Abstract
Accumulating evidence has demonstrated that S-nitrosation of proteins plays a critical role in several human diseases. Here, we explored the role of inducible nitric oxide synthase (iNOS) in the S-nitrosation of proteins involved in the early steps of the insulin-signaling pathway and insulin resistance in the skeletal muscle of aged mice. Aging increased iNOS expression and S-nitrosation of major proteins involved in insulin signaling, thereby reducing insulin sensitivity in skeletal muscle. Conversely, aged iNOS-null mice were protected from S-nitrosation–induced insulin resistance. Moreover, pharmacological treatment with an iNOS inhibitor and acute exercise reduced iNOS-induced S-nitrosation and increased insulin sensitivity in the muscle of aged animals. These findings indicate that the insulin resistance observed in aged mice is mainly mediated through the S-nitrosation of the insulin-signaling pathway.
In recent decades, S-nitrosation, the reaction of nitric oxide (NO) with cysteine residues in proteins to form S-nitrosothiol adducts, in addition to phosphorylation, acetylation, and ubiquitination, has become one of the main forms of post-translational modification of intracellular eukaryotic proteins (1). Deregulated S-nitrosation contributes to a range of chronic human diseases (1,2).
NO donor agents and the NO produced by inducible NO synthase (iNOS) induces insulin resistance through the S-nitrosation of proteins involved in the early steps of insulin action, such as insulin receptor β (IRβ), insulin receptor substrate 1 (IRS-1), and Akt (3–5). Although several studies suggest that NO and iNOS could be involved in several aging-related diseases (2,6), the participation of these molecules on aging-induced insulin resistance is unclear. Here, we hypothesized that iNOS could mediate the insulin resistance through the S-nitrosation of insulin-signaling pathway proteins during aging.
RESEARCH DESIGN AND METHODS
Animals.
Male C57BL/6 and iNOS-null mice were purchased from Jackson Laboratory (C57BL/6-Nos2tm1Lau colony), aged 3 months (young) and 28 months (old). Animals had free access to standard rodent chow and water. All experiments were approved by the ethics committee of the State University of Campinas.
L-N6-(l-iminoethyl) lysine (L-NIL) treatment.
The animals received an intraperitoneal injection of L-NIL (80 mg/kg body weight) or PBS, twice daily for 5 days (n = 8).
Exercise protocol.
The animals swam in for two 1.5-h-long bouts, separated by a 30-min rest period (n = 8), in water maintained at ∼34°C. This exercise protocol was adapted from a previously published procedure (7).
Glucose and serum insulin quantification.
Serum insulin concentration was determined using a commercially available enzyme linked immunosorbent assay kit (Crystal Chem Inc.; n = 12). Fasting glucose was measured using Accutrend Plus equipment (Roche); in this case, the blood sample was obtained from the tails of mice (n = 12).
Hyperinsulinemic-euglycemic clamp.
After a 6-h fast, the mice (n = 8) were anesthetized with a mixture of ketamine (100 mg) and diazepam (0.07 mg; 0.2 mL/100 g body weight). A 120-min hyperinsulinemic-euglycemic clamp procedure was conducted continuously infused at a rate of 5.0 mU/kg body weight ⋅ min to raise the plasma insulin concentration to ∼4.8–5.4 ng/mL. Glucose (5%) was infused at variable rates to maintain plasma glucose at 100 ± 10 mg/dL.
Glucose uptake measurement.
The glucose uptake in isolated soleus muscle was performed as previously described (3). At 1 h after the last L-NIL injection and 8 h after the exercise protocol, the mice (n = 8) were anesthetized and soleus muscles were isolated and incubated in Krebs-Ringer bicarbonate buffer and 0.011 MBq/mL d-[U-14C]glucose, with 95% O2/5% CO2, at 37°C and centrifuged at 1,000g. Incubation was performed for 1 h in the presence of 10 mUI/mL insulin and 2 or 20 mmol/L glutamine. Uptake of 2-deoxy-d-[2,6-3H]glucose was determined as previously described (8).
