Abstract
OBJECTIVE
To explore in France the relationship between insulin glargine use and overall and specific cancer risks in type 2 diabetic patients compared with other basal insulins.
RESEARCH DESIGN AND METHODS
Data were extracted from French health insurance information system (Système National d'Information Inter-Régimes de l'Assurance Maladie) linked with data from the French Hospital Discharge database (Programme de Médicalisation des Systèmes d'Information). Included were 70,027 patients aged 40–79 years who started a basal insulin in 2007–2009. Cox proportional hazards models with age as time-scale were used to calculate multivariate-adjusted hazard ratios for associations between type of basal insulin and risk of overall cancer, breast cancer, and seven other cancer sites.
RESULTS
The median follow-up was 2.67 years in patients exposed to insulin glargine. Absolute event rates for all cancer in patients exposed to glargine versus other basal insulin users were 1,622 and 1,643 per 100,000 person-years, respectively. No significant association was observed between glargine exposure and overall cancer incidence after adjustment for sex, with a hazard ratio of 0.97 (95% CI 0.87–1.07), or after additional adjustment for any other hypoglycemic agent use and duration of diabetes. No increased risk of breast cancer was observed for glargine users compared with other basal insulins users, with a fully adjusted hazard ratio of 1.08 (0.72–1.62).
CONCLUSIONS
In a large cohort of patients newly treated by basal insulin, no increased risk of any cancer was observed in insulin glargine users compared with other basal insulin users. Because follow-up did not exceed 4 years, longer-term studies are needed.
The publication in 2009 of four observational studies (1–4) raised the question of a possible relationship between the risk of developing tumors and the type of insulin (i.e., human insulin and insulin analogs), particularly insulin glargine. Previous in vitro studies comparing insulin glargine and human insulin concluded that glargine had a higher receptor affinity and mitogenic potency (5). However, the in vivo metabolism of insulin glargine in the blood suggests a low mitogenic potency due to the low affinity of its main metabolite for the IGF-1 receptor (6).
Overall, several differences concerning the methodology of published observational studies can be highlighted. In particular, some studies compared insulin glargine and human insulin monotherapy only and excluded patients exposed to both types of insulin in the main or a secondary analysis (1,2) and/or considered human insulin regardless of the duration of activity compared with a long-acting analog (1,2,4) and/or considered prevalent users (1–4), and one analysis included new users only (2). Several adjustments for various variables, according to the data available in the databases used, also often led to conflicting results within the same study. Moreover, multiple subgroup analyses increased the risk of spurious chance findings. The German study (1) that found a significant increased risk of total cancers for insulin glargine was criticized (7,8); in particular, Pocock and Smeeth (7) reported serious errors in the methodology that made the “article’s findings unsupportable.” They also pointed out that the main results of the first four cohort studies (1–4) and clinical trials (9) did not show any significant increase in the overall incidence of malignancy for insulin glargine compared with other insulins. However, the cancer risk associated with insulin glargine remained an ongoing debate in late 2010, particularly in relation to breast cancer.
We were therefore asked by the French Medicines Agency (Agence française de sécurité sanitaire des produits de santé [Afssaps]) in January 2011 to conduct a study based on national administrative databases. The main objective of this study was to estimate the relationship between insulin glargine use and cancer risk, especially breast cancer, in French patients with type 2 diabetes compared with other basal insulins.
RESEARCH DESIGN AND METHODS
Study design and data source
We conducted an historical cohort study based on data from the French National Health Insurance information system (Système National d'Information Inter-Régimes de l'Assurance Maladie [SNIIRAM]), which contains individualized, anonymous, and comprehensive data on health spending reimbursements (10). The main demographic data include age, sex, and vital status. Information on 100% reimbursed severe and costly chronic diseases (affection de longue durée [ALD] [11]) is available in the SNIIRAM and coded according to the ICD-10. These data can be linked to the French Hospital Discharge database (Programme de Médicalisation des Systèmes d'Information [PMSI]) (12). The PMSI database provides detailed medical information on all admissions in public and private hospitals, including discharge diagnoses ICD-10 codes and medical procedures performed during the hospital stay. The linkage between SNIIRAM and PMSI databases has been previously used to conduct several large epidemiological or postauthorization safety studies (13–15).
Data from SNIIRAM were only available from 2006. Specific approval was obtained from the French data protection agency (Commission nationale de l'informatique et des libertés [CNIL]) to perform this study during the planned period.
Study population
For reasons of complete availability of data during the whole study period, this study was based on the national health insurance general scheme, excluding students and civil servants, covering more than 49 million people (i.e., 75% of the French population).
