Abstract
OBJECTIVE
Although African American adolescents have the highest prevalence of obesity, they have the lowest prevalence of metabolic syndrome across all definitions used in previous research. To address this paradox, we sought to develop a model of the metabolic syndrome specific to African American adolescents.
RESEARCH DESIGN AND METHODS
Data from the National Health and Nutrition Examination Survey (2003–2010) of 822 nonpregnant, nondiabetic, African American adolescents (45% girls; aged 12 to 17 years) who underwent physical examinations and fasted at least 8 h were analyzed. We conducted a confirmatory factor analysis to model metabolic syndrome and then used latent profile analysis to identify metabolic syndrome risk groups among African American adolescents. We compared the risk groups on probability of prediabetes.
RESULTS
The best-fitting metabolic syndrome model consisted of waist circumference, fasting insulin, HDL, and systolic blood pressure. We identified three metabolic syndrome risk groups: low, moderate, and high risk (19% boys; 16% girls). Thirty-five percent of both boys and girls in the high-risk groups had prediabetes, a significantly higher prevalence compared with boys and girls in the low-risk groups. Among adolescents with BMI higher than the 85th percentile, 48 and 36% of boys and girls, respectively, were in the high-risk group.
CONCLUSIONS
Our findings provide a plausible model of the metabolic syndrome specific to African American adolescents. Based on this model, approximately 19 and 16% of African American boys and girls, respectively, are at high risk for having the metabolic syndrome.
The growing prevalence of obesity among American children and adolescents has raised concerns about metabolic consequences (1). There is evidence that childhood obesity increases risk for adult obesity, type 2 diabetes, hypertension, and dyslipidemia (2,3). To date, no standard definition of metabolic syndrome for children and adolescents has been created. According to an American Heart Association scientific statement (4), there have been approximately 15 different definitions of metabolic syndrome for children and adolescents used in previous research based on modifications of the adult definitions specified by the National Cholesterol Education Program—Adult Treatment Panel III, the International Diabetes Federation, and the World Health Organization. Because of the different definitions, the prevalence of metabolic syndrome among children and adolescents is not clear. In fact, the estimates from the National Health and Nutrition Examination Survey (NHANES) data range from 4.2 to 9.2% in 1988–1994 (5,6) and 2 to 9.4% in 1999–2002 (7).
Regardless of the definition, African American adolescents are consistently less likely to have metabolic syndrome compared with their non-Hispanic white counterparts (5,6,8,9), even after controlling for socioeconomic status and lifestyle (10). In contrast, African American adolescents have higher rates of obesity (1,11), insulin resistance (12), and elevated blood pressure (13) and a greater likelihood of diagnosis of type 2 diabetes and cardiovascular disease (CVD) in adulthood (14). This phenomenon, referred to as the “metabolic syndrome paradox,” suggests that the current metabolic thresholds or criteria for metabolic syndrome may not be appropriate for detecting risks for CVD and type 2 diabetes among African Americans (15–18). Thus, further examination of the metabolic syndrome within racial/ethnic groups is warranted.
The current study had three main objectives: 1) to develop a model of metabolic syndrome with construct validity and goodness of fit that is specific to African American adolescents; 2) to identify the existence of distinct metabolic syndrome risk groups among African American adolescents; and 3) to compare the prevalence of prediabetes between metabolic syndrome risk groups.
RESEARCH DESIGN AND METHODS
We used data from the cross-sectional NHANES 2003–2010 surveys. NHANES used a multistage, stratified, complex survey design, weighted to represent the population of noninstitutionalized U.S. civilians aged 2 months and older at the time the data were collected (19). Data were collected for a total of 5,403 individuals between the ages of 12 and 17 years during NHANES survey years 2003–2010, and approximately 1,679 (31%) of these adolescents identified as African American. For this study, our exclusion criteria were minimal: pregnancy, diagnosis of diabetes, and fasting for less than 8 h. Thus, this study included 822 (49%) nonpregnant, nondiabetic, African American adolescents aged 12–17 years who underwent physical examinations and fasted for ≥8 hours.