Protein analysis by immunoblotting.
Insulin (10−6 mol/L) or saline were infused in the portal vein, and 90 s later, both portions of gastrocnemius (red and white fibers) were ablated, pooled, minced coarsely, and homogenized immediately in extraction buffer (n = 8), and Western blot was performed, as previously described (9).
Detection of S-nitrosated proteins by biotin switch method.
The biotin-switch assay was performed essentially as previously described (10,11) to determine the IRβ, IRS-1, and Akt S-nitrosation in the gastrocnemius muscle (n = 8).
Statistical analysis.
Data were analyzed by the two-tailed unpaired Student t test or by one-way ANOVA, followed by post hoc analysis of significance (Bonferroni test) when appropriate, comparing experimental and control groups. The level of significance was set at P < 0.05.
RESULTS
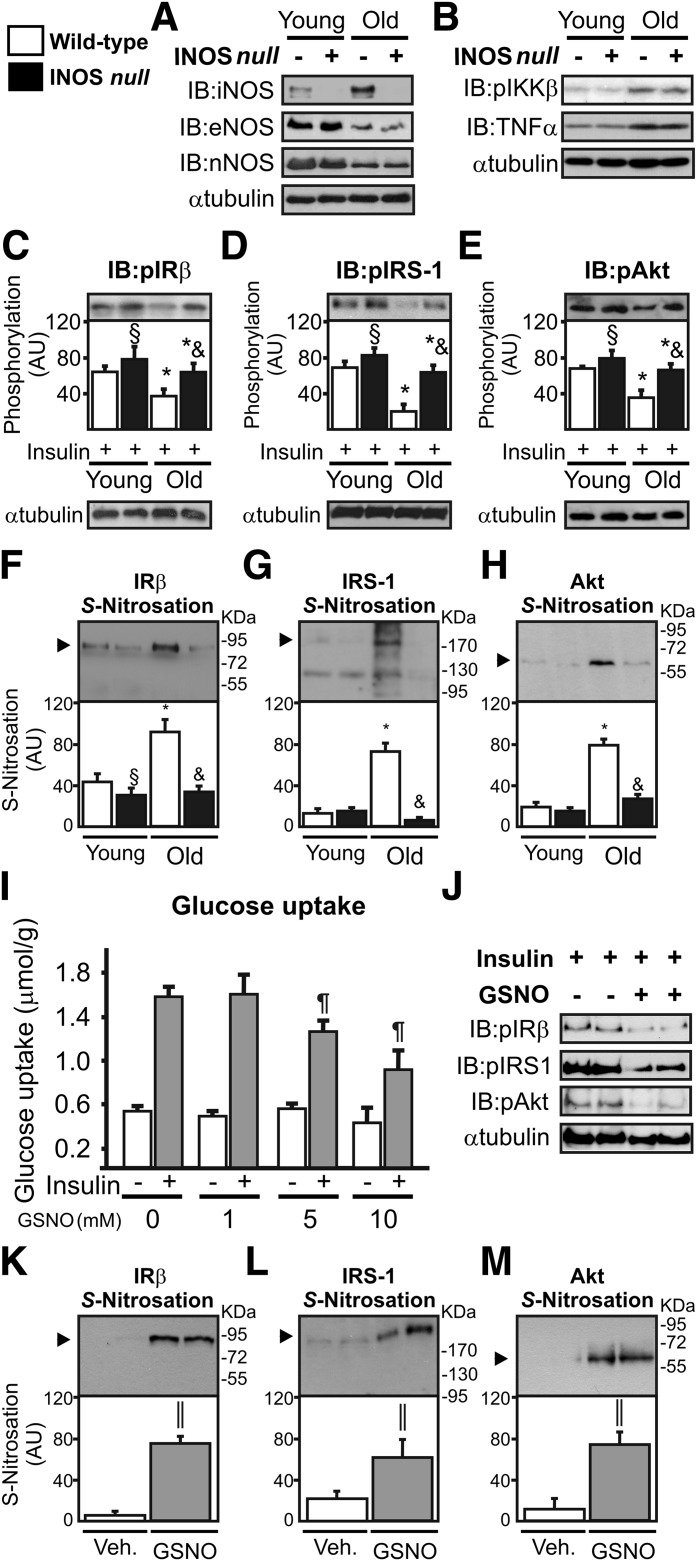
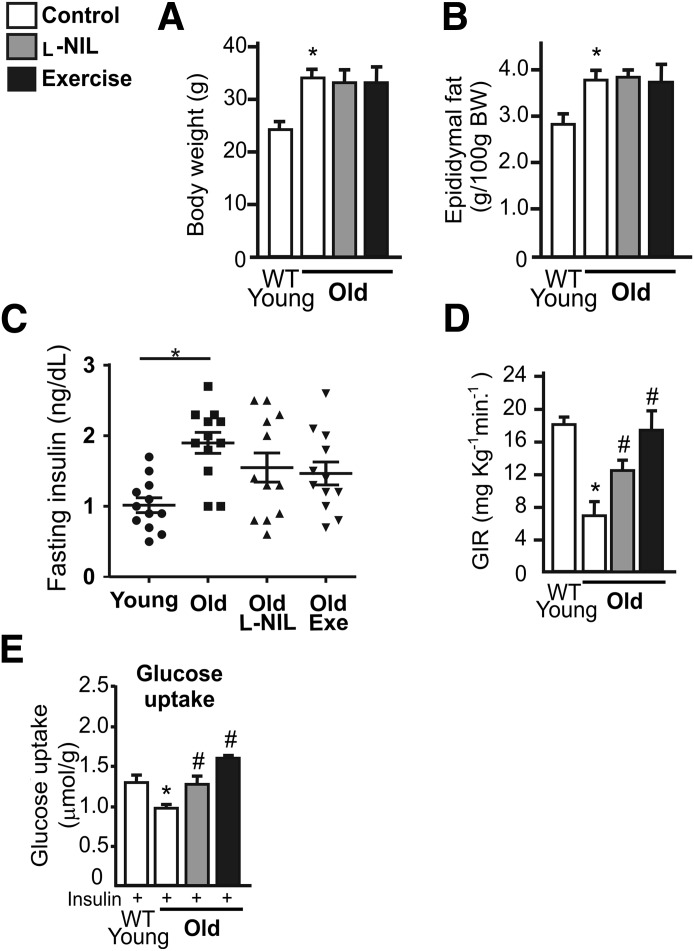
To explore the role of iNOS on insulin sensitivity during aging, we analyzed insulin sensitivity in young and old wild-type and iNOS-null male mice. During aging, wild-type and iNOS-null old mice presented similar values for body weight and epididymal fat weight (Fig. 1A and B). High levels of fasting serum insulin levels were found in wild-type old mice but not in iNOS-null mice (Fig. 1C), and fasting blood glucose levels were similar between the groups (data not shown). The hyperinsulinemic-euglycemic clamp revealed that the glucose infusion rate was 13% higher in iNOS-null young mice compared with wild-type young mice (Fig. 1D). In old mice, compared with the respective young group, there was a significant reduction in the insulin sensitivity of control mice compared with iNOS-null mice (Fig. 1D). We observed a strong reduction in the glucose infusion rate in wild-type mice compared with iNOS-null mice after the aging period (Fig. 1E). The reduction in the glucose infusion rate was ∼61% in wild-type mice and ∼31% in iNOS-null mice. Protection against the development of insulin resistance in iNOS-null mice has also been observed in previous studies involving obesity models (12,13).
FIG. 1.
