Abstract
OBJECTIVE
We sought to assess the association between maternal gestational physical activity and insulin action and body composition in early infancy.
RESEARCH DESIGN AND METHODS
At 28–32 weeks' gestation, pregnant women participating in an observational study in Sweden underwent assessments of height, weight, and body composition, an oral glucose tolerance test, and 10 days of objective physical activity assessment. Thirty mothers and infants returned at 11–19 weeks postpartum. Infants underwent assessments of weight, length, and body composition.
RESULTS
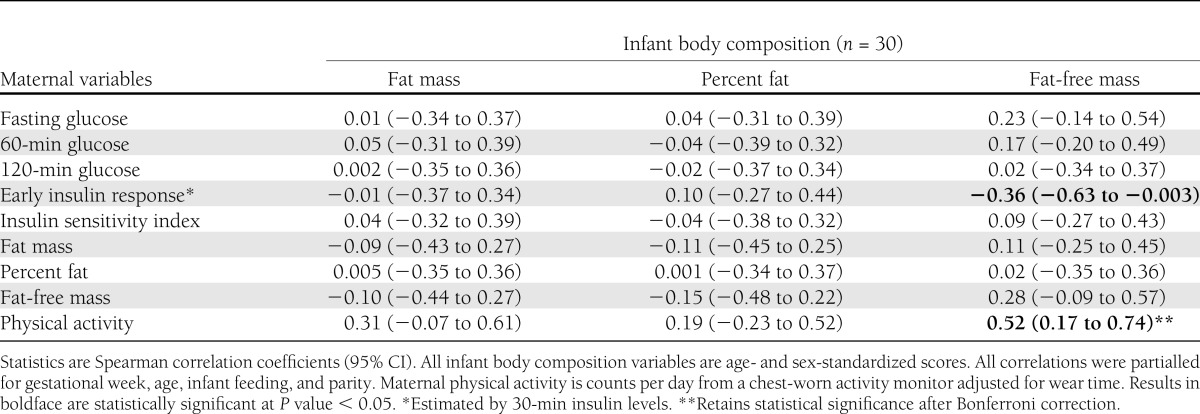
Early insulin response was correlated with total physical activity (r = −0.47; P = 0.007). Early insulin response (r = −0.36; P = 0.045) and total physical activity (r = 0.52; P = 0.037) were also correlated with infant fat-free mass. No maternal variable was significantly correlated with infant adiposity.
CONCLUSIONS
The relationships between maternal physical activity, insulin response, and infant fat-free mass suggest that physical activity during pregnancy may affect metabolic outcomes in the mother and her offspring.
Understanding the mechanisms by which intrauterine and early-life factors impact later type 2 diabetes risk may highlight intervention targets in pregnancy and infancy. Lifestyle modification facilitates short-term weight loss and lowers diabetes incidence in nonpregnant adults (1), but little is known about the effects of lifestyle in pregnancy on infant body composition. The primary purpose of this study was to examine how intrauterine factors influence body composition in early infancy.
RESEARCH DESIGN AND METHODS
Our primary aim was to examine the relationships between maternal measures of insulin and glucose during pregnancy and infant body composition. We further sought to establish whether maternal physical activity level mediates this relationship by examining the relationship of maternal physical activity in pregnancy with 1) gestational glucose and insulin levels and 2) the baby’s body composition at 4 months postpartum.
Thirty-five pregnant women from northern Sweden were enrolled in the study. Gestational data were collected at the Clinical Research Center (CRC), Umeå University Hospital, between 28 and 32 weeks' gestation. Three women withdrew from the study before giving birth. The remaining 32 women had successful pregnancies, one of which resulted in dizygotic twins. Eighteen boys and 15 girls were born. The mother and her dizygotic twins were excluded from this analysis. Mothers were asked to return with their babies to the CRC 11–19 weeks postpartum. One woman withdrew from the study at this stage. The remaining 30 women and their 30 infants completed the follow-up stage of the study. All women provided written informed consent for all aspects of the study protocol and provided written assent for their infants. The study was approved by the Regional Ethical Review Board in Umeå, Sweden.