Detailed descriptions of the NHANES protocol and assessment are described elsewhere (20). Blood sample collection and physical examinations were performed at the same time during each participant’s visit to a mobile examination center. In brief, blood specimens were frozen and stored at −70°C until analysis. Plasma glucose concentration was determined using an enzyme hexokinase method. A1C was measured in whole blood samples using Primus CLC330 in the 2003–2004 survey year, the A1c 2.2 Plus Glycohemoglobin Analyzer during 2005 to May 2007, and the Tosoh G7 HPLC Glycohemoglobin Analyzer from June 2007–2010. Laboratory method cross-over studies were conducted at the time of each laboratory instrument change. Fasting serum insulin was measured with a two-site immunoenzymometric assay. An oral glucose tolerance test (OGTT) was performed on adolescents starting in 2005. After the fasting plasma glucose sample was drawn, participants were asked to drink 75 g of glucose (Trutol), and 2 h later a second plasma glucose sample was drawn and tested for postload glucose levels. We used all available OGTT data from survey years 2005–2010 in the analyses.
Serum HDL cholesterol and triglycerides (using a series of coupled reactions) were measured using a Hitachi Analyzer. We used all available HDL and triglyceride data from survey years 2003–2010 in the current analyses. Mean blood pressure was calculated as the average of the second and third readings for those who had at least three readings, the average of two readings for those who only had two readings, and the only reading for those who had one reading. Standing height (in centimeters) was measured with a stadiometer. Weight (in kilograms) was measured in a standing position using a self-zeroing scale. Participant BMI (kg/m2), BMI percentile, and BMI z scores (age and sex specific) were calculated using the Center for Disease Control and Prevention’s Statistical Analysis System (SAS) syntax (21) based on the 2000 Center for Disease Control and Prevention’s growth charts. Waist circumference was measured during minimal respiration, to the nearest 0.1 cm at the midpoint between the bottom of the rib cage and the top of the iliac crest.
Statistical analysis
We ran descriptive statistics in SAS version 9.2 (SAS Institute, Inc., Cary, NC). Because they were not normally distributed, we log-transformed fasting triglyceride and fasting insulin values. Previous studies have examined the phenotype and stability of metabolic syndrome in adolescents using factor analysis (22–24). In this study, we also used confirmatory factor analysis (CFA) to test the goodness of fit of several hypothesized models to the data for African American adolescents. Similar to Goodman et al. (24), we evaluated the fit of a one-factor model with eight CVD risk factors—waist circumference, BMI z score, average systolic blood pressure, average diastolic blood pressure, fasting triglycerides, fasting HDL, fasting glucose, and fasting insulin—as indicators of metabolic syndrome. The CFAs were performed in Mplus software (version 6.11) (25). Each CFA model was tested in the overall sample and only in adolescents with BMI higher than the 85th percentile (overweight/obese subsample). Factor loadings were examined to determine the strength of the relationship between the CVD risk factors and the underlying factor (i.e., metabolic syndrome). To be consistent with previous studies (26), we defined factor loadings ranging from (±)0.2 to 0.4 as marginally correlated indicators and ±0.4 or greater as strongly correlated indicators with the factor. Full-information maximum likelihood was applied to handle missing data. We determined goodness of fit of the hypothesized models to the data by several fit indices, including a nonsignificant χ2 (P > 0.05) (27), comparative fit index (CFI) ≥0.90 (28), root mean square error approximation (RMSEA) ≤0.08 (29), and the standardized root mean square residual (SRMR) <0.10 (27).
Because we used CFA to model metabolic syndrome in African American adolescents, the CVD risk factor measures as well as the underlying factor are treated as continuous variables (23). Therefore, we needed to identify where individuals are on the continuum of metabolic syndrome (e.g., at high risk, low risk, or moderate risk for having metabolic syndrome) on the basis of the adolescent’s metabolic profile. To do this, we applied latent profile analysis (LPA) (30). Using LPA, individuals are grouped together based on having similar values across the CVD risk factors used to indicate metabolic syndrome. With LPA we are able to identify distinct groups of individuals on the metabolic syndrome continuum that may be characterized by level of risk for metabolic syndrome based on the mean of each CVD risk factor within the group. Using Mplus version 6.11, we ran several LPA models with the total sample as well as with a subsample of only the adolescents with BMI higher than the 85th percentile to see how many distinct groups emerged from the data. We ran the models separately for African American boys and girls. Fit indices were used to compare the fit of the models and aid in the selection of the optimal model for the data. Specifically, better model fit was suggested by a lower Akaike information criterion (31); a lower Bayesian information criterion (32); a significant Lo-Mendell-Rubin likelihood ratio test, suggesting the more complex model (i.e., model with more groups) fits the data better than the model with fewer groups (33); and entropy, the estimate of certainty of classification (ranging from 0 to 1). After model selection, groups were compared by mean age. Also, we estimated the probability of prediabetes and compared this value between each group. We defined prediabetes as impaired fasting glucose (fasting glucose ≥100 mg/dL), impaired glucose tolerance (2-h OGTT ≥140 mg/dL), A1C ≥5.7%, or any combination of the three (34).