Insulin sensitivity in wild-type (WT) and iNOS-null male mice after aging. Total body weight (BW) (A), epididymal fat weight (B), and fasting insulin (8-h fast) (C). Hyperinsulinemic-euglycemic clamp glucose infusion rate (GIR) (D) and variation in the GIR (E). F: Glucose uptake in the isolated soleus muscle after insulin incubation. G: GIR values are expressed as micromoles per gram of muscle variation. The bars represent the mean ± SEM of 8–12 mice. One-way ANOVA was used in A–D and F. Student t test was used in E and G. *P < 0.05 vs. the respective young group. §P < 0.05, young iNOS-null vs. young wild-type. #P < 0.05, vs. wild-type.
In addition, we observed that young iNOS-null mice showed higher (15%) insulin-induced glucose uptake in the soleus muscle compared with young wild-type mice (Fig. 1F). In the aged groups, both groups demonstrated impairment in the action of insulin in muscle compared with the respective younger groups (Fig. 1F). However, the variation in the reduction in insulin-induced glucose uptake in the skeletal muscle after aging was more pronounced in wild-type mice than in iNOS-null animals (Fig. 1G). The reduction in the glucose uptake was ∼44% in wild-type mice and ∼21% in iNOS-null mice. In aged animals, iNOS-null mice presented a higher gastrocnemius muscle weight than wild-type mice (169 ± 8.1 vs. 148.2 ± 7.4 mg, P < 0.05).
Western blot analysis showed that aging increased iNOS expression by ∼180% in the skeletal muscle of wild-type mice (Fig. 2A). We also evaluated the expression of the other NOS isoforms and inflammatory markers. Aging reduced endothelial NOS and neuronal NOS expression and increased inhibitor of nuclear factor κ-B (IKKβ) phosphorylation and tumor necrosis factor (TNF)-α expression in wild-type and iNOS-null animals in a similar fashion (Fig. 2A and B). We observed that insulin induced a 10–15% increase in IRβ, IRS-1, and Akt phosphorylation in the muscle of young iNOS-null mice compared with young wild-type animals (Fig. 2C–E). Wild-type mice presented reductions in IRβ, IRS-1, and Akt phosphorylation (43, 71, and 48%, respectively) after aging, whereas the impairment in insulin signaling in the skeletal muscle of iNOS-null mice was relatively minor (18, 22 and 16%, respectively; Fig. 2C–E). In parallel, aging increased IRβ, IRS-1, and Akt S-nitrosation in the muscle of wild-type but not in iNOS-null mice (Fig. 2F and G). In this genetic approach, we demonstrated that iNOS modulates IRβ, IRS-1, and Akt S-nitrosation and insulin sensitivity in the muscle of aged mice, without affecting other NOS isoforms and inflammatory signaling.
FIG. 2.
Insulin signaling and S-nitrosation of IR, IRS-1, and Akt in the skeletal muscle of wild-type and iNOS-null male mice after aging. A: Western immunoblot (IB) analysis was performed to evaluate iNOS, eNOS, and nNOS expression. B: IKKβ (Ser 180) phosphorylation and TNF-α expression. IRβ (Tyr 1162/1163) (C), IRS-1 (Tyr 971) (D), and Akt (Ser 473) (E) phosphorylation in the gastrocnemius muscle. The biotin switch method was used to determine IRβ (F), IRS-1 (G), and Akt S-nitrosation (H) in the gastrocnemius muscle. I: Glucose uptake in isolated soleus muscle inoculated with GSNO (0, 1, 5 and 10 mmol/L) during 30 min in the presence or absence of insulin (10 mUI/mL). J: IRβ (Tyr 1162/1163), IRS-1 (Tyr 971), and Akt (Ser 473) phosphorylation in the isolated soleus muscle in the presence or absence of GSNO (10 mmol/L). IRβ (K), IRS-1 (L), and Akt S-nitrosation (M) in the isolated soleus muscle incubated with GSNO (10 mmol/L). The results of scanning densitometry are expressed as arbitrary units (AU). Bars represent the mean ± SEM of eight mice. One-way ANOVA was used in C–I. The Student t test was used in K–M. *P < 0.05 vs. the respective young group. §P < 0.05 young iNOS-null vs. young wild-type. &P < 0.05 vs. aged wild-type. ¶P < 0.05 vs. insulin without GSNO. ‖P < 0.05 vs. vehicle.
