Abstract
OBJECTIVE
Progranulin has recently been introduced as a novel adipokine inducing insulin resistance and obesity. In the current study, we investigated renal elimination, as well as association of the adipokine with markers of the metabolic syndrome.
RESEARCH DESIGN AND METHODS
Progranulin serum levels were quantified by enzyme-linked immunosorbent assay and correlated to anthropometric and biochemical parameters of renal function and glucose and lipid metabolism, as well as inflammation, in 532 patients with stages 1–5 of chronic kidney disease (CKD).
RESULTS
Median serum progranulin levels adjusted for age, sex, and BMI were significantly different between CKD stages with highest values detectable in stage 5 (stage 1, 58.3 µg/L; stage 2, 63.0 µg/L; stage 3, 65.4 µg/L; stage 4, 68.8 µg/L; and stage 5, 90.6 µg/L). Furthermore, CKD stage was the strongest independent predictor of circulating progranulin in our cohort. In addition, high-sensitivity interleukin-6 and adiponectin remained significantly and independently correlated with the adipokine.
CONCLUSIONS
We demonstrate that progranulin serum levels increase with deteriorating renal function. These findings are in accordance with the hypothesis that renal clearance is a major elimination route for circulating progranulin. Furthermore, the adipokine is positively and independently associated with markers of inflammation and adiponectin.
The incidence of obesity and related disorders including insulin resistance, type 2 diabetes, dyslipidemia, and hypertension has increased dramatically in recent decades. Hyperplasia and hypertrophy of adipocytes during weight gain influence the secretion pattern of adipose tissue, and altered secretion of adipocyte-derived proteins, so-called adipokines, contributes to the increased risk for metabolic and cardiovascular diseases in obesity. Thus, the adipokine leptin strongly suppresses appetite by inducing anorexigenic and inhibiting orexigenic peptides in the hypothalamus (1). When body weight increases, leptin resistance occurs leading to diminished central, appetite-suppressive effects of this adipokine despite higher levels in obesity (1). Adiponectin, another adipokine, has potent insulin-sensitizing and anti-inflammatory effects (2). In contrast, tumor necrosis factor (TNF)-α and interleukin (IL)-6 are proinflammatory adipokines that correlate with increasing body weight and induce insulin resistance (3,4).
Progranulin (also referred to as proepithelin, PC-cell–derived growth factor, acrogranin, and granulin-epithelin precursor) has most recently been introduced as a novel adipokine inducing insulin resistance. Thus, Matsubara et al. (5) identified progranulin as an adipokine induced by TNF-α and dexamethasone by differential proteome analysis in cellular models of insulin resistance. Furthermore, the authors convincingly demonstrated that progranulin knockout mice are resistant to high-fat diet–induced insulin resistance, adipocyte hypertrophy, and obesity (5). Mechanistically, progranulin deficiency blocked elevation of IL-6 in blood and adipose tissue induced by high-fat feeding (5). Most interestingly, progranulin-induced insulin resistance was suppressed by neutralizing IL-6 in vivo (5). In addition, the authors demonstrate that progranulin levels in blood and adipose tissue were markedly increased in obese mice (5). In agreement with these findings, Youn et al. (6) revealed that serum concentrations of progranulin were also elevated in obese patients, as well as patients with type 2 diabetes, compared with individuals with normal glucose tolerance. Furthermore, a study by the same group demonstrated higher progranulin levels in insulin resistant compared with insulin-sensitive individuals with morbid obesity (7). Interestingly, a recent study by Tönjes et al. (8) suggests that progranulin is associated with impaired glucose tolerance rather than impaired fasting glucose. In addition, serum levels of progranulin decreased in overweight subjects during a long-term diet intervention indicating a mechanistic link between body weight and circulating levels of this adipokine (9). Most recently, an elegant study showed that a combination of four urinary proteins including progranulin could predict early renal damage in type 1 diabetes, suggesting that the adipokine might be involved in the pathogenesis of complications of the disease (10). Moreover, serum progranulin was higher in patients with nonalcoholic fatty liver disease compared with control subjects (11). With these studies taken into consideration, progranulin appears as a novel adipokine upregulated in obesity and inducing insulin resistance.