RESULTS
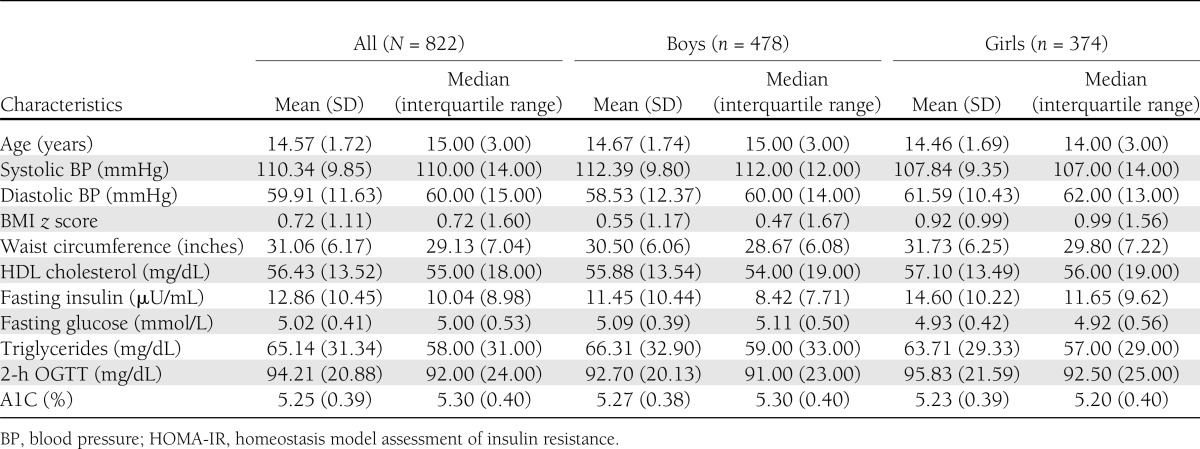
Table 1 presents the sample characteristics of the 822 African American adolescents (45% girls) from NHANES 2003–2010 who were included in the analyses. Forty percent (n = 325; 47% girls) of the adolescents had a BMI higher than the 85th percentile. The mean age was 15 years. Mean time spent fasting was 12.67 h (SD = 3.49); 80% of the participants were seen during the morning session of physical examinations, 16% during the afternoon session, and 3.5% during the evening session.
Table 1.
Characteristics of African American boys and girls from NHANES 2003–2010
In the CFA with the eight CVD risk factors, model fit was inadequate [χ2 (20) = 173.22; P < 0.001; RMSEA = 0.10; CFI = 0.91; SRMR = 0.06]. More specifically, factor loading for diastolic blood pressure (β = −0.02) was non-significant, fasting glucose (β = 0.17) was <0.2, and factor loadings for log-transformed triglycerides (β = 0.28) and systolic blood pressure (β = 0.29) indicated marginal correlations with the metabolic syndrome factor. Similarly, when we ran the eight CVD risk factor CFAs for adolescents with BMI higher than the 85th percentile, there was poor model fit [χ2 (20) = 120.96; P < 0.001; RMSEA = 0.13; CFI = 0.85; SRMR = 0.09], diastolic blood pressure did not significantly load onto the factor, fasting glucose fell below the threshold (β = 0.15), and triglycerides (β = 0.23) and systolic blood pressure (β = 0.22) were marginally correlated with the factor.