To determine whether NO leads to insulin resistance, isolated soleus muscle from young wild-type mice were incubated with increasing NO donor, S-nitrosoglutathione (GSNO). We observed that GSNO reduced insulin-induced glucose uptake in a dose-dependent manner (Fig. 2I). In parallel, GSNO reduced the insulin-induced IRβ, IRS-1, and Akt phosphorylation (Fig. 2J) and markedly increased the S-nitrosation of these proteins (Fig. 2K–M).
To extend our hypothesis, we used pharmacological (L-NIL treatment) and physiological (exercise) approaches to reduce iNOS activity and expression, respectively (14). We observed that neither L-NIL treatment nor exercise changed the total body weight, epididymal fat weight, or fasting insulin serum levels in aged mice compared with aged mice treated with vehicle (Fig. 3A–C). However, the clamp procedure revealed that L-NIL treatment and exercise were able to increase insulin sensitivity in aged mice compared with aged mice treated with vehicle (Fig. 3D). In addition, L-NIL treatment and exercise significantly increased insulin-stimulated glucose uptake in the soleus muscle in old mice (Fig. 3E).
FIG. 3.
Pharmacological and physiological iNOS inhibition improves insulin sensitivity in aged mice. Total body weight (A), epididymal fat weight (B), and fasting insulin (8-h fast) (C) were determined 1 h after the last L-NIL injection or 8 h after the exercise protocol D: Hyperinsulinemic-euglycemic clamp. E: Glucose uptake in the isolated soleus muscle after insulin incubation is expressed as micromoles per gram of muscle. One-way ANOVA was used. Bars represent the mean ± SEM of 8–12 mice. *P < 0.05 vs. young wild-type (WT). #P < 0.05 vs. old mice without L-NIL or exercise.
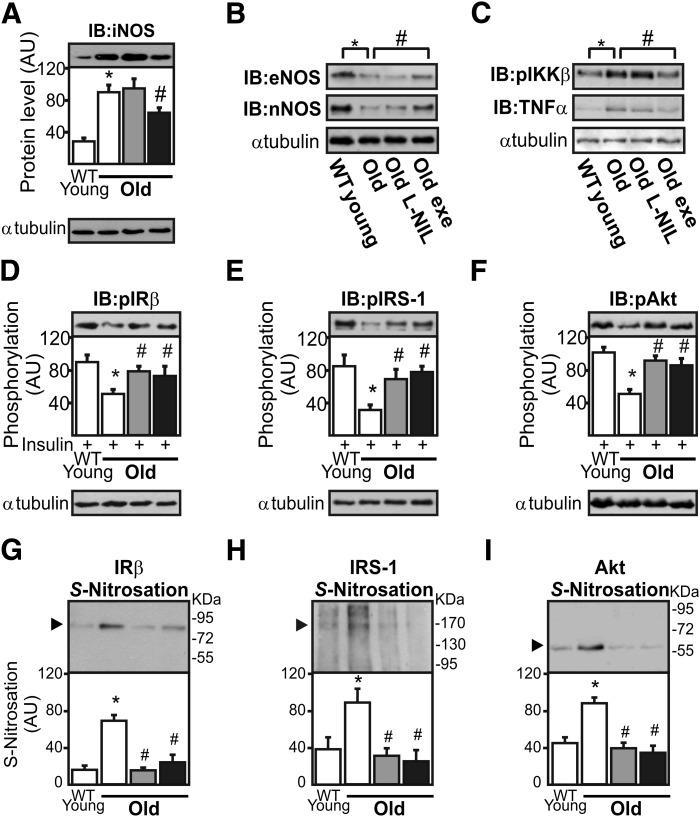
We observed that exercise was able to reduce iNOS and increase endothelial NOS and neuronal NOS expression in the skeletal muscle of aged mice, whereas L-NIL treatment did not change the expression of these enzymes (Fig. 4A and B). In parallel, we observed that L-NIL did not change IKKβ phosphorylation and TNF-α expression, whereas exercise decreased IKKβ phosphorylation and TNF-α expression (Fig. 4C). L-NIL treatment and exercise increase insulin-induced IRβ, IRS-1, and Akt phosphorylation to a similar degree (by ∼45, 130, and 70%, respectively) compared with aged wild-type animals (Fig. 4D–F). In addition, L-NIL treatment and physical exercise reduced age-induced IRβ, IRS-1, and Akt S-nitrosation in the skeletal muscle of aged wild-type mice (Fig. 4G–I).
