Prologue
Name giving is part of human nature as an attempt to classify objects and structure the world around us. In a scientific context such name giving is ideally organized according to strict rules of nomenclature that form a system of terms and principles allowing accurate communication between specialists in particular disciplines. However, for reasons of convenience or eloquence, such strict conventions are often disregarded, sometimes to the extent that a name no longer evokes a clear idea of the object. A good example is the ‘CD' naming system in immunology, where many casual users would be hard pressed to recall the molecules corresponding to any but the most frequently used CD denominators. Nevertheless, as Shakespeare reminds us in Romeo and Juliette, ‘What's in a name? That what we call a rose by any other name would smell as sweet.' Indeed, what matters is the object rather than its name. However, when it comes to naming compounds, a question arises: To what extent can we yield to the pressures of convenience, eloquence or discovery history rather than follow the agreed-upon nomenclature rules? What is more important, a molecule's chemical composition or its intended use? Very often, compounds are named after the biological target against which they were discovered. This provides an easy and attractive way of naming molecules, but it risks causing a bias in research aims and the way in which results are interpreted. Indeed, names can clarify, but they can also blur or confuse. Two papers ‘Activity and specificity of Necrostatin-1, small molecule inhibitor of RIP1 kinase' in Cell Death and Differentiation1 and ‘Necrostatin-1 analogs: Critical issues on the specificity, activity and in vivo use in experimental disease models' in Cell Death and Disease2 raise important considerations on the use of necrostatin-1 (Nec-1) and its analogs to study the implication of RIPK1 kinase activity in experimental disease models.
Face 1: Nec-1 as RIPK1 kinase inhibitor
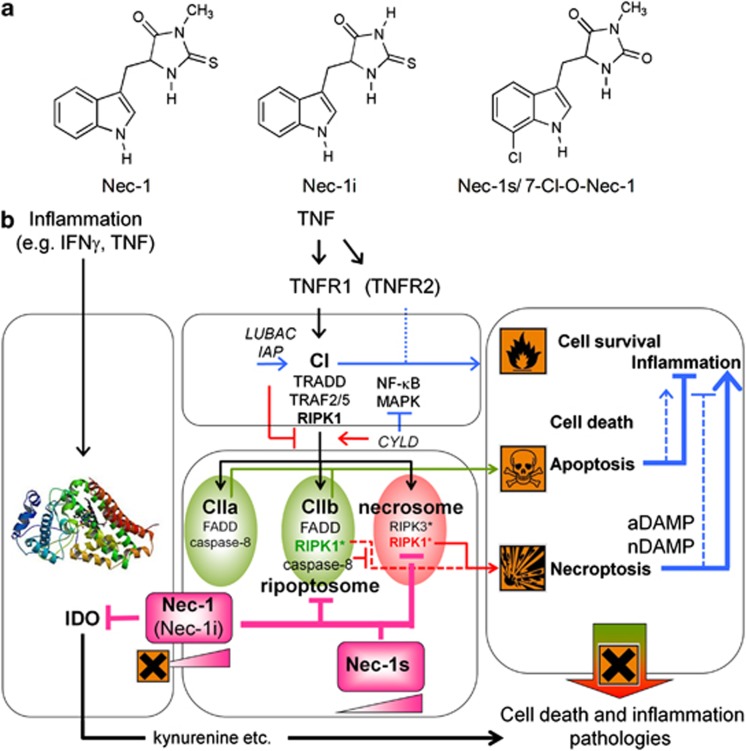
Necrostatins are a family of compounds of diverse chemical structure that have been named for their ability to block necrotic cell death. Some of these necrostatins target RIPK1 activity, whereas others block necrosis by yet unidentified mechanisms.3 But even in the case of the well-characterized and widely used Nec-1 and its inactive control (Nec-1i), which lacks a methyl group in the thiohydantoin moiety (Figure 1a), the clear picture is blurred by some issues concerning specificity, in vivo activity and dose–responses. Nec-1 was identified in 2005 by Alexei Degterev and Junying Yuan as a compound that blocks necrotic cell death in human and murine cells.4 In a subsequent study, Nec-1 was identified as an allosteric inhibitor of RIPK1 kinase activity.3 Nec-1 is now widely used to target RIPK1 kinase activity (Figure 1b) in various experimental disease models, such as ischemia -reperfusion injury in brain, heart and kidney, systemic inflammatory response syndrome, sepsis and retinal cell death.4, 5, 6, 7, 8
Figure 1.