The cohort contained new users of long-acting or intermediate-acting insulin during the years 2007, 2008, and 2009, identified by the Anatomical Therapeutic Chemical (ATC) code. Three groups were defined according to first exposure to insulin glargine (A10AE04), insulin detemir (A10AE05), or human intermediate-acting or long-acting insulin (i.e., basal human insulin [BHI]; A10AC01, A10AD01, and A10AE01). Two distinct prescriptions for the same type of basal insulin were required within a 6-month period to define exposure. To select a population with type 2 diabetes, inclusion was limited to patients aged 40 to 79 years with at least three prescriptions for any other hypoglycemic agent (OHA; i.e., ATC code A10B) in the calendar year preceding insulin exposure. Patients were defined as new users if they had no prescription for any basal or bolus insulin in 2006.
Excluded were patients with any discharge diagnosis of cancer (ICD-10 codes C00-C97, D00-D09, and D37-D48) from 2005 to inclusion or within the 12 months after inclusion, or with cancer identified from ALD status since 1987.
Exposure
Exposure to insulin glargine or other basal insulin was considered a time-dependent variable. A patient was defined as really exposed to a specific insulin after 12 months from the first reimbursement until the end of follow-up, even after treatment discontinuation. Moreover, any switch to another type of basal insulin was taken into account similarly. Exposure to any class of OHA was defined in a similar way. The actual cumulative insulin dose from study entry to the last date of prescription was considered to be a time-dependent variable and calculated by evenly distributing the total dose of each basal insulin prescription over all of the days between the date of the prescription and the date of the subsequent prescription.
Outcomes
Outcomes analysis started from the 13th month after the date of the first eligible insulin reimbursement to the earliest of the following events: occurrence of the studied event (all cancer or specific cancer), loss to follow-up, defined by more than 6 consecutive months without having filled any drug prescription, death from any cause, or end of the observation period on 31 December 2010. Patients lost to follow-up were censored 2 months after the start of the minimum 6-month gap to allow enough time to observe any incident cancer.
Cancer cases were identified by hospital discharge diagnoses (ICD-10 codes). The two main outcomes were “all malignancies” (C00 to C97) and “breast cancer” in women (C50). The incidence of cancers at seven other sites was also studied for insulin glargine users: prostate (C61), colorectal (C18 to C21), liver (C22), kidney (C64), bladder (C67), head and neck (C00 to C14), and lung (C33 and C34) cancers.
Confounding factors
The main potential confounders were age at study entry, sex, use of any class of OHA after inclusion, and information on duration of diabetes, which was estimated from the date of initiation of the ALD for diabetes (when available). The following classes of OHA were considered separately: metformin, pioglitazone, rosiglitazone, sulfonylureas, and other OHA. Together with OHA comedication, screening tests for breast and prostate cancer, hormone replacement therapy (HRT), and hospitalizations for cardiovascular or cerebrovascular disease were identified during the calendar year before inclusion. Women aged 50 years or older were considered as being treated with HRT (ATC codes G03CA, G03FB, or G03HB) if they had two or more annual prescriptions. Screening tests were collected by the corresponding codes in SNIIRAM for men aged 50 years or older (blood prostate-specific antigen test) and women aged 50 to 74 years (mammography). Hospitalizations for acute coronary syndrome and stroke were identified from discharge diagnoses (ICD-10 codes I21 to I23 and I60 to I64, respectively).
Statistical analyses
Age, sex, OHA class use, and duration of diabetes were compared using the χ2 test for categorical variables and the Wilcoxon test for continuous variables. Other baseline characteristics (including admission rates and screening tests) were compared using the Mantel-Haenszel method with adjustment for age and sex. Percentages were age- and sex-standardized, including overall mortality rate during the first year after study entry. Patients were classified into insulin glargine, insulin detemir, or BHI groups according to the first insulin prescribed.
Crude incidence rates and incidence rate ratios (IRRs) of overall and site-specific cancers were calculated with the number of cancer cases and the corresponding exposure or nonexposure periods. Cox proportional hazards models were used to calculate adjusted hazard ratio (HR) estimates of cancer incidence with insulin glargine, insulin detemir, BHI, or OHA class and their 95% CI. Age in calendar months was used as the time scale. Several models with adjustment for potential confounders were performed: sex in model 1, sex and OHA class use in model 2, and sex, OHA class use, and duration of diabetes in model 3. The same models were applied for cumulative doses classified into terciles. Other potential confounders, such as screening tests and HRT, were considered in model 2 for the main analysis only (i.e., insulin glargine vs. other basal insulin users) when the P value was ≤ 0.2 for comparison of prevalences. Two sensitivity analyses were conducted: one used length of follow-up in calendar months as the time scale and age as covariate (10-year classes); another considered only a 6-month period instead of a 12-month period for the exclusion of cancers after inclusion and for the defined time lag for exposure to insulin glargine.
Associations were considered statistically significant for a P value <0.05. Analyses were performed with SAS 9.1 software (SAS Institute, Cary, NC).