However, no data have been published thus far concerning progranulin in relation to renal function in contrast to other adipokines including leptin and adiponectin (12,13). If renal function influenced levels of circulating progranulin, we would anticipate 1) accumulation of the adipokine with deteriorating renal function and 2) correlation of progranulin with estimated glomerular filtration rate (eGFR) independent of metabolic and inflammatory markers. To test our hypothesis, we quantified progranulin serum concentrations in 532 patients with chronic kidney disease (CKD) stages 1–5 and correlated this adipokine to clinical and biochemical markers of renal function, glucose, and lipid metabolism, as well as inflammation.
RESEARCH DESIGN AND METHODS
For this cross-sectional study, 532 patients (men, n = 305; women, n = 227) were recruited by the Department of Endocrinology and Nephrology, University of Leipzig, as well as from three outpatient Nephrology Care units (KfH Renal Unit, Division of Nephrology, Hospital St. Georg, and outpatient Nephrology Care units, Leipzig). All patients were classified into CKD stages 1–5 according to the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative guidelines (14). CKD stages were based on eGFR (milliliters per minute) assessed by the Modification of Diet in Renal Disease formula using four parameters (creatinine, age, race, and sex) (15). The following eGFR thresholds were used for the different CKD stages as previously described (14): ≥90 mL/min (stage 1), 60–89 mL/min (stage 2), 30–59 mL/min (stage 3), 15–29 mL/min (stage 4), and <15 mL/min or hemodialysis (stage 5). BMI was determined as weight divided by the square of height in meters. Waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) were calculated after waist and hip circumference, as well as height, were assessed. Age of the study population ranged from 19 to 91 years and BMI from 14.3 to 49.0 kg/m2. Homeostasis model assessment of insulin resistance (HOMA-IR), as well as triglycerides and glucose (TyG) index [ln (fasting glucose in mmol/L × 18.015/2 × triglycerides in mmol/L × 87.719)] and McAuley index {exp [2.63–0.28 ln (fasting insulin in pmol/L × 0.1386) − 0.31 ln (triglycerides in mmol/L)]}, was calculated as previously described (16–18). The following inclusion and exclusion criteria were applied: inclusion criteria, age >18 years, nonpregnant, and provided written informed consent; exclusion criteria, end-stage malignant diseases, acute generalized inflammation, acute infectious disease, and history of drug abuse. The study was approved by the local ethics committee.
Assays
Blood samples were taken after an overnight fast. In hemodialysis patients, blood was obtained just before hemodialysis started. Progranulin (R&D Systems, Minneapolis, MN), high-sensitivity IL-6 (hsIL-6) (R&D Systems, Minneapolis, MN), leptin (Mediagnost, Reutlingen, Germany), and adiponectin (Mediagnost) were determined with enzyme-linked immunosorbent assays according to the manufacturers’ instructions. Serum creatinine; fasting glucose; fasting insulin; triglycerides; total, HDL, and LDL cholesterol, and high-sensitivity C-reactive protein (hsCRP) were measured in a certified laboratory by standard methods.
Statistical analysis
SPSS software, version 18.0 (IBM, Armonk, NY), was used for all statistical analyses. Overall group differences for continuous parameters were assessed by Kruskal-Wallis test followed by post hoc analysis with prior adjustment for age, sex, and BMI. Categorical parameters were analyzed using the χ2 test. Univariate correlations were assessed by Spearman rank correlation method followed by Bonferroni adjustment for multiple testing. Furthermore, 95% CIs were determined using Analyze-it 2.26 (Leeds, U.K.). For adjustment for the effects of covariates and identification of independent relationships, multivariate linear regression analyses were performed. Before multivariate analysis, distribution of continuous variables was tested for normality using Shapiro-Wilk test and nonnormally distributed parameters were logarithmically transformed. A P value of <0.05 was considered statistically significant in all analyses.