Actions of Nec-1 and its derivatives in a mouse system. (a) Chemical structures of Nec-1 derivatives targeting RIPK1: active compound Nec-1, inactive variant Nec-1i and stable variant Nec-1s/7-Cl-O-Nec-1. (b) Effect of Nec-1 derivatives on two pathways involving cell death and inflammation. The TNFR1 signaling pathway mediated by RIPK1 versus the IDO–kynurenine pathway are shown in parallel. TNFR1 signaling can lead to cell survival, apoptosis or necroptosis mediated by formation of three distinct signaling complexes: the membrane-bound Complex I (CI), the cytosolic Complex II or the necrosome, respectively. RIPK1 ubiquitination by cIAPs or LUBAC within the CI functions as a platform to initiate activation of NF-kB and MAPK, resulting in cell survival and inflammation through gene induction. Deubiquitination of RIPK1 by CYLD leads to formation of either CIIa or CIIb (the latter in cIAP-depleted conditions, also called ripoptosome), both leading to apoptosis mediated by caspase-8. Only apoptosis mediated by CIIb is dependent on RIPK1 activity and is thus inhibited by Nec-1. RIPK1 and RIPK3 pro-necrotic activity is restrained by caspase-8 activity in the pro-apoptotic CIIa and CIIb, but inhibition or loss of caspase-8 results in necrosome formation and subsequent necroptosis. This process is dependent on RIPK1 kinase activity and is inhibited by Nec-1. Excess apoptosis or necroptosis may induce inflammation, though in general apoptosis is anti-inflammatory (represented by arrows of different size) through release of specific DAMPs. Thus, all three TNF-induced pathways may result in pathologies associated with cell death and inflammation (X representing harmful effects). Both Nec-1 and Nec-1i also inhibit IDO. The IDO–kynurenine pathway is activated under inflammatory conditions and exerts immunomodulatory functions. Consequently, inhibition of this pathway may also result in pathologies associated with cell death and inflammation. At higher concentrations both Nec-1 and Nec-1i inhibit RIPK1 with equal potency but at low concentration both are toxic (this concentration effect is represented by gradient in a triangle and X represents harmful effects). Nec-1s, which inhibits RIPK1 and subsequent necroptosis, lacks the IDO inhibitory activity and concentration-dependent toxicity in vivo. DAMPs, damage-associated molecular patterns, a, apoptotic, n, necrotic
Face 2: Nec-1 as IDO inhibitor
Methyl-thiohydantoin-tryptophan (MTH-Trp) has been described as an inhibitor of indoleamine-2,3-dioxygenase,9 which catabolizes the essential amino acid Trp into kynurenine. The IDO–kynurenine pathway leads to modulation of the innate and adaptive immune system, but is also implicated in neuroprotection. Targeting IDO using MTH-Trp is used to interfere in inflammation-associated tumorigenesis, breaking the tumor immunotolerance and sensitizing the tumor to cell death.10 1-M-Tryptophane (1-MT), an analog of MTH-Trp, is now in phase I/II clinical trials to test its ability to break tumor tolerance.11
For almost 7 years these two research domains evolved independently, with scientists purchasing either Nec-1 to target RIPK1 or MTH-Trp to block IDO. However, to be honest, at least one company also mentions Nec-1 in the list of IDO inhibitors. Degterev et al.1 and Takahashi et al.2 now clearly notify the scientific community that Nec-1 and MTH-Trp are chemically identical and, consequently, have the same IUPAC name 5-((1H-indol-3-yl)methyl)-3-methyl-2-thioxoimidazolidin-4-one (http://www.nature.com/nchembio/journal/v1/n2/compound/nchembio711_ci.html). The use of three different names for the same chemical entity has temporarily blurred the cell death research domain, especially as both targeted enzymes (RIPK1 and IDO) are implicated in modulation of inflammation and neuroprotection, two important processes in which cell death is implicated (Figure 1b). The only discrepancy between both reports regarding the activities of Nec-1 derivatives on IDO is that in Degterev et al., Nec-1i apparently did not block human IDO,1 while it did in the report of Takahashi et al., in line with the molecular modelling.2
Nec-1: what's in a name?