Pregnant women first attended the CRC the morning after a 10-h overnight fast at 28–32 gestational weeks. After the collection of an antecubital venous blood sample, a 75-g oral glucose load was administered; additional blood samples were drawn into different storage media (EDTA-plasma and serum) 30, 60, and 120 min later and immediately placed on ice, processed, and sent to the hospital clinical biochemistry laboratory for analysis. The insulin sensitivity index was calculated from the oral glucose tolerance test using the Matsuda composite model, which has been shown to be strongly correlated with hyperinsulinemic-euglycemic clamp–measured insulin sensitivity during pregnancy (2). The 30-min insulin level (3) was used to estimate early insulin response.
Height was measured to the nearest 0.5 cm using a calibrated wall-mounted stadiometer and weight to the nearest 0.1 kg using a calibrated digital scale with participants in light clothing and no shoes. Body composition (fat and fat-free mass) was estimated using the doubly labeled water isotope dilution method (4). Total body water was calculated as the average of the linearly regressed isotope dilution spaces at time 0, correcting by 1.01 and 1.04, respectively, to account for the exchange of isotopes with nonaqueous components within the body. Fat-free mass was calculated by dividing by the hydration factor of 0.747, with the difference between body weight and lean tissue equating to fat mass (5).
Physical activity at 28–32 weeks of gestation was measured using a combined heart rate–movement sensor (Actiheart; CNT Ltd., Papworth, U.K.) for 10 days as described previously (6). Total accelerometry counts adjusted for wear time were used as an index of total physical activity volume.
At 11–19 weeks postpartum, mothers and their infants attended the CRC for a follow-up visit. Infant weight was measured using a calibrated digital scale. Infant length was assessed from the top of the head to the heel of the foot to the nearest 0.1 cm using a measuring board (CMS Weighing Equipment Ltd., London, U.K.) with the infant lying supine and legs held outstretched. Fat mass and fat-free mass were assessed (without clothing or diaper) using air displacement plethysmography (PeaPod; Life Measurement Inc., Concord, CA). Body composition in the infants is expressed as percent fat, as well as fat mass and fat-free mass standardized by age and sex.
A questionnaire was administered from which detailed information on the infant’s feeding habits up until the follow-up visit at 4 months of age was obtained.
Statistical analyses were performed using the SAS software (V9.2; SAS Institute, Cary, NC). Bivariate relationships between maternal characteristics and infant body composition were evaluated using Spearman partial correlations adjusted for relevant covariables. Bonferroni and false discovery rate (FDR) corrections were made for each predefined set of hypothesis tests.
RESULTS
The study included healthy women 20–35 years of age at baseline, with first trimester BMIs ranging from 18 to 46 kg/m2. No one was classified as having impaired glucose tolerance or diabetes by World Health Organization criteria (7).
Total physical activity (counts per day at 28–32 gestational weeks) was inversely related to insulin area under the curve (r = −0.41; P = 0.027) and, as previously reported (6), early insulin response (r = −0.47; P = 0.007).
Maternal early insulin response (estimated by 30-min insulin) was significantly negatively related to infant fat-free mass (Table 1). No other measure of insulinemia or glycemia was related to infant body composition (P > 0.05). Maternal total physical activity was significantly positively correlated with infant fat-free mass, but not with infant fat mass, which was statistically significant after Bonferroni and FDR correction.
Table 1.
Relationships between maternal physical activity and metabolic characteristics and infant body composition 4 months postpartum
CONCLUSIONS
The offspring of diabetic mothers are prone to obesity in childhood (8) and diabetes later in life (9), but little is known about the underlying biological mechanisms. Here, we show that a woman’s early insulin response to glucose challenge at 28–32 weeks' gestation may be inversely related to her offspring’s early infancy fat-free mass. This relationship appears to be mediated by gestational physical activity levels in the mother, thus highlighting a logical target for intervention in pregnancy. The relationship between objectively measured physical activity in pregnancy and infant body composition is a novel observation to our knowledge. However, although observational data are useful for estimating the effects of lifestyle exposures on health-related traits, inferring causality from these data is difficult, owing to the potential for confounding and reverse causality.