RESULTS
Description of the cohort population
From the 89,471 individuals potentially eligible, 13,782 were excluded for cancer before study entry or within the 12 months after study entry and 5,662 patients for follow-up of less than 12 months (see Supplementary Fig. 1). The cohort included 70,027 patients; of these, 47,432 (67.7%) were treated with insulin glargine only, 12,506 (17.9%) were treated with insulin detemir only, 4,564 (6.5%) were treated with BHI only, and 5,525 (7.9%) were treated successively with at least two distinct long-acting or intermediate-acting insulins. Among patients who initiated their insulin therapy with insulin glargine, 5.4% subsequently switched to another basal insulin. This percentage was higher for insulin detemir (12.9%) and BHI (18.5%). BHI actually corresponded to intermediate-acting insulin only (i.e., neutral protamine Hagedorn [NPH]) insulin, showing that long-acting human insulin was already no longer prescribed in France and has totally been replaced by analogs. Information on duration of diabetes was available for 58,889 patients (78.3%).
Patients exposed to insulin glargine were slightly but significantly older than those exposed to other basal insulins (P < 0.0001; Table 1). The duration of diabetes was comparable between the insulin glargine, insulin detemir, and BHI groups, and did not differ significantly between insulin glargine users and other basal insulins users. Patients exposed to insulin glargine were more frequently exposed to any OHA (87.2%) than patients exposed to BHI (70.3%), but less frequently exposed than insulin detemir users (91.3%).
Table 1.
Demographic data and use of antidiabetic drugs during follow-up by long-acting or intermediate-acting insulin
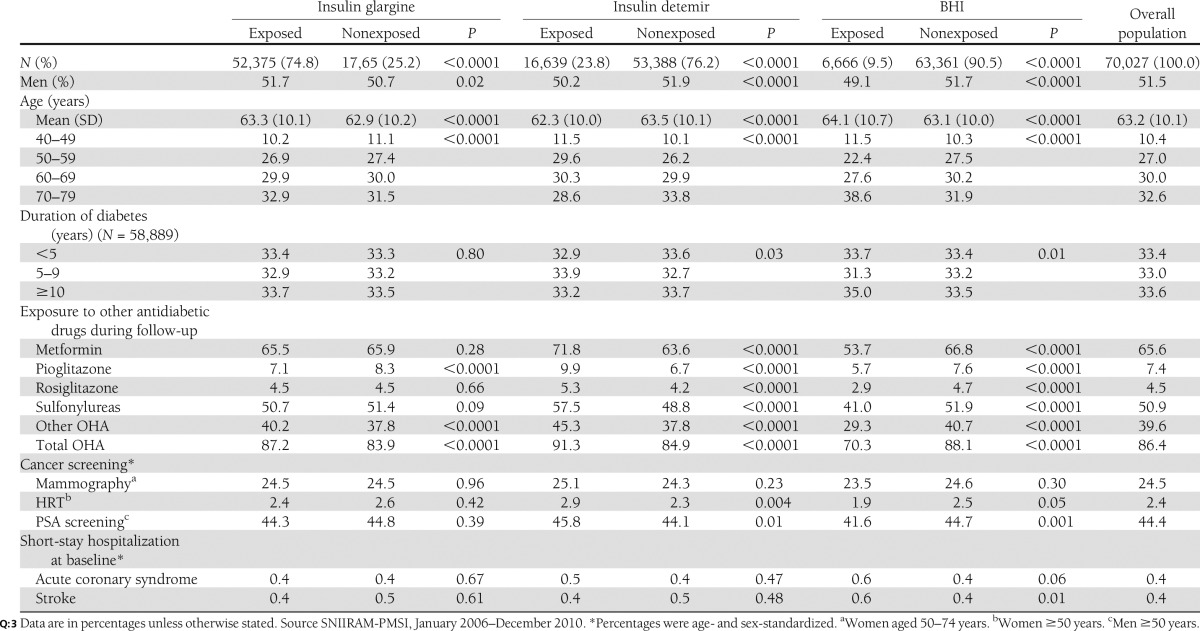
The all-cause mortality rate was higher in BHI users (8.9%) than in insulin glargine users (4.7%) and insulin detemir users (3.5%; P < 0.0001). No significant difference in hospitalizations for acute coronary syndrome and stroke, or for screening tests and HRT was observed between insulin glargine users and other basal insulin users (Table 1).
Median follow-up was 2.67, 2.75, and 2.83 years in patients exposed to insulin glargine, insulin detemir, and BHI, respectively. The median monthly and cumulative doses were 722 and 21,000, 679 and 18,000, and 606 and 16,500 IU, respectively.