FIG. 4.
Pharmacological and physiological iNOS inhibition improves insulin signaling and reduces S-nitrosation of IRβ, IRS-1, and Akt in the skeletal muscle of aged mice. iNOS expression (A), endothelial eNOS and neuronal nNOS expression (B), and IKKβ (Ser 180) phosphorylation and TNFα expression (C) were determined 1 h after the last L-NIL injection or 8 h after the exercise protocol by immunoblotting (IB). IRβ (Tyr 1162/1163) (D), IRS-1 (E), and Akt (Ser 473) (F) phosphorylation in the gastrocnemius muscle. The biotin switch method was used to determine IRβ (G), IRS-1 (H), and Akt (I) S-nitrosation in the muscle. The results of scanning densitometry are expressed as arbitrary units (AU). White bars indicate control group; grey bars indicate old mice plus L-NIL treatment; and black bars indicate old mice plus exercise. Bars represent the mean ± SEM of eight mice. One-way ANOVA was used. *P < 0.05 vs. young wild-type (WT). #P < 0.05 vs. aged iNOS-null.
DISCUSSION
Here we demonstrated that aging increased iNOS expression, leading to insulin resistance in the skeletal muscle through the S-nitrosation mechanism. Genetic, pharmacological, and physiological iNOS inhibition reduced IRβ, IRS-1, and Akt S-nitrosation and increased insulin signaling in old mice. Our results reinforce the idea that iNOS plays a crucial role in inducing insulin resistance, as previously shown in different animal models such as diet-, lipopolysaccharide-, and lipid-induced skeletal muscle insulin resistance (3,13,15,16).
In contrast to our data, Cha et al. (17) reported that iNOS deficiency does not prevent age-associated insulin resistance. In contrast to the previous study, we did not observe an increase in TNF-α expression in the skeletal muscle of aged iNOS-null mice compared with the aged wild-type group; these data were confirmed by analyzing IKKβ phosphorylation. In accordance with our data, a previous study showed iNOS knockout mice were protected from insulin resistance induced by a high-fat diet (13). Furthermore, iNOS-null mice fed a high-fat diet do not present increased TNF-α levels in the muscle and are protected against lipopolysaccharide-induced S-nitrosation of IRβ/IRS-1 and Akt (12,15).
Accumulating evidence has demonstrated that S-nitrosation negatively modulates insulin signaling (3,5). Our data reveal that high levels of iNOS expression were accompanied by IRβ, IRS-1, and Akt S-nitrosation and insulin resistance in aged male mice. In accordance with our results in male mice, high levels of iNOS expression and IR and Akt S-nitrosation were reported in the liver and adipose tissue of aged female rodents. Altogether, these data suggest that this phenomenon may also occur in female mice (18,19). Wu et al. (20) reported that Akt S-nitrosation was associated with a reduction in Akt activity and with skeletal muscle disorders in aged mice. Although the S-nitrosation of Akt at cysteine 224 leads to inactivation of this kinase, the reactive cysteine residue(s) that undergo(es) S-nitrosation in IR and IRS-1 are unknown and deserve further investigation.
