Abstract
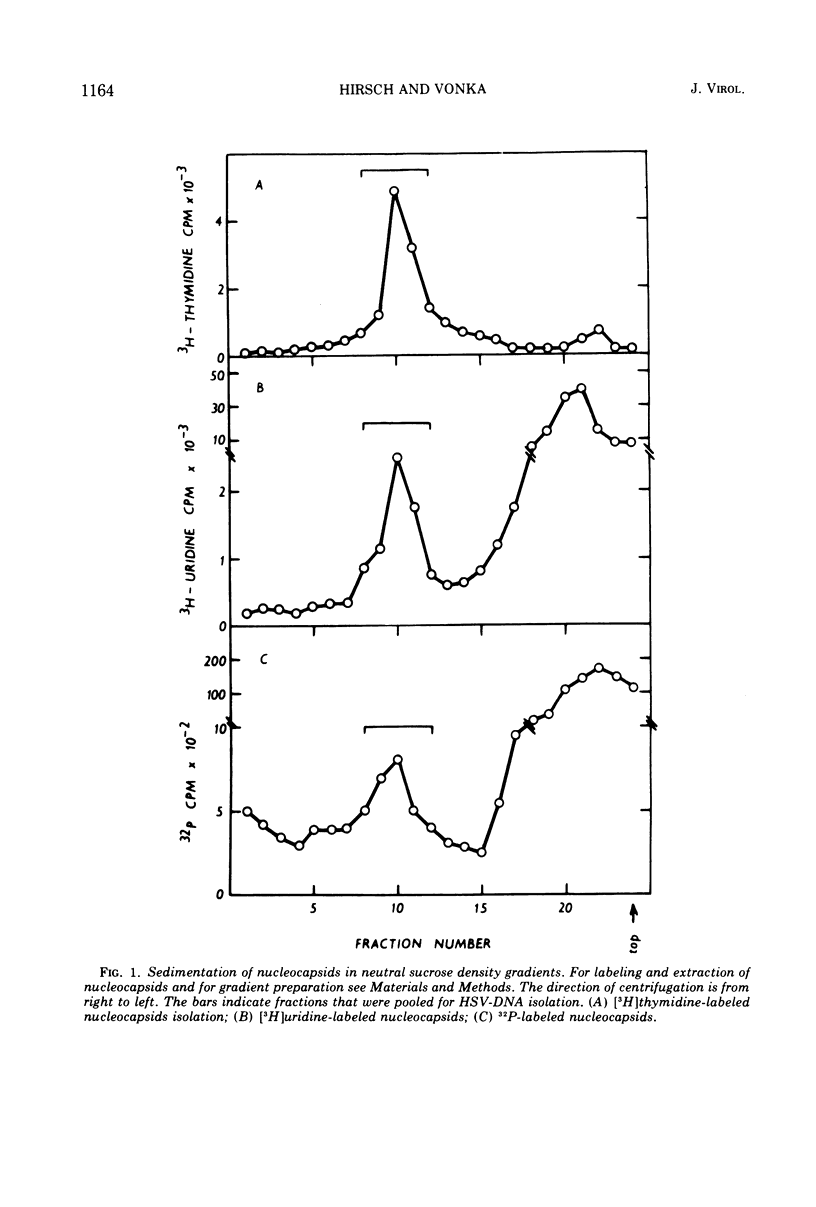
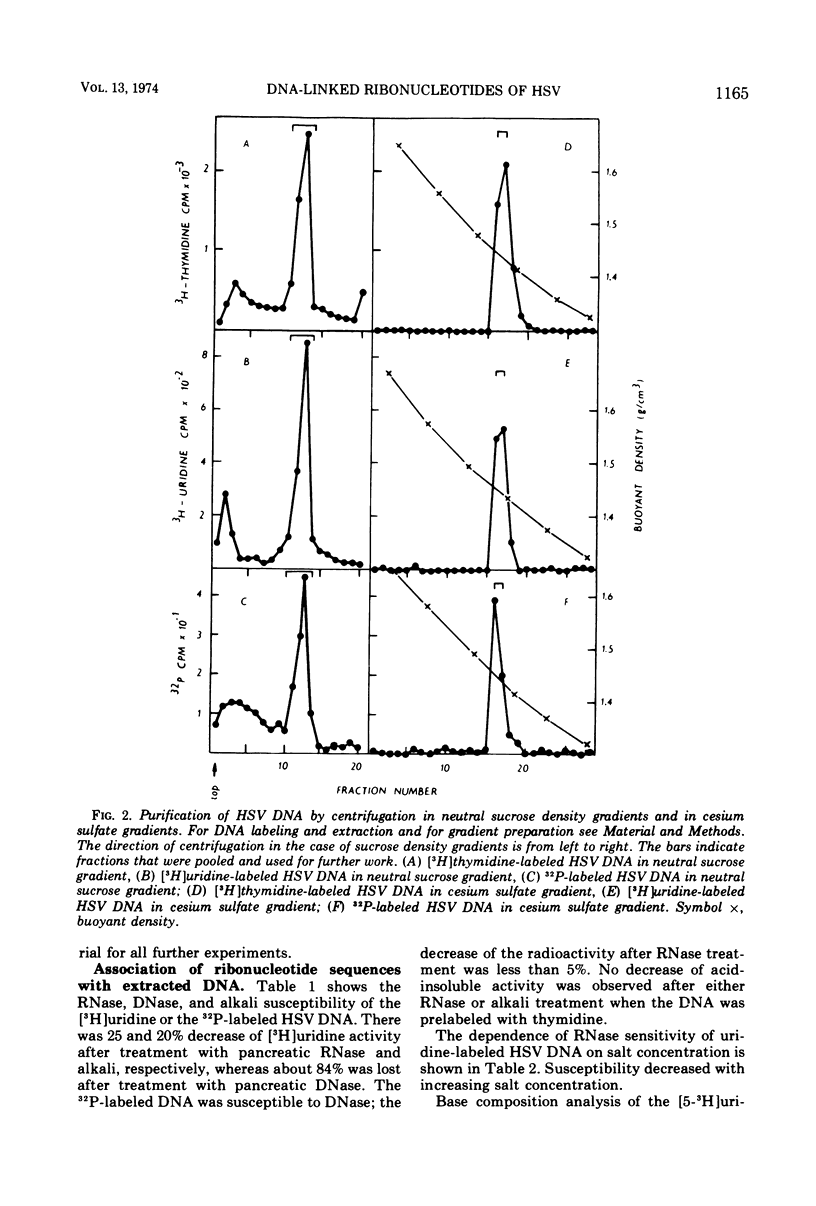
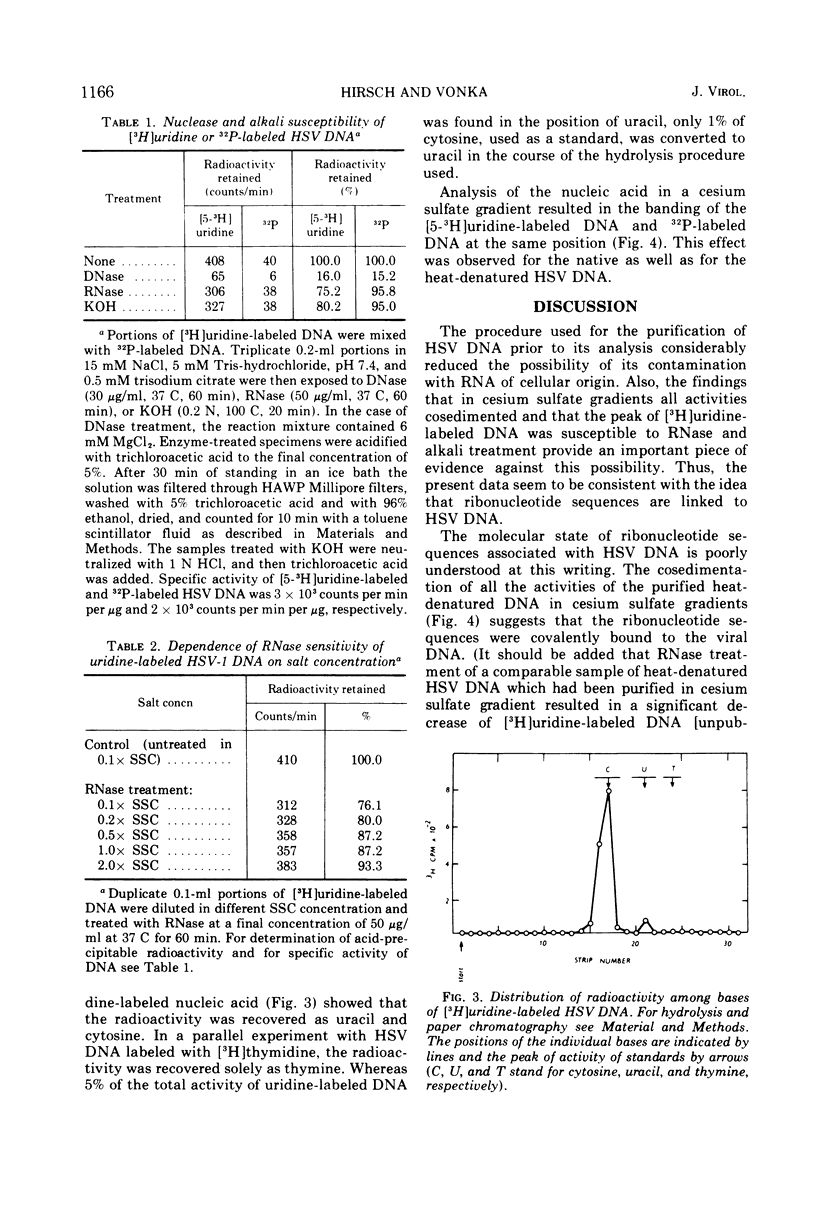
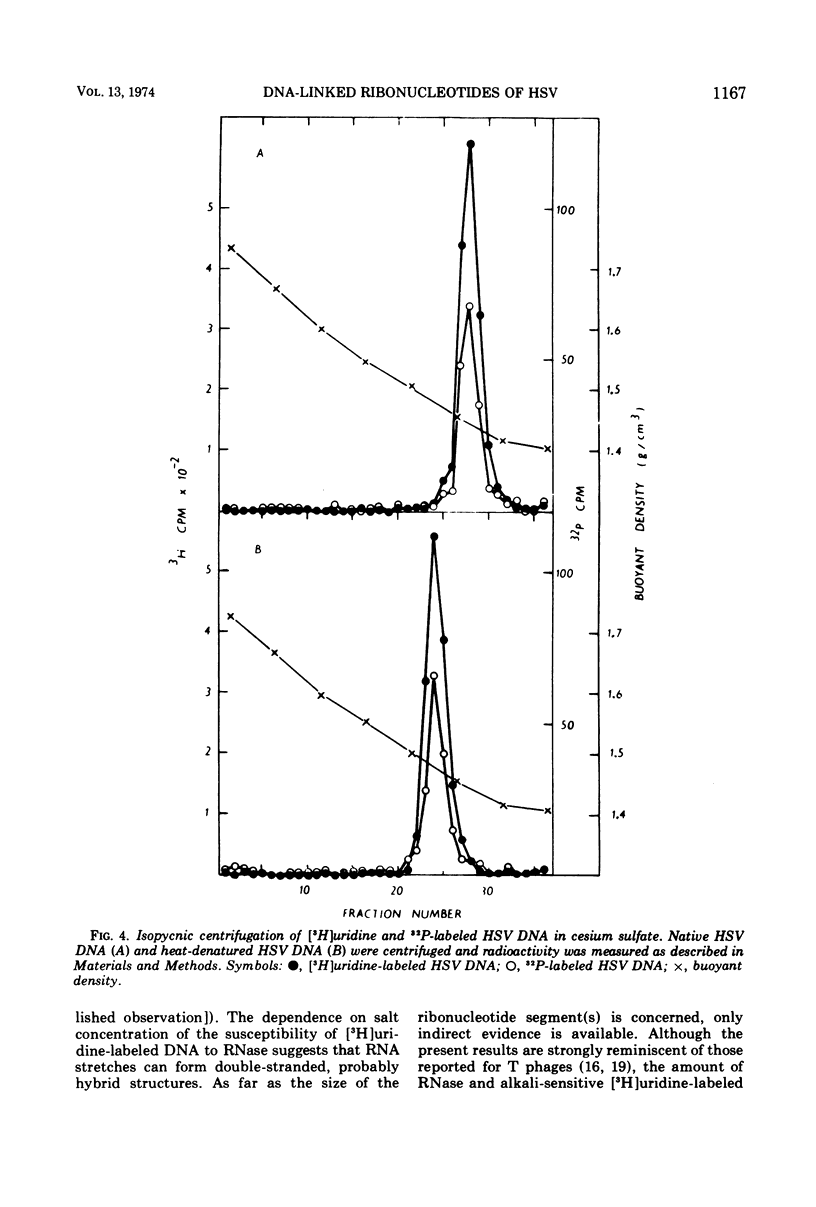
Cells of a continuous cell line derived from rabbit embryo fibroblasts were infected with herpes simplex type 1 virus (HSV-1) and maintained in the presence of either [5-3H]uridine or [methyl-3H]thymidine or 32PO43−. Nucleocapsids were isolated from the cytoplasmic fraction, partially purified, and treated with DNase and RNase. From the pelleted nucleocapsids, DNA was extracted and purified by centrifugation in sucrose and cesium sulfate gradients. The acid-precipitable radioactivity of [5-3H]uridine-labeled DNA was partially susceptible to pancreatic RNase and alkaline treatment; the susceptibility to the enzyme decreased with increasing salt concentration. No drop of activity of DNA labeled with [3H]thymidine was observed either after RNase or alkali treatment. Base composition analysis of [5-3H]uridine-labeled DNA showed that the radioactivity was recovered as uracil and cytosine. In the cesium sulfate gradient, the purified [5-3H]uridine-labeled DNA banded