Association with cancer
A total of 1,391 malignancies of any type were reported for patients exposed to insulin glargine versus 405 in patients exposed to insulin detemir and 191 in BHI users. Crude incidence rates for individuals exposed and not exposed to insulin glargine were 1,622 and 1,643 per 100,000 person-years, respectively (crude IRR 0.99 [95% CI 0.89–1.09]; Table 2). No significant association was observed between insulin glargine exposure and incidence of any malignancy after adjustment for sex (HR 0.97 [0.87–1.07]) or after additional adjustment for any OHA class use and duration of diabetes (Table 3). Comparable results were observed in a similar analysis with insulin detemir and BHI. A borderline significant risk decrease was observed for metformin, with HR 0.87 (0.79–0.96) in model 2 and 0.87 (0.78–0.97) in model 3, and sulfonylureas as well, with 0.89 (0.81–0.97) and 0.86 (0.77–0.95). A stratified analysis—not initially planned—on metformin users versus nonusers led to comparable results in both groups for the three models (Supplementary Table 1). During follow-up, crude incidence rates of breast cancer were 271 and 264 per 100,000 person-years for insulin glargine versus nonusers, respectively (crude IRR 1.02 [0.71–1.47]; Table 3). No increased risk of breast cancer was observed in any model; in particular, the HR for glargine was 1.01 (0.71–1.45) in model 1 and 1.08 (0.72–1.62) in model 3. Comparable results were observed for insulin detemir and BHI (Table 3).
Table 2.
Crude incidence rate ratios by long-acting or intermediate-acting insulin for all malignancies and breast cancer
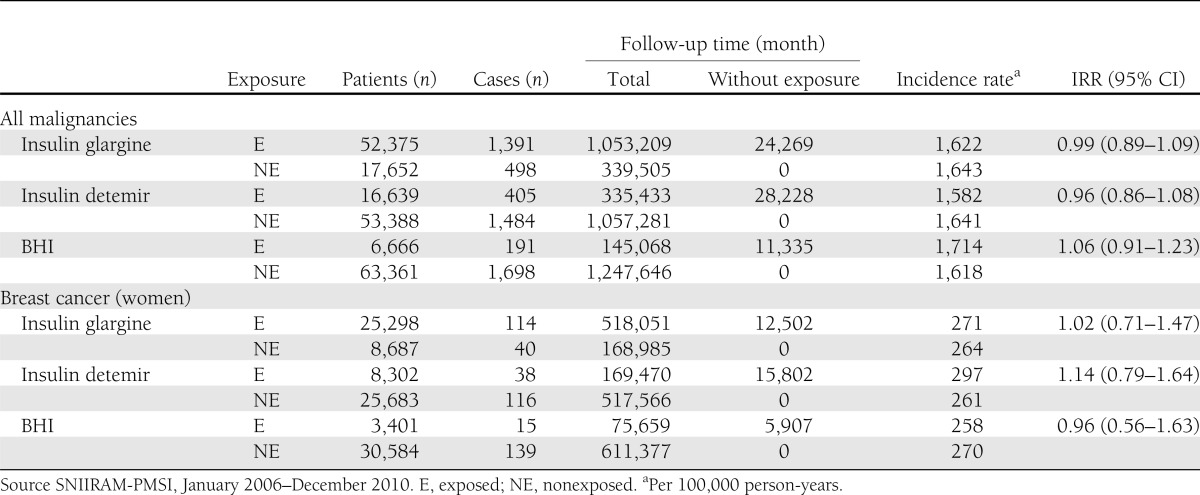
Table 3.