In experimental disease models, rescue by Nec-1 has often been interpreted as the involvement of necroptosis. However, a Nec-1/MTH-Trp inhibited process cannot be reduced to necroptosis as the in vivo context may involve a contribution of IDO. The challenge is to analyze the extent to which the protective effect of Nec-1 or MTH-Trp in different disease models can be attributed to inhibition of RIPK1, IDO or both. The papers by Degterev et al.1 and Takahashi et al.2 clearly indicate that we have a tool for this. Indeed, Nec-1s or 7-Cl-O-Nec-1 [5-((7-chloro-1H-indol-3-yl)methyl)-3-methylimidazolidine-2,4-dione] does not target IDO and is a RIPK1 inhibitor with improved in vivo pharmacokinetic properties.3 As 1-MT (IDO inhibitor) and Nec-1/MTH-Trp share a common indol moiety it was conceivable that the classical IDO inhibitor 1-MT could also target RIPK1, and subsequent necroptosis. This is clearly not the case. Both papers demonstrate that neither in human nor mouse cells necroptosis is affected by other IDO inhibitors such as 1-MT.1, 2
A second major issue is that the name ‘necrostatin' refers to the initial screening of a compound library in a necrotic cell death assay.4 Such a name of course emphasizes necroptosis as the main target and may bias the consideration of the involvement of other processes. Indeed, despite its name, Nec-1/1-MTH-Trp can also target RIPK1-mediated apoptosis when cIAPs are blocked by Smac mimetics or are downregulated.12, 13 Conditions of caspase-8 deficiency favor necroptosis induction14, 15, 16, 17 in which RIPK1/RIPK3 are implicated (Figure 1b). Therefore, in pathological conditions it cannot a priori be said whether Nec-1 mediated inhibition targets RIPK1-mediated apoptosis or RIPK1-mediated necroptosis. The key question is indeed, ‘What are the pathological conditions or particular cell types in which cIAP or caspase-8 downmodulation occurs which could favor RIPK1-mediated apoptosis or RIPK1-mediated necroptosis, respectively?'
Nec-1 and Nec-1i: some more paradoxical findings
The paper of Takahashi et al.2 also points to two additional issues regarding the use of Nec-1 and its inactive demethylated derivative Nec-1i [5-((1H-indol-3-yl)methyl)-2-thioxoimidazolidin-4-one], which apparently is still active in a necroptosis assay based on mouse cells2 but not on human cells.1 Moreover, and this makes the interpretation of Nec-1-based studies really difficult, Nec-1 and Nec-1i show paradoxical dose–response curves. Indeed, low concentrations of Nec-1 and Nec-1i in vivo even sensitize to lethality during TNF-induced SIRS, suggesting that these compounds are somehow toxic and that the balance is tipped to improved survival only at the higher doses.2 This toxicity may explain some controversial reports in the literature about the role of RIPK1 in TNF-induced SIRS and sepsis.18, 19 This toxicity of low doses was not observed for Nec-1s, again suggesting that Nec-1s is a preferred tool for targeting RIPK1 in vivo (Figure 1b).
