this article were reported.
The funding organizations and sponsors had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, or approval of the manuscript.
K.K.D. contributed to the discussion and wrote the manuscript. B.H., K.K., and J.O. researched data, contributed to the discussion, and reviewed and edited the manuscript. B.K., M.Q., and E.B. contributed to the discussion and reviewed and edited the manuscript. J.M. and A.M. researched data and reviewed and edited the manuscript. K.K.D. and J.O. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract and oral form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors thank William R. Clarke (University of Iowa, Iowa City, Iowa) for feedback on the statistical analysis.
Footnotes
Clinical trial reg. no. NCT00679042, clinicaltrials.gov.
References
- 1.Moss SE, Klein R, Klein BE. Cause-specific mortality in a population-based study of diabetes. Am J Public Health 1991;81:1158–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760–765 [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM, Lachin J, Cleary P, et al. Diabetes Control and Complications Trial. Epidemiology of Diabetes Interventions and Complications Research Group Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003;348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polak JF, Backlund JY, Cleary PA, et al. DCCT/EDIC Research Group Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes 2011;60:607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan DM, Zinman B, Cleary PA, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications Experience (1983-2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen JL, Colling CW, Ratanasuwan T, et al. Pancreas transplantation improves vascular disease in patients with type 1 diabetes. Diabetes Care 2004;27:1706–1711 [DOI] [PubMed] [Google Scholar]
- 8.Larsen JL. Pancreas transplantation: indications and consequences. Endocr Rev 2004;25:919–946 [DOI] [PubMed] [Google Scholar]
- 9.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes 2005;54:2060–2069 [DOI] [PubMed] [Google Scholar]
- 10.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 11.Gangemi A, Salehi P, Hatipoglu B, et al. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant 2008;8:1250–1261 [DOI] [PubMed] [Google Scholar]
- 12.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care 2012;35:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant 2008;8:1990–1997 [DOI] [PubMed] [Google Scholar]
- 14.Fiorina P, Gremizzi C, Maffi P, et al. Islet transplantation is associated with an improvement of cardiovascular function in type 1 diabetic kidney transplant patients. Diabetes Care 2005;28:1358–1365 [DOI] [PubMed] [Google Scholar]
- 15.Mazzone T, Meyer PM, Feinstein SB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA 2006;296:2572–2581 [DOI] [PubMed] [Google Scholar]
- 16.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Effect of intensive diabetes treatment on carotid artery wall thickness in the epidemiology of diabetes interventions and complications. Diabetes 1999;48:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Kidney Foundation K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification and Stratification: Part 5. Evaluation of laboratory measurements for clinical assessment of kidney disease. Am J Kidney Dis 2002;39:S76–S110 [PubMed] [Google Scholar]
- 18.Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation 2002;106:2055–2060 [DOI] [PubMed] [Google Scholar]
- 19.Hovingh GK, Brownlie A, Bisoendial RJ, et al. A novel apoA-I mutation (L178P) leads to endothelial dysfunction, increased arterial wall thickness, and premature coronary artery disease. J Am Coll Cardiol 2004;44:1429–1435 [DOI] [PubMed] [Google Scholar]
- 20.Salonen JT, Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation 1993;87(Suppl.):II56–II65 [PubMed] [Google Scholar]
- 21.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med 2008;168:1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garvey WT, Kwon S, Zheng D, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003;52:453–462 [DOI] [PubMed] [Google Scholar]
- 23.Fiorina P, Folli F, Maffi P, et al. Islet transplantation improves vascular diabetic complications in patients with diabetes who underwent kidney transplantation: a comparison between kidney-pancreas and kidney-alone transplantation. Transplantation 2003;75:1296–1301 [DOI] [PubMed] [Google Scholar]
- 24.Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011;365:2078–2087 [DOI] [PubMed] [Google Scholar]
- 25.Riccioni G, Cipollone F, Santovito D, et al. Effect of 2-year treatment with low-dose rosuvastatin on intima-media thickness in hypercholesterolemic subjects with asymptomatic carotid artery disease. Expert Opin Pharmacother 2011;12:2599–2604 [DOI] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration. Guidance for industry: considerations for allogenic pancreatic islet cell products [Internet], 2012. Available at http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/cellularandgenetherapy/ucm182440.htm Accessed 27 June 2012
- 27.Tiwari JL, Schneider B, Barton F, Anderson SA. Islet cell transplantation in type 1 diabetes: an analysis of efficacy outcomes and considerations for trial designs. Am J Transplant 2012;12:1898–1907 [DOI] [PubMed] [Google Scholar]
- 28.Ekser B, Ezzelarab M, Hara H, et al. Clinical xenotransplantation: the next medical revolution? Lancet 2012;379:672–683 [DOI] [PubMed] [Google Scholar]
- 29.Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant 2012;12:1576–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]