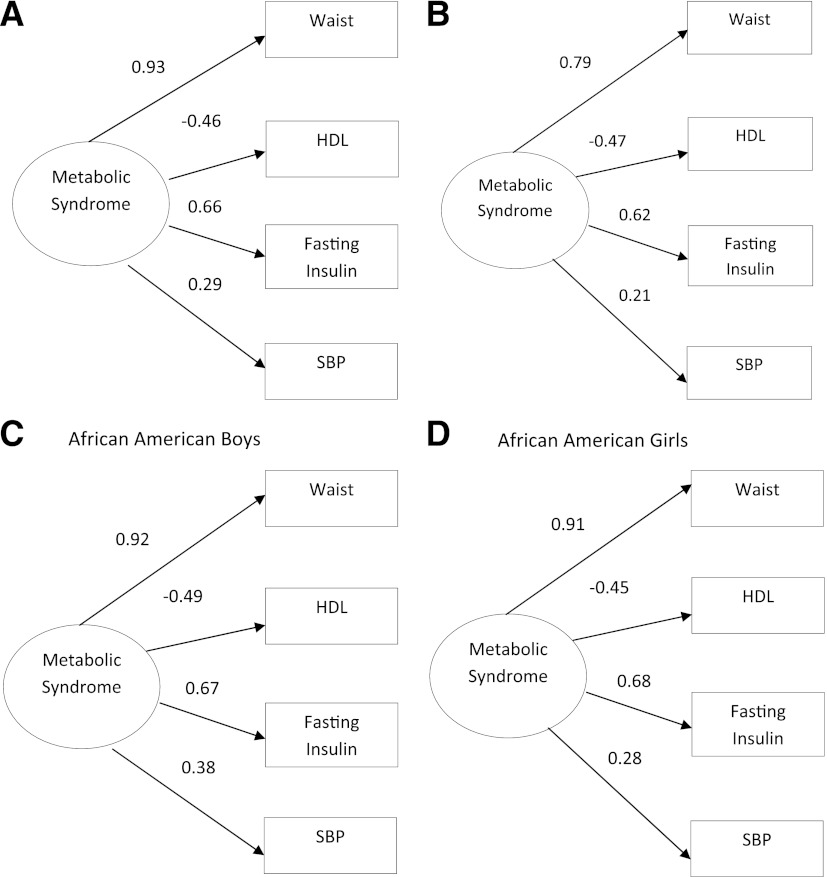
On the basis of these findings, we examined additional reduced factor models consisting of only the risk factors with significant factor loadings ±0.2 or higher. The best-fitting model to the data (Fig. 1) consisted of waist circumference, fasting insulin, HDL, and systolic blood pressure [χ2 (2) = 3.31; P = 0.19; RMSEA = 0.03; CFI = 1.00; SRMR = 0.01]. Model fit for African American boys [χ2 (2) = 3.55; P = 0.17; RMSEA = 0.04; CFI = 0.99; SRMR = 0.02] and African American girls [χ2 (2) = 3.44; P = 0.18; RMSEA = 0.04; CFI = 0.99; SRMR = 0.02] was excellent for this final model (Fig. 1). Results from a multiple group analysis indicated that African American boys and girls differed on the factor loading for systolic blood pressure [χdiff2 (1) = 4.33; P < 0.05] in this model. The best-fitting model for the total sample was also the best model for overweight/obese African American adolescents [χ2 (2) = 1.39; P = 0.50; RMSEA = 0.00; CFI = 1.00; SRMR = 0.02] (Fig. 1).
Figure 1.
Standardized factor loadings of one-factor confirmatory factor analysis models for metabolic syndrome in African American adolescents aged 12–17 years from NHANES 2003–2010. Measurement errors are not shown in figure. A: Model fit for final reduced model for total sample: χ2 (2) = 3.31; P = 0.19; RMSEA = 0.03; CFI = 1.00; SRMR = 0.01. B: Model fit for adolescents with a BMI higher than the 85th percentile: χ2 (2) = 1.39; P = 0.50; RMSEA = 0.00; CFI = 1.00; SRMR = 0.02. C: Model fit for final reduced model in all boys: χ2 (2) = 3.55; P = 0.17; RMSEA = 0.04; CFI = 0.99; SRMR = 0.02. D: Model fit for final reduced model in all girls: χ2 (2) = 3.44; P = 0.18; RMSEA = 0.04; CFI = 0.99; SRMR = 0.02.