RESULTS
Progranulin serum levels increase with deteriorating renal function
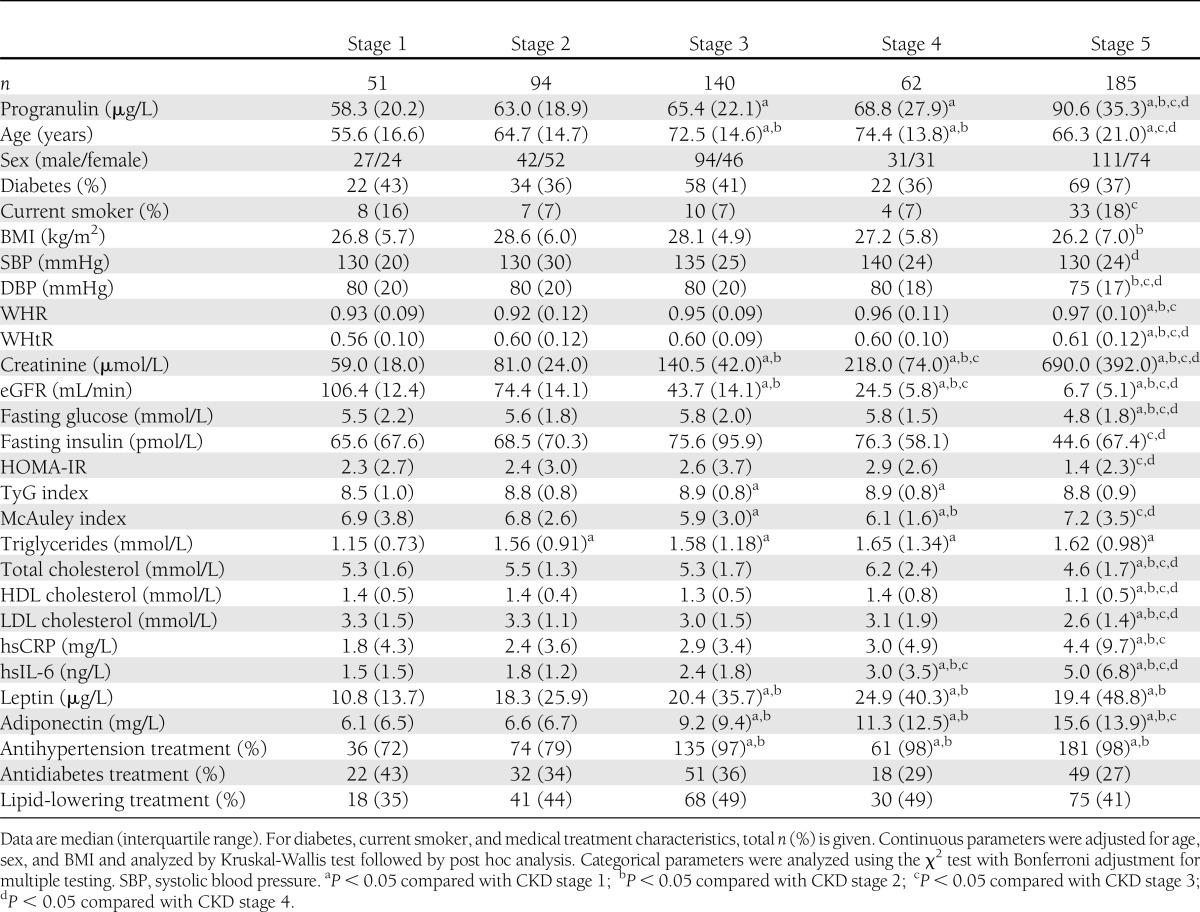
Table 1 summarizes clinical characteristics of the five subgroups studied (CKD 1–5), and all continuous variables are given as median (interquartile range). Serum progranulin levels were 70.8 µg/L (interquartile range 27.8) in the total sample. Circulating progranulin was significantly different between the five subgroups even after adjustment for age, sex, and BMI (P < 0.001) (Table 1). Furthermore, progranulin serum levels increased with deteriorating renal function, with highest levels seen in stage 5 of CKD (Table 1). In contrast,a significant difference in progranulin concentrations could not be demonstrated depending on sex (female 69.9 µg/L [interquartile range 25.4]; male 71.8 µg/L [31.5]), type 2 diabetes (type 2 diabetic patients 71.6 µg/L [29.8]; nondiabetic subjects 70.4 µg/L [27.0]), or current smoking (smoker µg/L 75.4 [35.7]; nonsmoker µg/L 70.5 [26.9]).