at the same position as the 32P-labeled DNA. The present data tend to suggest that ribonucleotide sequences are present in HSV DNA, that they are covalently attached to the viral DNA, and that they can form double-stranded structures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEN-PORAT T., KAPLAN A. S. The chemical composition of herpes simplex and pseudorabies viruses. Virology. 1962 Mar;16:261–266. doi: 10.1016/0042-6822(62)90246-5. [DOI] [PubMed] [Google Scholar]
- DARLINGTON R. W., RANDALL C. C. The nucleic acid content of equine abortion virus. Virology. 1963 Mar;19:322–327. doi: 10.1016/0042-6822(63)90071-0. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Separation of the herpesvirus deoxyribonucleic acid duplex into unique fragments and intact strand on sedimentation in alkaline gradients. J Virol. 1972 Oct;10(4):565–572. doi: 10.1128/jvi.10.4.565-572.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordin M., Olshevsky U., Rosenkranz H. S., Becker Y. Studies on herpes simplex virus DNA: denaturation properties. Virology. 1973 Sep;55(1):280–284. doi: 10.1016/s0042-6822(73)81031-1. [DOI] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D., de Rudder J., Privat de Garilhe M. A propos de la composition du DNA du virus herpétique. Bull Soc Chim Biol (Paris) 1965;47(6):1033–1042. [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Lee L. F., Kieff E. D., Bachenheimer S. L., Roizman B., Spear P. G., Burmester B. R., Nazerian K. Size and composition of Marek's disease virus deoxyribonucleic acid. J Virol. 1971 Mar;7(3):289–294. doi: 10.1128/jvi.7.3.289-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H. Untersuchungen am genetischen Material von Herpesviren. I. Biophysikalisch-chemische Charakterisierung von Herpesvirus=Desoxyribonucleinsäuren. Med Microbiol Immunol. 1972;157(3):186–211. doi: 10.1007/BF02121161. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Hudson J. B. Some properties of the genome of murine cytomegalovirus (MCV). Virology. 1973 Jul;54(1):135–149. doi: 10.1016/0042-6822(73)90123-2. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Detection of Epstein-Barr viral genome in nonproductive cells. Nat New Biol. 1971 Sep 22;233(38):103–106. doi: 10.1038/newbio233103a0. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. C. Herpes virus nucleic acid. Virology. 1962 Mar;16:355–357. doi: 10.1016/0042-6822(62)90262-3. [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. S. RNA in coliphage T5. Nature. 1973 Mar 30;242(5396):327–329. doi: 10.1038/242327a0. [DOI] [PubMed] [Google Scholar]
- Roubal J., Vonka V. Multiplicity reactivation in UV-irradiated herpes simplex type 1 virus. Intervirology. 1973;1(2):73–79. doi: 10.1159/000148834. [DOI] [PubMed] [Google Scholar]
- Ríman J., Beaudreau G. S. Viral DNA-dependent DNA polymerase and the properties of thymidine labelled material in virions of an oncogenic RNA virus. Nature. 1970 Oct 31;228(5270):427–430. doi: 10.1038/228427a0. [DOI] [PubMed] [Google Scholar]
- Speyer J. F., Chao J., Chao L. Ribonucleotides covalently linked to deoxyribonucleic acid in T4 bacteriophage. J Virol. 1972 Nov;10(5):902–908. doi: 10.1128/jvi.10.5.902-908.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Hirose S., Okazaki R. RNA-linked nascent DNA fragments in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1863–1867. doi: 10.1073/pnas.69.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]