Risk of all malignancies and breast cancer, overall and with increasing level of insulin glargine, insulin detemir, or BHI exposure during follow-up: adjusted HR estimated from the three multivariate Cox models with age as the time scale
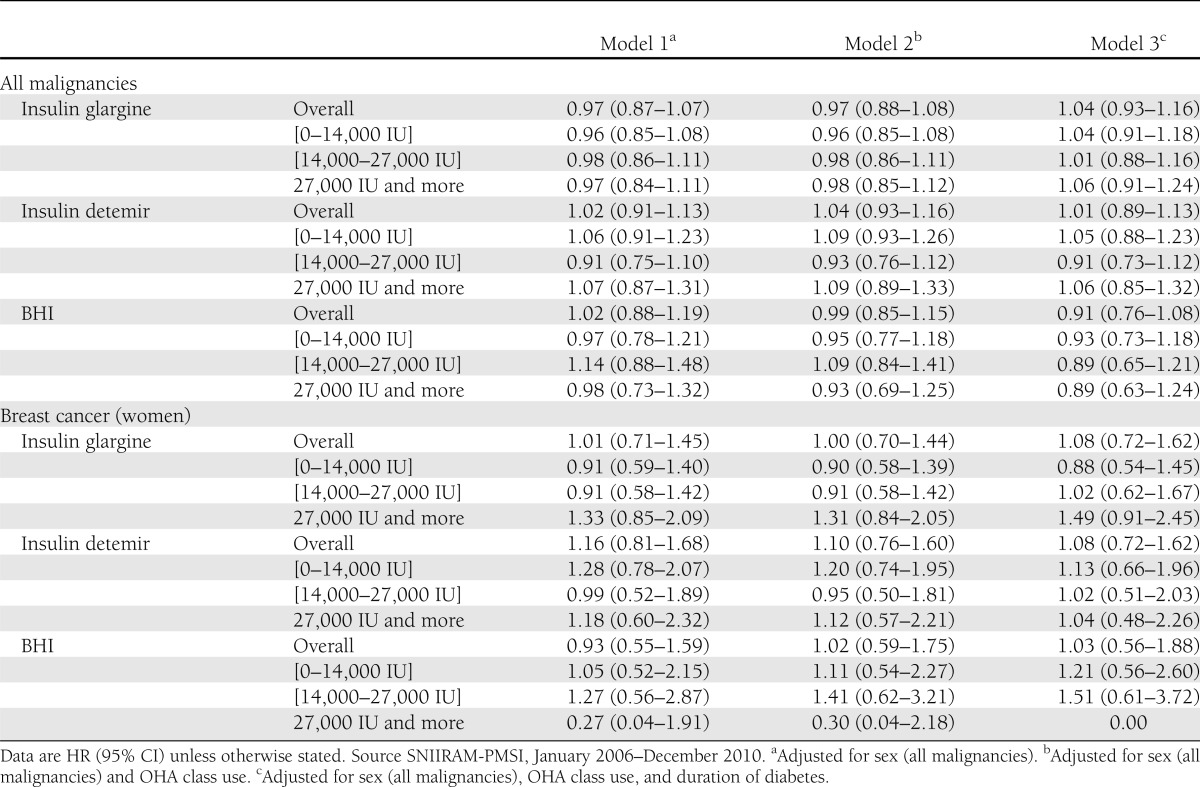
Similar results for insulin glargine were observed when length of follow-up was used as the time scale, with HR 0.97 (95% CI 0.88–1.08) for all cancers and 1.00 (0.70–1.44) for breast cancer (model 1; Supplementary Table 2). When considering a 6-month period instead of a 12-month period for the exclusion of cancers after inclusion and for the defined time lag for exposure to insulin, the results for insulin glargine remained nonsignificant for all cancers (0.95 [0.87–1.04] in model 1) and breast cancer (1.00 [0.72–1.37] in model 1; Supplementary Table 3).
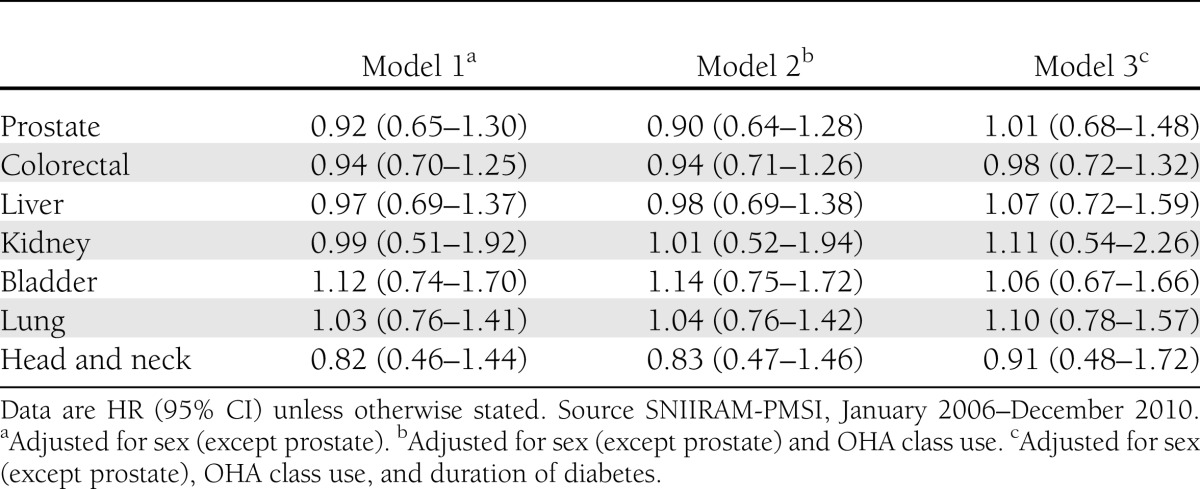
Evaluation of a possible dose-effect showed that no increased risk of cancer was specifically observed in any model for higher doses of insulin glargine (27,000 IU and more), with HR in model 1 of 0.97 (0.84–1.11) for all malignancy and 1.33 (0.85–2.09) for breast cancer in women. Similar results were observed for higher doses of insulin detemir or BHI. However, a slight nonsignificant increase in point estimates was observed in the three models for increased doses of glargine and the occurrence of breast cancer. No association was observed between insulin glargine use and the seven other tumor sites compared with the use of other basal insulins in any model (Table 4).