Epilogue
Targeting necroptosis apparently is alive and kicking. The discovery of necrostatins3, 4 has given the scientific community invaluable tools to target RIPK1-mediated processes in vivo. The results are very promising and have promoted RIPK1/necroptosis targeting as a central theme in biomedical research on inflammatory and degenerative disease models. Although the name ‘necrostatin-1' suggests that necroptosis is the main targeted process, other cellular processes such as RIPK1-dependent apoptosis and IDO targeting cannot a priori be excluded. Science is the result of a continuous process of error elimination and knowledge adaptation resulting in the evolution of deeper insights and novel paradigms. The papers by Degterev et al.1 and Takahashi et al.2 have clearly contributed to evolution of our knowledge of these compounds and demonstrate that Nec-1s, which is devoid of IDO inhibitory activity1, 2 and which does not show a paradoxical dose–response curve by becoming toxic at low concentrations,2 is an improved alternative for studying the functions of RIPK1 in experimental disease models. Commercially available Nec-1 is still instrumental when the involvement of IDO is ruled out by use of 1-MT.
Acknowledgments
We thank Amin Bredan for editorial help. Research in the Vandenabeele group is supported by European grants (FP6 ApopTrain, MRTN-CT-035624; FP7 EC RTD Integrated Project, Apo-Sys, FP7-200767; Euregional PACT II), Belgian grants (Interuniversity Attraction Poles, IAP 6/18, IAP 7/32), Flemish grants (Research Foundation Flanders, FWO G.0875.11, FWO G.0973.11 and FWO G.0A45.12N), Ghent University grants (MRP, GROUP-ID consortium) and grants from Flanders Institute for Biotechnology (VIB). PV holds a Methusalem grant (BOF09/01M00709) from the Flemish Government. SG was supported by the ‘Institute for the promotion of Innovation by Science and Technology in Flanders' (IWT SB63424), a ‘Special Research Fund' from Ghent University (BOF B/09683/02), and a university contract as part of a Methusalem grant awarded to professor PV by the Flemish Government (BOF09/01M00709).
The authors declare no conflict of interest.
References
- Degterev A, Maki JL, Yuan J. Cell Death Differ. 2013. p. 366. [DOI] [PMC free article] [PubMed]
- Takahashi N, et al. Cell Death Dis. 2012. p. e437. [DOI] [PMC free article] [PubMed]
- Degterev A, et al. Nat Chem Biol. 2008. pp. 313–321. [DOI] [PMC free article] [PubMed]
- Degterev A, et al. Nat Chem Biol. 2005. pp. 112–119. [DOI] [PubMed]
- Linkermann A, et al. Kidney Int. 2012. pp. 751–761. [DOI] [PubMed]
- Smith CC, et al. Cardiovasc Drugs Ther. 2007. pp. 227–233. [DOI] [PubMed]
- Trichonas G, et al. Proc Natl Acad Sci USA. 2010. pp. 21695–21700. [DOI] [PMC free article] [PubMed]
- Duprez L, et al. Immunity. 2011. pp. 908–918. [DOI] [PubMed]
- Muller AJ, et al. Nat Med. 2005. pp. 312–319. [DOI] [PubMed]
- Prendergast GC, Metz R, Muller AJ. Am J Pathol. 2010. pp. 2082–2087. [DOI] [PMC free article] [PubMed]
- Institute HLMCCaR A Phase 1 Study of 1-Methyl-D-tryptophan in Patients with Advanced Malignancies. 2012.
- Feoktistova M, et al. Mol Cell. 2011. pp. 449–463. [DOI] [PMC free article] [PubMed]
- Tenev T, et al. Mol Cell. 2011. pp. 432–448. [DOI] [PubMed]
- Kaiser WJ, et al. Nature. 2011. pp. 368–372. [DOI] [PMC free article] [PubMed]
- Oberst A, et al. Nature. 2011. pp. 363–367. [DOI] [PMC free article] [PubMed]
- Gunther C, et al. Nature. 2011. pp. 335–339. [DOI] [PMC free article] [PubMed]
- Kovalenko A, et al. J Exp Med. 2009. pp. 2161–2177. [DOI] [PMC free article] [PubMed]
- Linkermann A, et al. Mol Med. 2012. pp. 577–586. [DOI] [PMC free article] [PubMed]
- McNeal SI, et al. Shock. 2011. pp. 499–505. [DOI] [PMC free article] [PubMed]