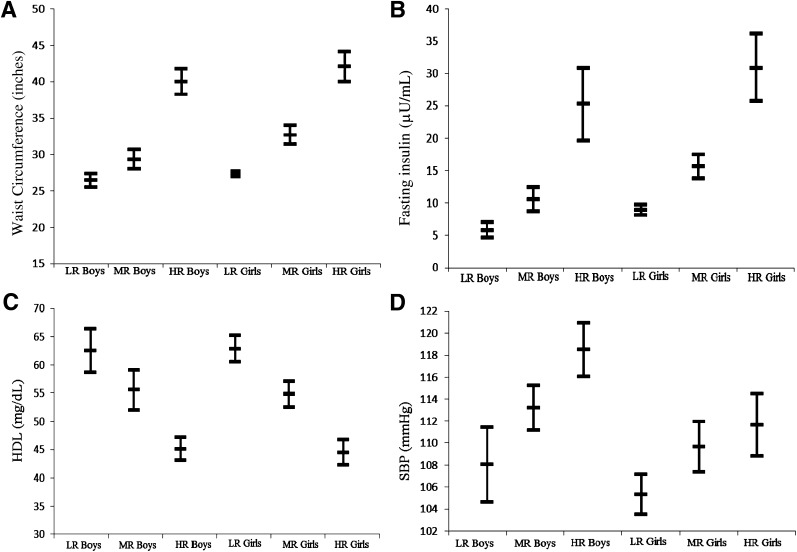
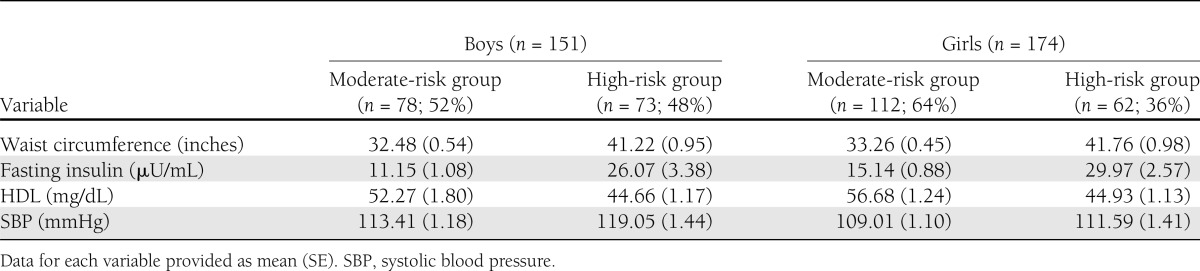
Metabolic syndrome components in the final best-fitting model (i.e., waist circumference, fasting insulin, HDL, and systolic blood pressure) were the indicators for the LPA, which was conducted separately for African American boys and girls. Models with two or more groups were tested and compared based on relative model fit indices (i.e., Akaike information criterion, Bayesian information criterion, and Lo-Mendell-Rubin test). On the basis of these three values, as well as entropy and interpretation, a three-group model was selected for both boys and girls for the total sample and a two-group model was selected from the subsample with BMI higher than the 85th percentile for both boys and girls. From the total sample, the low-risk group comprised approximately 38% of boys and 51% of girls, the moderate-risk group comprised 43% of boys and 33% of girls, and the high-risk group comprised approximately 19% of boys and 16% of girls (Fig. 2). Among the adolescents with a BMI higher than the 85th percentile, 48% of boys and 36% of girls were in the high-risk group (Table 2). Adolescents in the group at low risk of having metabolic syndrome were considered as such because, on average, individuals in this group had a waist circumference, fasting insulin, and HDL cholesterol level considered to be in the normal range (Fig. 2). On the other hand, adolescents in the groups at high risk for having metabolic syndrome were centrally obese, hyperinsulinemic, and had low HDL cholesterol (Fig. 2; Table 2). Mean values for the moderate-risk groups fell between those of the low- and high-risk groups. We labeled the second risk group for adolescents with BMI higher than the 85th percentile “moderate risk” because the mean values for each risk factor in this group were approximate to the means of the moderate-risk group for the total sample. Mean systolic blood pressure was not considered to be elevated (i.e., higher than the 90th percentile for age, sex, and height) for any of the risk groups (Fig. 2; Table 2).
Figure 2.
Latent profile analysis for total sample: metabolic syndrome risk groups by sex. Means and CIs for waist circumference (A), fasting insulin (B), HDL cholesterol (C), and systolic blood pressure (SBP) (D). LR, low risk (38% boys, 51% girls); MR, moderate risk (43% boys, 33% girls); HR, high risk (19% boys, 16% girls).
Table 2.