Table 4.
Risk of other cancer sites for glargine vs. other basal insulins: adjusted HR estimated from the three multivariate Cox models with age as the time scale
CONCLUSIONS
Insulin glargine was first marketed in France in mid-2003. Using a large nationwide cohort including more than 70,000 basal insulin incident users—to our knowledge the largest study to date on this topic—we did not find any significant difference in the risk of all cancers between new insulin glargine users and nonusers. We also did not observe any difference in breast cancer risk or the risk for seven other malignancies between these two groups. Interestingly, a significantly decreased risk was observed for metformin and sulfonylureas users compared with nonusers.
The two databases used are independent in data collection, resulting in a decreased risk of selection biases. These databases are considered to be comprehensive, because all French pharmacists and all physicians in French hospitals routinely contribute to data collection, with annual quality control of coding.
This study presents several limitations. The maximum duration of follow-up was 4 years, and we therefore had no information on the occurrence of cancers after longer-term exposure. Besides, data from French cancer registries established in a few French areas (16) cannot be linked to SNIIRAM and PMSI data. Consequently, stage of cancer and date of first symptoms were not available in this study. In some studies, however, regional estimations of cancer incidence from the PMSI database have been compared with the incidence reported by local cancer registries (17,18). One study reported a good concordance between breast cancer data from 12 departmental registries and PMSI discharge diagnoses (17). Moreover, insulin users may differ in several criteria according to insulin type, and other adjustments, such as BMI, would have been useful to improve this comparison. BMI and diabetes duration were comparable among insulin users in two U.K. studies (2,3), but BMI could not be compared in French patients.
Another study limitation is the lack of information on tobacco use, which is a known risk factor for many cancers, particularly lung, head and neck, and bladder cancers. This may also be an important factor affecting the generalizability of our results. However, further analysis concerning other cancer sites showed that there was no difference between insulin glargine incident users and nonusers in the incidence of lung cancer and head and neck cancer, which supports the hypothesis that the prevalence of smokers was comparable in the two groups, as observed in the Scottish study (2).
Our analysis only attributed a case of cancer when this event occurred at least 12 months after start of exposure, a period that we considered to be possibly relevant for inducing a cancer. However, no consensus has been reached concerning the duration of cancer induction. We therefore also considered a shorter period of 6 months as a sensitive analysis, which provided similar results. Furthermore, a recent study (19) suggested that the higher risks of cancer shortly after starting insulin treatment might be explained by protopathic bias. We also took into account any subsequent change in insulin exposure status and observed that only a small proportion of patients in this cohort (7.5%) were exposed to more than one basal insulin. We also analyzed BHI users versus nonusers and insulin detemir users versus nonusers and found no difference for cancer risks in these two analyses. In addition, we only selected patients exposed to long-acting or intermediate-acting insulin, as was done in a Taiwanese study (20), because patients exposed to fast-acting insulin only might present several differences, such as severity of diabetes, level of residual insulin, or comorbidities, compared with basal insulin users. We also tried to improve group comparability by not applying the monotherapy comparisons performed in some studies (1,21) (i.e., users only exposed to the same basal insulin during the study period), and conversely, we took into account all switches from a basal insulin to another one. Moreover, we compared basal insulins with or without short-acting insulin, and we did not include individuals exposed to short-acting insulin only.
Because information on drug exposure was available only from 2006 onward, leading to a possible lack of data for patients who initiated insulin therapy a long time ago, we also selected new users only rather than a fixed cohort. Moreover, these patients were more likely to be comparable in severity of diabetes or stage of progression of the disease.
We considered that age was the most appropriate time scale in the main Cox regression model, because age is a known risk factor for many cancers. For such outcomes, the hazard is more likely to be a function of age than time-on-study (22). However an analysis was also performed with time-on-study as the time scale and showed similar results, as in the Taiwanese study (20).
Besides, the results of the multicenter randomized Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial have been recently published (23). They were based on 12,537 people who received insulin glargine or standard care—in an open-label design—and mainly provided an analysis of cardiovascular morbidity and/or mortality. The results of the safety analysis on cancer were comparable with our study findings, as there was no significant difference in the incidence of any cancer (HR 1.00 [95% CI 0.88–1.13]) with a median follow-up of 6.2 years.