Our study used state-of-the art methods to assess physical activity and body composition. However, such measures are costly and difficult to perform, which limited our sample size. This limitation is important, as it impacts the extent to which we can be confident that a finding that we declared as not statistically significant is truly negative. Bonferroni correction is overly conservative when hypothesis tests are correlated, and there is strong a priori evidence supporting the hypothesis tests, as is the case here. Existing evidence from epidemiological studies raises the prior probability that associations exist between the variables we examined here, hence mitigating the extent to which our findings are false positive. However, when conservative correction for multiple testing was used (FDR and Bonferroni correction), the only finding that remained statistically significant was the association between the mothers’ gestational physical activity levels and the infants’ fat-free mass. Nevertheless, our study was adequately powered to test the primary hypotheses, in part because we used methods that are both precise and accurate.
Our findings suggest that a pregnant woman’s physical activity level is an important determinant of her insulin response to glucose challenge in pregnancy and that this in turn impacts fetal growth. These data elucidate prior observations that the offspring of glucose-intolerant women tend to be large for gestational age at birth and are prone to obesity during childhood.
Acknowledgments
This study was a preparatory project for the LifeGene Study (www.lifegene.se) and was funded by the Torsten and Ragnar Söderberg foundations, Fredrik and Ingrid Thurings Foundation, and Västerbotten regional health authority (all grants to P.W.F.). The stable isotope measurements were conducted under the Medical Research Council Unit Program U1059. J.P. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program. S.E.K. was supported in part by the U.S. Department of Veterans Affairs.
No potential conflicts of interest relevant to this article were reported.
J.P., F.R., and P.W.F. researched data and wrote the manuscript. A.M.G., I.M., M.P., L.B., and A.W. researched data and reviewed and edited the manuscript. S.E.K. and M.D. contributed to the discussion and reviewed and edited the manuscript. P.W.F. and J.P. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors are grateful to the study participants and thank Monica Holmgren, other staff at the Clinical Research Center (Umeå University Hospital), and Carina Forslund (Umeå University Hospital) for skillful assistance in data collection.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catalano PM, Kirwan JP. Clinical utility and approaches for estimating insulin sensitivity in pregnancy. Semin Perinatol 2002;26:181–189 [DOI] [PubMed] [Google Scholar]
- 3.Hanson RL, Pratley RE, Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol 2000;151:190–198 [DOI] [PubMed] [Google Scholar]
- 4.Schoeller DA, van Santen E, Peterson DW, Dietz W, Jaspan J, Klein PD. Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr 1980;33:2686–2693 [DOI] [PubMed] [Google Scholar]
- 5.Lof M, Forsum E. Hydration of fat-free mass in healthy women with special reference to the effect of pregnancy. Am J Clin Nutr 2004;80:960–965 [DOI] [PubMed] [Google Scholar]
- 6.Gradmark A, Pomeroy J, Renström F, et al. Physical activity, sedentary behaviors, and estimated insulin sensitivity and secretion in pregnant and non-pregnant women. BMC Pregnancy Childbirth 2011;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Definitions, Diagnosis and Classification of Diabetes Mellitus and its Complications: Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Organization, 1999 [Google Scholar]
- 8.Pettitt DJ, Knowler WC, Bennett PH, Aleck KA, Baird HR. Obesity in offspring of diabetic Pima Indian women despite normal birth weight. Diabetes Care 1987;10:76–80 [DOI] [PubMed] [Google Scholar]
- 9.Franks PW, Looker HC, Kobes S, et al. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes 2006;55:460–465 [DOI] [PubMed] [Google Scholar]