Latent profile analysis among adolescents with BMI higher than the 85th percentile: metabolic syndrome risk groups by sex
Within the total sample, boys in the high-risk group (mean age, 14.97 years; SE, 0.19 years) were significantly older than those in the low-risk group (mean age, 14.33 years; SE, 0.16 years; P = 0.01). There was no significant age difference among the risk groups for girls. In NHANES 2003–2010, approximately 22 and 12% of African American boys and girls, respectively, had prediabetes. Among adolescents with a BMI higher than the 85th percentile, 26% of boys and 19% of girls had prediabetes. Within the total sample high-risk group, 35% of boys had prediabetes compared with 25% in the moderate-risk group and 11% in the low-risk group. There was a significant difference in the prevalence of prediabetes between the high- and low-risk groups (P < 0.001) and the moderate- and low-risk groups (P < 0.05); however, there was no significant difference between the high- and moderate-risk groups for prevalence of prediabetes (P = 0.16) among boys. For girls, 35% of the high-risk group had prediabetes compared with 11% of the moderate-risk group and only 5% of the low-risk group. The prevalence of prediabetes was significantly higher in the high-risk group compared with the moderate-risk (P = 0.01) and low-risk groups (P < 0.001). There was no significant difference in prediabetes between the low-risk and moderate-risk groups for girls (P = 0.20). Within the overweight/obese subsample, 38% of high-risk boys compared with 14% of moderate-risk boys had prediabetes (P = 0.02). The prevalence of prediabetes among overweight/obese high-risk girls (32%) was also significantly higher (P = 0.02) than the prevalence for overweight/obese moderate-risk girls (11%).
CONCLUSIONS
Given the excellent fit of the model and construct validity, waist circumference, HDL cholesterol, fasting insulin, and systolic blood pressure seem to be significant indicators of metabolic syndrome among African American adolescents. In addition, African American boys and girls at high risk for having metabolic syndrome are more likely to have prediabetes (35% for each sex) than boys and girls at low risk (11 and 5%, respectively). Waist circumference and fasting insulin seem to be the strongest indicators of metabolic syndrome among African American adolescents, which is consistent with previous reports of metabolic syndrome in adolescents (23). Findings also are consistent with recent reports of African American adults, among whom central obesity, low HDL cholesterol, insulin resistance, and elevated blood pressure have been shown to be the strongest markers for having metabolic syndrome (18,35).
Our findings address the metabolic syndrome paradox in African American adolescents and demonstrate the need to model the metabolic syndrome within racial/ethnic groups. The metabolic syndrome model developed in this study differs from models that have been developed to fit across racial/ethnic groups (23), specifically by our inclusion of HDL rather than triglyceride levels. Li and Ford (23) included triglycerides rather than HDL in their final model because factor models with HDL were more variant across race/ethnicity, particularly between African American and Mexican American adolescents. Yet when we included triglycerides in our CFA models, model fit was poor according to at least two fit indices. These findings suggest that models attempting to fit criteria across racial/ethnic groups may fail to identify disease markers specific to race/ethnicity. In fact, triglycerides have been found to be the least predictive indicator of metabolic syndrome among African American adults (35). Despite higher rates of insulin resistance, which is known to be associated with dyslipidemia, African American youth and adults are more likely to have low triglycerides and high HDL compared with their white counterparts (15–18). The literature suggests that the metabolic syndrome paradox may be due to this lack of dyslipidemia among African Americans and that the current cutoffs for triglycerides and HDL for metabolic syndrome may need to be revised to be more indicative of CVD risk for African Americans (17).
Examining means for the high-risk groups from the total sample provides important clinical information about metabolic syndrome in African American boys and girls (Fig. 2). The mean waist circumferences for both boys (∼42 inches) and girls (∼44 inches) indicate central obesity given that the values were well above the 90th percentile across the age cutoffs for African American adolescents aged 12–17 years (36). Mean fasting insulin values for boys (∼25 µU/mL) and girls (∼30 µU/mL) suggest hyperinsulinemia, regardless of whether puberty stage is considered (37). Also, boys in the high-risk group had a mean HDL level of approximately 45 mg/dL. This falls above the criteria for low HDL (HDL <40 mg/dL) that is often cited in the literature (5); however, perhaps much higher HDL levels (above 45 mg/dL) are needed to be considered cardioprotective for African American boys (15). For systolic blood pressure, clinically, it is difficult to determine whether the means (∼118 mmHg for boys and ∼112 mmHg for girls) are objectively elevated because systolic blood pressure percentiles are based on age and height in addition to sex.