Table 1.
Baseline characteristics of the study population divided into five stages of CKD
Univariate correlations
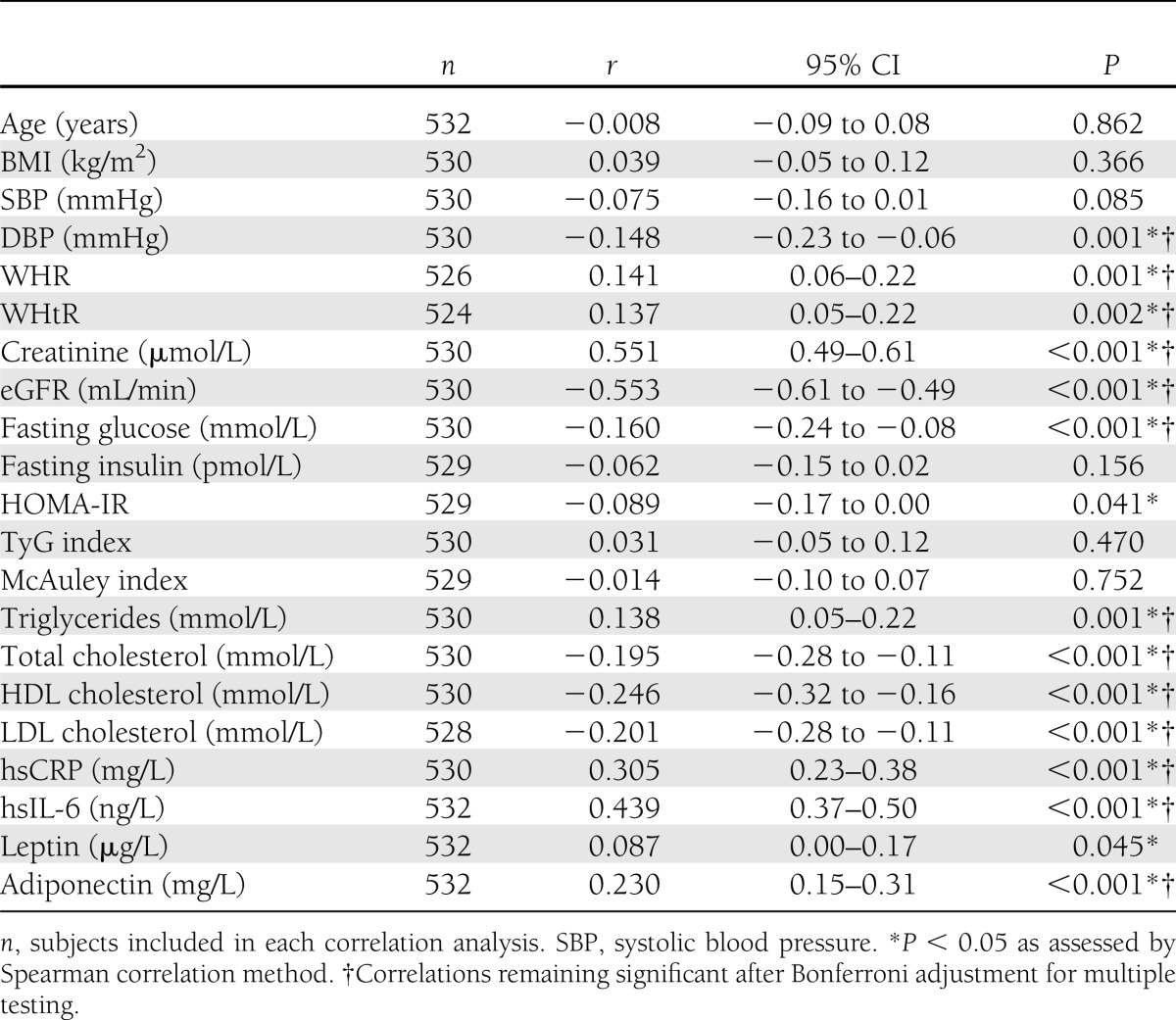
Serum progranulin concentrations in all individuals positively and significantly correlated with WHR, WHtR, serum creatinine, triglycerides, hsCRP, hsIL-6, and adiponectin (Table 2). In addition, progranulin was negatively and significantly associated with diastolic blood pressure (DBP), eGFR, fasting glucose, total cholesterol, HDL cholesterol, and LDL cholesterol (Table 2). Furthermore, in a subset of 351 individuals, circulating progranulin was not significantly associated with fat mass, body fat percentage, lean body mass, or basic metabolic rate assessed by bioelectrical impedance analysis (data not shown).
Table 2.
Univariate correlations with serum progranulin in the study population
Multivariate regression analysis
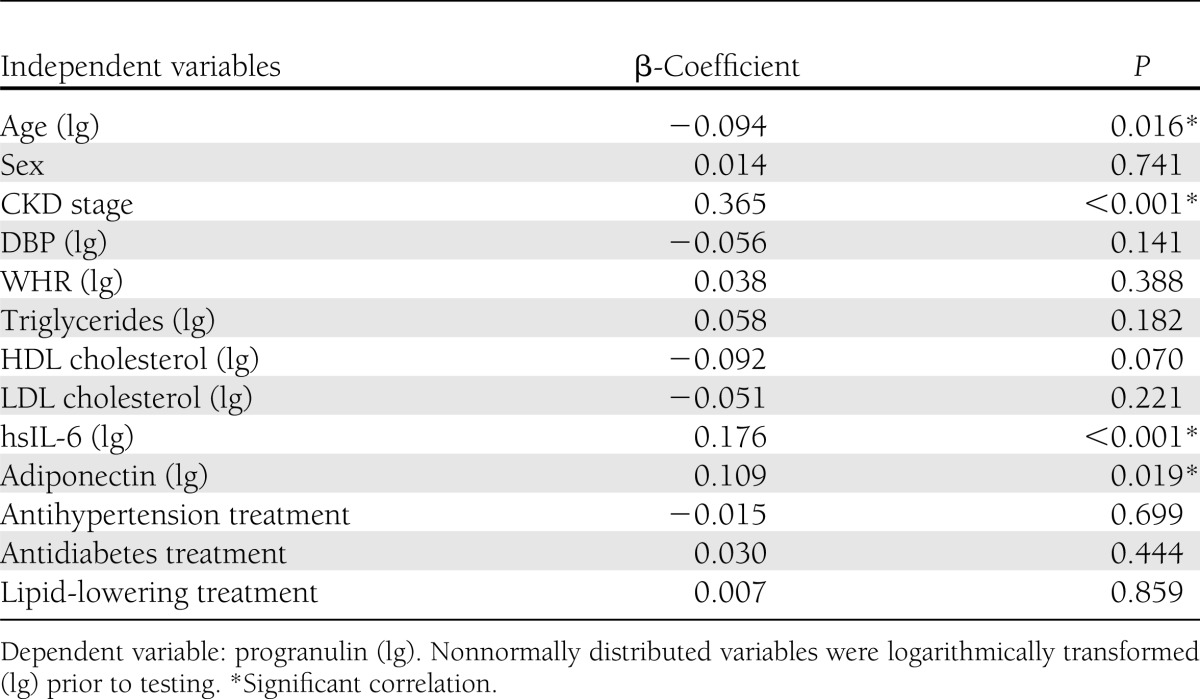
Multiple linear regression analysis revealed that renal function assessed as CKD stage remained independently associated with circulating progranulin levels after adjustment for age, sex, DBP, WHR, triglycerides, HDL cholesterol, LDL cholesterol, hsIL-6, and adiponectin, as well as antihypertension, antidiabetes, and lipid-lowering treatment (Table 3). Similar results were obtained when eGFR instead of CKD stage was included in the model (data not shown). Besides CKD stage, age, hsIL-6, and adiponectin remained independent predictors of progranulin serum levels in this analysis (Table 3). Of note, all variables remaining significant in multivariate analysis except age; i.e., eGFR, hsIL-6, and adiponectin, were significantly associated with progranulin serum