No increased risk for breast cancer was observed in our study, but a slight nonsignificant trend was noted with increased doses of glargine. A recent in vitro study showed that insulin glargine stimulated proliferation of breast adenocarcinoma cell line (MCF-7) compared with regular human insulin (24). However, it is unknown whether this finding supports an increased risk of developing a breast tumor in immunocompetent women. A subgroup analysis in the first Swedish study (4) showed a significantly increased risk of breast cancer for glargine versus no glargine use, but nonsignificant results were observed for glargine and overall cancer risk. A significant result was also reported in the Scottish study (2) for the prevalent users and for insulin glargine monotherapy only. However, such a risk was not found in three other analyses of the Scottish study (2) or in the second Swedish study published in early 2011 (25). The Swedish authors concluded that the increased risk of breast cancer observed in the 2006–2007 period in Sweden and not in 2007–2008 was possibly due to chance. Four studies reported more recent results (26–29). A moderate increase was reported by Ruiter et al. (27) for insulin glargine compared with human insulin, despite a significantly lower risk of all malignancies for insulin glargine versus human insulin. This was not observed for overall glargine in the three other studies (26,28,29), but some subgroup analyses provided borderline significant results, including use over 5 years in a subgroup of women who had been on insulin before starting glargine (HR 2.7 [95% CI 1.1–6.5]) (26). However, there is no evidence of concordance, as these significant results for the association of insulin glargine and breast cancer were mainly observed in subgroups that differed between studies. Moreover, such a risk was not identified in the ORIGIN trial (23) with a follow-up of up to 7 years.
No significant difference was observed among incident users between insulin glargine and other basal insulins for the risk of prostate, colorectal, liver, kidney, bladder, lung, and head and neck cancers, in accordance with the great majority of previously published findings, including recent results from the General Practice Research Database (GPRD) database (19). The Taiwanese study recently reported a significant risk increase for prostate cancer (20) for insulin glargine users versus nonusers. Conversely, a significant protection for colorectal cancer by insulin glargine was reported in two recent studies (27,28). These findings were not identified in other studies and could have resulted from multiple comparisons or residual confounding factors. At this time, the U.S. Food and Drug Administration and the European Medicines Agency have not concluded that insulin glargine increases the risk of any cancer, and the review of this safety concern is still ongoing (30,31).
This cohort study was conducted at the request of the French Medicines Agency (Afssaps) in response to a pharmacovigilance signal. No associations were observed in incident users monitored up to 4 years between insulin glargine and increased or decreased risk of all malignancies, or for higher doses of insulin glargine, compared with other basal insulins. No risk increase was observed for breast cancer, specifically, or for seven other tumor sites. This study, based on data from large nationwide administrative databases, therefore confirms the nonsignificant results of most previous studies, although follow-up was limited. Further studies with a longer follow-up are needed to ensure continuous monitoring of cancer risk in patients with diabetes mellitus.
Supplementary Material
Acknowledgments
The authors are employees of the French National Health Insurance and received no funding.
No potential conflicts of interest relevant to this article were reported.
J.-P.F. was in charge of the literature search and critical analysis, wrote the manuscript, and contributed to defining the used methodology and to data interpretation. P.-O.B. was in charge of data extraction from databases and statistical analysis, contributed to defining the used methodology and to data interpretation, and wrote the results report with figures and tables. P.R., A.W., F.A., and H.A. contributed to defining the used methodology and to data interpretation. J.-P.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Saskia Van Der Erf from National Health Insurance for assistance in writing the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0506/-/DC1.
References
- 1.Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009;52:1732–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colhoun HM, SDRN Epidemiology Group Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2009;52:1755–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–1777 [DOI] [PubMed] [Google Scholar]
- 4.Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia 2009;52:1745–1754 [DOI] [PubMed] [Google Scholar]
- 5.Kurtzhals P, Schäffer L, Sørensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000;49:999–1005 [DOI] [PubMed] [Google Scholar]
- 6.Agin A, Jeandidier N, Gasser F, Grucker D, Sapin R. Glargine blood biotransformation: in vitro appraisal with human insulin immunoassay. Diabetes Metab 2007;33:205–212 [DOI] [PubMed] [Google Scholar]