There are limitations to this study that should be addressed. First, the cross-sectional nature of NHANES data limits the ability to examine questions regarding temporality of the mechanisms involved in developing metabolic syndrome. Furthermore, because of the cross-sectional data, we are not able to test the stability of our final model over time. Second, not since the NHANES III 1988–1994 has the NHANES collected data on sexual maturation of children and adolescents to calculate Tanner and determine pubertal stage. Therefore, we were not able to control for pubertal development in our analysis, which may have implications for our final conceptual model and the characteristics and distinctions between risk groups, particularly when considering the prevalence of prediabetes. Fasting insulin levels typically are elevated during puberty and return to normal after puberty (4). We do not know if the elevated fasting insulin in the high-risk groups were due to pubertal development or if they actually were indicative of risk for metabolic syndrome. Third, the risk factors we included in our CFA were based on previous studies that applied CFA to examine metabolic syndrome; thus, we may have excluded other viable indicators of metabolic syndrome in adolescents (4).
The strengths of this study include being one of a few to examine markers of metabolic syndrome based on the continuous distribution of individual CVD risk factors (38), and it is the first study to apply LPA to examine metabolic syndrome risk groups. By using the continuous distribution of individual CVD risk factors, applicability of cutoffs for lipids and other components by race/ethnicity or sex is no longer an issue. Future studies using the CFA and LPA approaches are needed to replicate these findings, particularly with longitudinal data, to establish a unique and perhaps more appropriate definition and set of criteria for metabolic syndrome in African American adolescents. In fact, the means within each metabolic syndrome risk group identified using LPA could be examined as potential cutoffs specific to African American adolescents in a sensitivity/specificity analysis (39). In addition, future studies may include establishing metabolic syndrome risk scores using formative measurement (40) to continue to study metabolic syndrome as a continuous variable.
Our findings have implications in clinical, epidemiologic, and public health research in terms of how we conceptualize metabolic syndrome in African American adolescents. The best-fitting model for African American adolescents consisted of four measures that can be obtained easily in a clinic setting. A standard definition of pediatric metabolic syndrome would be ideal in both research and clinical practice, but the findings of this study further support that there are physiological differences that need to be accounted for so African American adolescents’ risk for CVD is not undermined. Metabolic syndrome has not been viewed as helpful in clinical practice, given that patients still are treated for the individual risk factors. However, modeling the syndrome allows us to see how these risk factors may cluster together differently and in different proportions to increase risk for disease, therefore shedding light on the pathophysiology of CVD specific to a racial/ethnic group.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
S.L.F. compiled the data, performed analyses, and wrote the manuscript. B.S.L. wrote the manuscript, contributed to the discussion, and reviewed and edited the manuscript. F.L.B., S.H.G., and F.H.-B. reviewed and edited the manuscript. S.L.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
References
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA 2010;303:242–249 [DOI] [PubMed] [Google Scholar]
- 2.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011;365:1876–1885 [DOI] [PubMed] [Google Scholar]
- 3.Orio F, Jr, Palomba S, Cascella T, Savastano S, Lombardi G, Colao A. Cardiovascular complications of obesity in adolescents. J Endocrinol Invest 2007;30:70–80 [DOI] [PubMed] [Google Scholar]
- 4.Steinberger J, Daniels SR, Eckel RH, et al. American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young. Council on Cardiovascular Nursing. Council on Nutrition, Physical Activity, and Metabolism Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2009;119:628–647 [DOI] [PubMed] [Google Scholar]
- 5.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med 2003;157:821–827 [DOI] [PubMed] [Google Scholar]
- 6.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation 2004;110:2494–2497 [DOI] [PubMed] [Google Scholar]
- 7.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999-2002. J Pediatr 2008;152:165–170 [DOI] [PubMed] [Google Scholar]
- 8.Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes Care 2008;31:587–589 [DOI] [PubMed] [Google Scholar]
- 9.Zimmet P, Alberti KG, Kaufman F, et al. IDF Consensus Group The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes 2007;8:299–306 [DOI] [PubMed] [Google Scholar]