Pharmacological iNOS inhibition has been investigated in animal models of insulin resistance and diabetes. In accordance with the results obtained from aged mice, L-NIL restored insulin sensitivity involving the S-nitrosation mechanism in the muscle of obese rats (3). Beyond the skeletal muscle, high levels of iNOS expression were also reported in the liver of ob/ob mice, whereas L-NIL treatment was sufficient to enhance insulin-induced IRS-1- and IRS-2 phosphorylation (21). Moreover, aspirin treatment improved insulin signaling in the muscle of obese rats by reducing iNOS activity (12). Interestingly, a nonacetylated salicylate treatment, salsalate, also improved glycemic control in diabetic patients in parallel with reductions in the inflammatory response, including reduced levels of serum nitrite, which at least in part may be secondary to reduced iNOS activation (22).
In the current study, we also demonstrated that after a single bout of exercise, iNOS expression and IRβ, IRS-1, and Akt S-nitrosation were diminished; conversely, insulin sensitivity was increased in the skeletal muscle of old mice. These data are in accordance with previous results observed in obese exercised rats (14). Therefore, beyond the pharmacological and genetic approach, the physiological reduction of iNOS levels induced by exercise reversed the deregulation of insulin signaling and insulin resistance observed in old mice.
Beyond S-nitrosation, NO metabolites can also induce tyrosine nitration (i.e., the covalent addition of NO2 to the tyrosine residues of proteins) (23). It has been demonstrated that tyrosine nitration reduces tyrosine phosphorylation and the activation of downstream insulin-signaling intermediates (24). Serine phosphorylation of IRSs mediated by proinflammatory stimuli and increased protein tyrosine phosphatase 1B (PTP1B) activity have been reported as central molecular mechanisms involved in the development of insulin resistance with aging (25). Thus, aging elicits all of these mechanisms, which converge to cause insulin-signaling disruption.
Collectively, our study provides evidence that the age-related increase in muscle iNOS expression and activity is an important contributing factor to the S-nitrosation of insulin signaling proteins and insulin resistance in the skeletal muscle of aged rodents.
ACKNOWLEDGMENTS
This study was supported by grants from Fundacão de Amparo à Pesquisa do Estado de São Paulo and Conselho Nacional de desenvolvimento científico e tecnológico.
No potential conflicts of interest relevant to this article were reported.
E.R.R. researched data and wrote the manuscript. J.R.P., D.E.C., A.S.d.S., C.T.D.S., D.G., B.M.C., A.M.C., C.K.K., M.A.C.-F., and S.H. researched data. R.C. contributed to discussion. L.A.V. and M.J.A.S. contributed to discussion and reviewed and edited the manuscript. J.B.C.C. contributed to discussion and wrote, reviewed, and edited the manuscript. E.R.R. is the guarantor of this work and, as such, had full access to all the data and takes full responsibility for the integrity of data and the accuracy of data analysis.
The authors thank Dr. Marcelo G. de Oliveira from the Chemistry Institute (UNICAMP) for the discussion of some data regarding NO chemical reactions.
Footnotes
See accompanying commentary, p. 346.