- 7.Pocock SJ, Smeeth L. Insulin glargine and malignancy: an unwarranted alarm. Lancet 2009;374:511–513 [DOI] [PubMed] [Google Scholar]
- 8.Hernández-Díaz S, Adami HO. Diabetes therapy and cancer risk: causal effects and other plausible explanations. Diabetologia 2010;53:802–808 [DOI] [PubMed] [Google Scholar]
- 9.Home PD, Lagarenne P. Combined randomised controlled trial experience of malignancies in studies using insulin glargine. Diabetologia 2009;52:2499–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merlière Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique 2010;58:286–290 [DOI] [PubMed] [Google Scholar]
- 11.Païta M, Weill A. Patients with long term disease on 31 December 2008 [Internet]. Available from http://www.ameli.fr/fileadmin/user_upload/documents/27_-_ALD_2008.pdf Accessed 12 August 2011 [in French]
- 12.Website of Technical Hospitalization Information Agency (ATIH) [Internet]. Available from http://www.atih.sante.fr Accessed 12 August 2011 [in French]
- 13.Tuppin P, Neumann A, Marijon E, et al. Implantation and patient profiles for pacemakers and cardioverter-defibrillators in France (2008-2009). Arch Cardiovasc Dis 2011;104:332–342 [DOI] [PubMed] [Google Scholar]
- 14.Weill A, Païta M, Tuppin P, et al. Benfluorex and valvular heart disease: a cohort study of a million people with diabetes mellitus. Pharmacoepidemiol Drug Saf 2010;19:1256–1262 [DOI] [PubMed] [Google Scholar]
- 15.Fagot JP, Boutrelle A, Ricordeau P, Weill A, Allemand H. HPV vaccination in France: uptake, costs and issues for the National Health Insurance. Vaccine 2011;29:3610–3616 [DOI] [PubMed] [Google Scholar]
- 16.Bélot A, Velten M, Grosclaude P, et al. Estimation nationale de l’incidence et de la mortalité par cancer en France entre 1980 et 2005 [Internet]. Saint-Maurice, France, Institut de veille sanitaire, December 2008. Available at http://www.invs.sante.fr/publications/2009/estimation_cancer_1980_2005/estimation_cancer_1980_2005.pdf Accessed 12 August 2011 [in French]
- 17.Remontet L, Mitton N, Couris CM, et al. Is it possible to estimate the incidence of breast cancer from medico-administrative databases? Eur J Epidemiol 2008;23:681–688 [DOI] [PubMed] [Google Scholar]
- 18.Mitton N, Colonna M, Trombert B, et al. A suitable approach to estimate cancer incidence in area without cancer registry. J Cancer Epidemiol 2011;2011:l418968 [DOI] [PMC free article] [PubMed]
- 19.Van Staa TP, Patel D, Gallagher AM, de Bruin ML. Glucose-lowering agents and the patterns of risk for cancer: a study with the General Practice Research Database and secondary care data. Diabetologia 2012;55:654–665 [DOI] [PubMed] [Google Scholar]
- 20.Chang CH, Toh S, Lin JW, et al. Cancer risk associated with insulin glargine among adult type 2 diabetes patients—a nationwide cohort study. PLoS ONE 2011;6:e21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blin P, Lassalle R, Dureau-Pournin C, et al. Insulin glargine and risk of cancer: a cohort study in the French National Healthcare Insurance Database. Diabetologia 2012;55:644–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 1997;145:72–80 [DOI] [PubMed] [Google Scholar]
- 23.The ORIGIN Trial Investigators Basal Insulin and Cardiovascular and Other Outcomes in Dysglycemia. N Engl J Med 11 June 2012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Teng JA, Hou RL, Li DL, Yang RP, Qin J. Glargine promotes proliferation of breast adenocarcinoma cell line MCF-7 via AKT activation. Horm Metab Res 2011;43:519–523 [DOI] [PubMed] [Google Scholar]
- 25.Ljung R, Talbäck M, Haglund B, Jonasson JM, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies - a three-year population-based observation. Acta Oncol 2011;50:685–693 [DOI] [PubMed] [Google Scholar]
- 26.Suissa S, Azoulay L, Dell’Aniello S, Evans M, Vora J, Pollak M. Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia 2011;54:2254–2262 [DOI] [PubMed] [Google Scholar]
- 27.Ruiter R, Visser LE, van Herk-Sukel MP, et al. Risk of cancer in patients on insulin glargine and other insulin analogues in comparison with those on human insulin: results from a large population-based follow-up study. Diabetologia 2012;55:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morden NE, Liu SK, Smith J, Mackenzie TA, Skinner J, Korc M. Further exploration of the relationship between insulin glargine and incident cancer: a retrospective cohort study of older Medicare patients. Diabetes Care 2011;34:1965–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lind M, Fahlén M, Eliasson B, Odén A. The relationship between the exposure time of insulin glargine and risk of breast and prostate cancer: an observational study of the time-dependent effects of antidiabetic treatments in patients with diabetes. Prim Care Diabetes 2012;6:53–59 [DOI] [PubMed] [Google Scholar]
- 30.FDA Drug Safety Communication. Update to ongoing safety review of Lantus (insulin glargine) and possible risk of cancer [Internet]. Available from http://www.fda.gov/Drugs/DrugSafety/ucm239376.htm Accessed 21 October 2011
- 31.European Medicines Agency update on safety of insulin glargine—update [Internet]. Available from http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2009/11/news_detail_000066.jsp&murl=menus/news_and_events/news_and_events.jsp&mid=WC0b01ac058004d5c1 Accessed 21 October 2011
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