- 10.Walker SE, Gurka MJ, Oliver MN, Johns DW, Deboer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis 2012;22:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006. JAMA 2008;299:2401–2405 [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Bao W, Begum S, Elkasabany A, Srinivasan SR, Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects: the Bogalusa Heart Study. Diabetes 2000;49:1042–1048 [DOI] [PubMed] [Google Scholar]
- 13.Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation 2007;116:1488–1496 [DOI] [PubMed] [Google Scholar]
- 14.Crawford AG, Cote C, Couto J, et al. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: findings from the GE Centricity Electronic Medical Record database. Popul Health Manag 2010;13:151–161 [DOI] [PubMed] [Google Scholar]
- 15.Gaillard T, Schuster D, Osei K. Metabolic syndrome in Black people of the African diaspora: the paradox of current classification, definition and criteria. Ethn Dis 2009;19(2 Suppl. 2):S2-1-7 [PubMed] [Google Scholar]
- 16.Osei K. Metabolic syndrome in blacks: are the criteria right? Curr Diab Rep 2010;10:199–208 [DOI] [PubMed] [Google Scholar]
- 17.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr 2009;155:S7.e7–e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumner AE, Zhou J, Doumatey A, et al. Low HDL-cholesterol with normal triglyceride levels is the most common lipid pattern in West Africans and African Americans with metabolic syndrome: Implications for cardiovascular disease prevention. CVD Prev Contr 2010;5:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Analytic note regarding 2007-2010 survey design changes and combining data across other survey cycles. Available from http://www.cdc.gov/nchs/data/nhanes/analyticnote_2007-2010.pdf Accessed 6 December 2011
- 20.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: questionnaires, datasets, and related documentation. Available from http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm Accessed 6 December 2011
- 21.Centers for Disease Control and Prevention. Growth chart training: a SAS program for the CDC growth charts. Available from http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm Accessed 6 December 2011
- 22.Goodman E, Daniels SR, Meigs JB, Dolan LM. Instability in the diagnosis of metabolic syndrome in adolescents. Circulation 2007;115:2316–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Ford ES. Is there a single underlying factor for the metabolic syndrome in adolescents? A confirmatory factor analysis. Diabetes Care 2007;30:1556–1561 [DOI] [PubMed] [Google Scholar]
- 24.Goodman E, Li C, Tu YK, Ford E, Sun SS, Huang TT. Stability of the factor structure of the metabolic syndrome across pubertal development: Confirmatory factor analyses of three alternative models. J Pediatr 2009;155:S5.e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muthen LK, Muthen BO. Mplus user’s guide. 6th ed. Los Angeles, CA: Muthen & Muthen; 1998–2010
- 26.Lawlor DA, Ebrahim S, May M, Davey Smith G. (Mis)use of factor analysis in the study of insulin resistance syndrome. Am J Epidemiol 2004;159:1013–1018 [DOI] [PubMed] [Google Scholar]
- 27.Kline R. Principles and practice of structural equation modeling . 2nd ed New York, The Guilford Press, 2005 [Google Scholar]
- 28.Hu LT, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling 1999;6:1–55 [Google Scholar]
- 29.Browne MW, Cudeck R. Alternative ways of assessing model fit. In Testing structural equation models. Bollen KA, Long JS, Eds. Newbury Park, CA, Sage, 1993, p. 136–162 [Google Scholar]
- 30.Vermunt JK, Magidson J. Latent class cluster analysis. In Applied latent class analysis. Hagenaars JA, McCutcheon AL, Eds. Cambridge, U.K., Cambridge University Press, 2002, p. 89–106 [Google Scholar]
- 31.Akaike H. Factor analysis and AIC. Psychometrika 1987;52:317–332 [Google Scholar]
- 32.Sclove L. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika 1987;52:333–343 [Google Scholar]
- 33.Nylund KL, Asparouhov T, Muthen B. Deciding on the number of classes in latent class analysis and growth mixture modeling: A monte carlo simulation study. Struct Equ Modeling 2007;14:535–569 [Google Scholar]
- 34.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor H, Liu J, Wilson G, et al. Distinct component profiles and high risk among African Americans with metabolic syndrome: the Jackson Heart Study. Diabetes Care 2008;31:1248–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 2004;145:439–444 [DOI] [PubMed] [Google Scholar]
- 37.Viner RM, Segal TY, Lichtarowicz-Krynska E, Hindmarsh P. Prevalence of the insulin resistance syndrome in obesity. Arch Dis Child 2005;90:10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thivel D, Malina RM, Isacco L, Aucouturier J, Meyer M, Duché P. Metabolic syndrome in obese children and adolescents: dichotomous or continuous? Metab Syndr Relat Disord 2009;7:549–555 [DOI] [PubMed] [Google Scholar]
- 39.Llabre MM, Fitzpatrick SL. Revisiting measurement models in psychosomatic medicine research: a latent variable approach. Psychosom Med 2012;74:169–177 [DOI] [PubMed] [Google Scholar]
- 40.Bollen K, Lennox R. Conventional wisdom on measurement: a structural equation perspective. Psychol Bull 1991;110:305–314 [Google Scholar]