concentrations in both female and male subjects in univariate analysis (data not shown). Sex, DBP, WHR, triglycerides, HDL cholesterol, and LDL cholesterol, as well as antihypertension, antidiabetes, and lipid-lowering treatment, were not significantly associated with progranulin serum concentrations in multivariate analysis (Table 3). Furthermore, HOMA-IR, TyG index, and McAuley index were not independent predictors of progranulin when included in multivariate analysis instead of adiponectin (data not shown).
Table 3.
Correlation between progranulin (dependent variable) and CKD stage adjusted for age and sex, as well as DBP, WHR, triglycerides, HDL cholesterol, LDL cholesterol, hsIL-6, adiponectin, antihypertension treatment, antidiabetes treatment, and lipid-lowering treatment (total n = 516)
CONCLUSIONS
In the current study, we show for the first time that renal function assessed as eGFR is a strong, independent predictor of circulating levels of the adipokine progranulin. Furthermore, progranulin serum concentrations significantly increase with deteriorating renal function assessed as CKD stage independent of age, sex, and BMI. It is interesting to note in this context that various studies have reported an association between renal function and multiple adipokines. Thus, circulating levels of the adipokines leptin (12), adiponectin (13), retinol-binding protein-4 (19), zinc-α2-glycoprotein (20), adipocyte fatty acid–binding protein (21), fibroblast growth factor 21 (22), and chemerin (23) are significantly elevated in end-stage renal disease. With these results taken into consideration, it seems that the association between adipokines and renal function is not restricted to progranulin. Furthermore, our findings are in accordance with the hypothesis that impaired renal clearance function might account for this association.
Interestingly, progranulin can be found in significant amounts in spot urine samples (data not shown). However, urinary progranulin is not significantly correlated with serum progranulin in a subgroup (n = 145) of our study population (r = 0.048; P = 0.564). These findings are in agreement with the hypothesis that progranulin is at least partly degraded during renal elimination. Besides renal elimination, hepatic clearance of adipokines including adiponectin (24) has been suggested and needs to be further assessed for progranulin in future studies. Since median serum progranulin levels are not significantly different before and immediately after hemodialysis in a subcohort (n = 30) of our CKD stage 5 patients (data not shown), the adipokine does not appear to be dialyzable.
The physiological relevance of increased progranulin levels in renal disease remains to be established. It is interesting to note in the context of our study that Matsubara et al. (5) present evidence that progranulin induces proinflammatory IL-6 in adipose tissue. In agreement with these results, progranulin correlates independently and positively with circulating IL-6 in our cohort. Furthermore, inflammation is one of the key factors promoting vascular disease when renal function deteriorates (25). Taking these results into consideration, it is tempting to speculate that progranulin might directly contribute to the proinflammatory state frequently seen in renal dysfunction. Clearly, more work is needed to elucidate the role of this adipokine in renal disease.