REFERENCES
- 1.Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol 2009;10:721–732 [DOI] [PubMed] [Google Scholar]
- 2.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 2009;15:391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho-Filho MA, Ueno M, Hirabara SM, et al. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes 2005;54:959–967 [DOI] [PubMed] [Google Scholar]
- 4.Sugita H, Fujimoto M, Yasukawa T, et al. Inducible nitric-oxide synthase and NO donor induce insulin receptor substrate-1 degradation in skeletal muscle cells. J Biol Chem 2005;280:14203–14211 [DOI] [PubMed] [Google Scholar]
- 5.Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem 2005;280:7511–7518 [DOI] [PubMed] [Google Scholar]
- 6.Lewinska A, Macierzynska E, Grzelak A, Bartosz G. A genetic analysis of nitric oxide-mediated signaling during chronological aging in the yeast. Biogerontology 2011;12:309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ropelle ER, Flores MB, Cintra DE, et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biol 2010;8:e1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Challiss RA, Lozeman FJ, Leighton B, Newsholme EA. Effects of the beta-adrenoceptor agonist isoprenaline on insulin-sensitivity in soleus muscle of the rat. Biochem J 1986;233:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ropelle ER, Pauli JR, Prada PO, et al. Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation. J Physiol 2006;577:997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001;2001:pl1. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Ruiz A, Lamas S. Detection and proteomic identification of S-nitrosylated proteins in endothelial cells. Arch Biochem Biophys 2004;423:192–199 [DOI] [PubMed] [Google Scholar]
- 12.Carvalho-Filho MA, Ropelle ER, Pauli RJ, et al. Aspirin attenuates insulin resistance in muscle of diet-induced obese rats by inhibiting inducible nitric oxide synthase production and S-nitrosylation of IRbeta/IRS-1 and Akt. Diabetologia 2009;52:2425–2434 [DOI] [PubMed] [Google Scholar]
- 13.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med 2001;7:1138–1143 [DOI] [PubMed] [Google Scholar]
- 14.Pauli JR, Ropelle ER, Cintra DE, et al. Acute physical exercise reverses S-nitrosation of the insulin receptor, insulin receptor substrate 1 and protein kinase B/Akt in diet-induced obese Wistar rats. J Physiol 2008;586:659–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho-Filho MA, Ueno M, Carvalheira JB, Velloso LA, Saad MJ. Targeted disruption of iNOS prevents LPS-induced S-nitrosation of IRbeta/IRS-1 and Akt and insulin resistance in muscle of mice. Am J Physiol Endocrinol Metab 2006;291:E476–E482 [DOI] [PubMed] [Google Scholar]
- 16.Cha HN, Song SE, Kim YW, Kim JY, Won KC, Park SY. Lack of inducible nitric oxide synthase prevents lipid-induced skeletal muscle insulin resistance without attenuating cytokine level. J Pharmacol Sci 2011;117:77–86 [DOI] [PubMed] [Google Scholar]
- 17.Cha HN, Kim YW, Kim JY, et al. Lack of inducible nitric oxide synthase does not prevent aging-associated insulin resistance. Exp Gerontol 2010;45:711–718 [DOI] [PubMed] [Google Scholar]
- 18.Kireev RA, Tresguerres AC, Garcia C, et al. Hormonal regulation of pro-inflammatory and lipid peroxidation processes in liver of old ovariectomized female rats. Biogerontology 2010;11:229–243 [DOI] [PubMed] [Google Scholar]
- 19.Ovadia H, Haim Y, Nov O, et al. Increased adipocyte S-nitrosylation targets anti-lipolytic action of insulin: relevance to adipose tissue dysfunction in obesity. J Biol Chem 2011;286:30433–30443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M, Katta A, Gadde MK, et al. Aging-associated dysfunction of Akt/protein kinase B: S-nitrosylation and acetaminophen intervention. PLoS ONE 2009;4:e6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujimoto M, Shimizu N, Kunii K, Martyn JA, Ueki K, Kaneki M. A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes 2005;54:1340–1348 [DOI] [PubMed] [Google Scholar]
- 22.Goldfine AB, Silver R, Aldhahi W, et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci 2008;1:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun 2003;305:776–783 [DOI] [PubMed] [Google Scholar]
- 24.Charbonneau A, Marette A. Inducible nitric oxide synthase induction underlies lipid-induced hepatic insulin resistance in mice: potential role of tyrosine nitration of insulin signaling proteins. Diabetes 2010;59:861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Rodriguez A, Mas-Gutierrez JA, Mirasierra M, et al. Essential role of protein tyrosine phosphatase 1B in obesity-induced inflammation and peripheral insulin resistance during aging. Aging Cell 2012;11:284–296 [DOI] [PMC free article] [PubMed] [Google Scholar]