Various studies demonstrate a positive association between circulating progranulin and components of the metabolic syndrome including insulin resistance, obesity, and dyslipidemia (6–9,11). In agreement with these findings, progranulin positively correlates with anthropometric markers of adverse fat distribution (WHR and WHtR), as well as triglycerides, and is negatively associated with HDL cholesterol in univariate analysis in our cohort. However, these associations are all lost after controlling for renal function. Based on the results of our study, serum creatinine or eGFR should always be included in future studies concerning progranulin physiology. It is interesting to note in this context that renal function was not assessed in recent papers on progranulin regulation in the metabolic syndrome in humans (6–9,11). Furthermore, progranulin is positively associated with circulating levels of the insulin-sensitizing and anti-inflammatory adipokine adiponectin in our cohort independent of renal function. It needs to be established in future studies whether increased circulating adiponectin might be a compensatory response to limit proinflammatory effects of progranulin.
It is counterintuitive that HOMA-IR is reduced in patients with end-stage renal disease despite higher hsCRP levels. However, improvements in insulin sensitivity after initiation of hemodialysis have been reported by different groups (26,27). It is interesting to note in this context that CKD stage 5 patients in our cohort not on chronic hemodialysis tend to have higher HOMA-IR values compared with subjects on hemodialysis (1.76 vs. 1.22). However, this difference does not reach statistical significance as assessed by Mann-Whitney U test (P = 0.140).
To investigate effects of common genetic variation in the granulin (GRN) gene on levels of progranulin, seven HapMap tagging single nucleotide polymorphisms covering the common genetic variation within GRN have been genotyped in a subset of 412 of 532 patients of our study population. Although one of the single nucleotide polymorphisms (rs4792939) shows nominal association with circulating progranulin, this association would not withstand a correction for multiple testing (e.g., Bonferroni correction; data not shown). Furthermore, rs4792939 does not remain a significant predictor of progranulin in multivariate analysis (data not shown). Therefore, genetic variation within GRN does not seem to have a major impact on circulating progranulin.
There are some limitations of the study that need to be emphasized: First, the study has a cross-sectional design, and, therefore, causality cannot be established. Second, the sample size is rather small, and it is quite possible that various nonsignificant associations in multivariate analyses would have become statistically significant if larger samples were studied.
Taken together, we describe for the first time that renal function is a strong independent predictor of serum levels of the adipokine progranulin. Our results are compatible with the hypothesis that renal filtration is an important route of progranulin elimination and markers of renal function should be included in studies on progranulin physiology. Prospective studies are needed to better elucidate the role of progranulin in deteriorating renal function, as well as metabolic and cardiovascular disease.
Supplementary Material
Acknowledgments
This study was supported by grants to M.F. from the Deutsche Forschungsgemeinschaft (DFG, KFO 152 “Atherobesity”, FA476/4-1); the Federal Ministry of Education and Research (BMBF), Germany, FKZ 01EO1001 (IFB Adiposity Diseases, projects K7-3, K7-9, and K7-31); and the Deutsche Hochdruckliga e.V. Furthermore, T.E. was supported by a junior research grant from the Medical Faculty, University of Leipzig, and BMBF, Germany, FKZ 01EO1001 (IFB AdiposityDiseases, MetaRot program).
No potential conflicts of interest relevant to this article were reported.
J.R., D.F., and T.E. wrote the manuscript and researched data. P.K. and A.B. researched data and reviewed and edited the manuscript. U.L. researched data. S.K. reviewed and edited the manuscript. J.K., J.B., M.A., and I.B. researched data and reviewed and edited the manuscript. M.B. and M.S. contributed to the discussion and reviewed and edited the manuscript. M.F. wrote the manuscript and researched data. M.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Münzberg H, Björnholm M, Bates SH, Myers MG., Jr Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci 2005;62:642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fasshauer M, Paschke R, Stumvoll M. Adiponectin, obesity, and cardiovascular disease. Biochimie 2004;86:779–784 [DOI] [PubMed] [Google Scholar]
- 3.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001;280:E745–E751 [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995;95:2409–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsubara T, Mita A, Minami K, et al. PGRN is a key adipokine mediating high fat diet-induced insulin resistance and obesity through IL-6 in adipose tissue. Cell Metab 2012;15:38–50 [DOI] [PubMed] [Google Scholar]
- 6.Youn B-S, Bang S-I, Klöting N, et al. Serum progranulin concentrations may be associated with macrophage infiltration into omental adipose tissue. Diabetes 2009;58:627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klöting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 2010;299:E506–E515 [DOI] [PubMed] [Google Scholar]
- 8.Tönjes A, Fasshauer M, Kratzsch J, Stumvoll M, Blüher M. Adipokine pattern in subjects with impaired fasting glucose and impaired glucose tolerance in comparison to normal glucose tolerance and diabetes. PLoS ONE 2010;5:e13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blüher M, Rudich A, Klöting N, et al. Two patterns of adipokine and other biomarker dynamics in a long-term weight loss intervention. Diabetes Care 2012;35:342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlatzer D, Maahs DM, Chance MR, et al. Novel urinary protein biomarkers predicting the development of microalbuminuria and renal function decline in type 1 diabetes. Diabetes Care 2012;35:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yilmaz Y, Eren F, Yonal O, et al. Serum progranulin as an independent marker of liver fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease. Dis Markers 2011;31:205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merabet E, Dagogo-Jack S, Coyne DW, et al. Increased plasma leptin concentration in end-stage renal disease. J Clin Endocrinol Metab 1997;82:847–850 [DOI] [PubMed] [Google Scholar]
- 13.Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol 2002;13:134–141 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–147 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 17.McAuley KA, Williams SM, Mann JI, et al. Diagnosing insulin resistance in the general population. Diabetes Care 2001;24:460–464 [DOI] [PubMed] [Google Scholar]
- 18.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 2010;95:3347–3351 [DOI] [PubMed] [Google Scholar]
- 19.Ziegelmeier M, Bachmann A, Seeger J, et al. Serum levels of adipokine retinol-binding protein-4 in relation to renal function. Diabetes Care 2007;30:2588–2592 [DOI] [PubMed] [Google Scholar]
- 20.Philipp A, Kralisch S, Bachmann A, et al. Serum levels of the adipokine zinc-α2-glycoprotein are increased in chronic hemodialysis. Metabolism 2011;60:669–672 [DOI] [PubMed] [Google Scholar]
- 21.Sommer G, Ziegelmeier M, Bachmann A, et al. Serum levels of adipocyte fatty acid-binding protein (AFABP) are increased in chronic haemodialysis (CD). Clin Endocrinol (Oxf) 2008;69:901–905 [DOI] [PubMed] [Google Scholar]
- 22.Stein S, Bachmann A, Lössner U, et al. Serum levels of the adipokine FGF21 depend on renal function. Diabetes Care 2009;32:126–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfau D, Bachmann A, Lössner U, et al. Serum levels of the adipokine chemerin in relation to renal function. Diabetes Care 2010;33:171–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tacke F, Wüstefeld T, Horn R, et al. High adiponectin in chronic liver disease and cholestasis suggests biliary route of adiponectin excretion in vivo. J Hepatol 2005;42:666–673 [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int 1999;55:648–658 [DOI] [PubMed] [Google Scholar]
- 26.DeFronzo RA, Tobin JD, Rowe JW, Andres R. Glucose intolerance in uremia. Quantification of pancreatic beta cell sensitivity to glucose and tissue sensitivity to insulin. J Clin Invest 1978;62:425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi S, Maejima S, Ikeda T, Nagase M. Impact of dialysis therapy on insulin resistance in end-stage renal disease: comparison of haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 2000;15:65–70 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


