Abstract
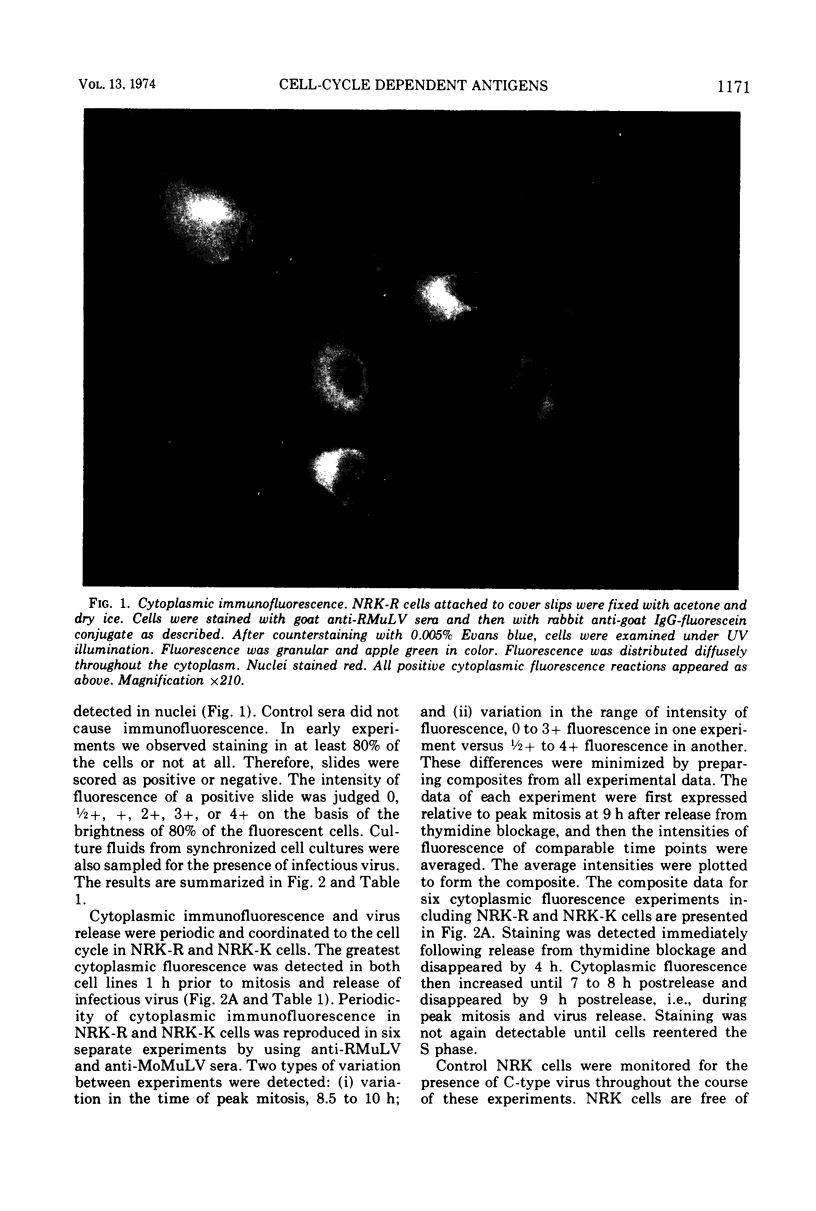
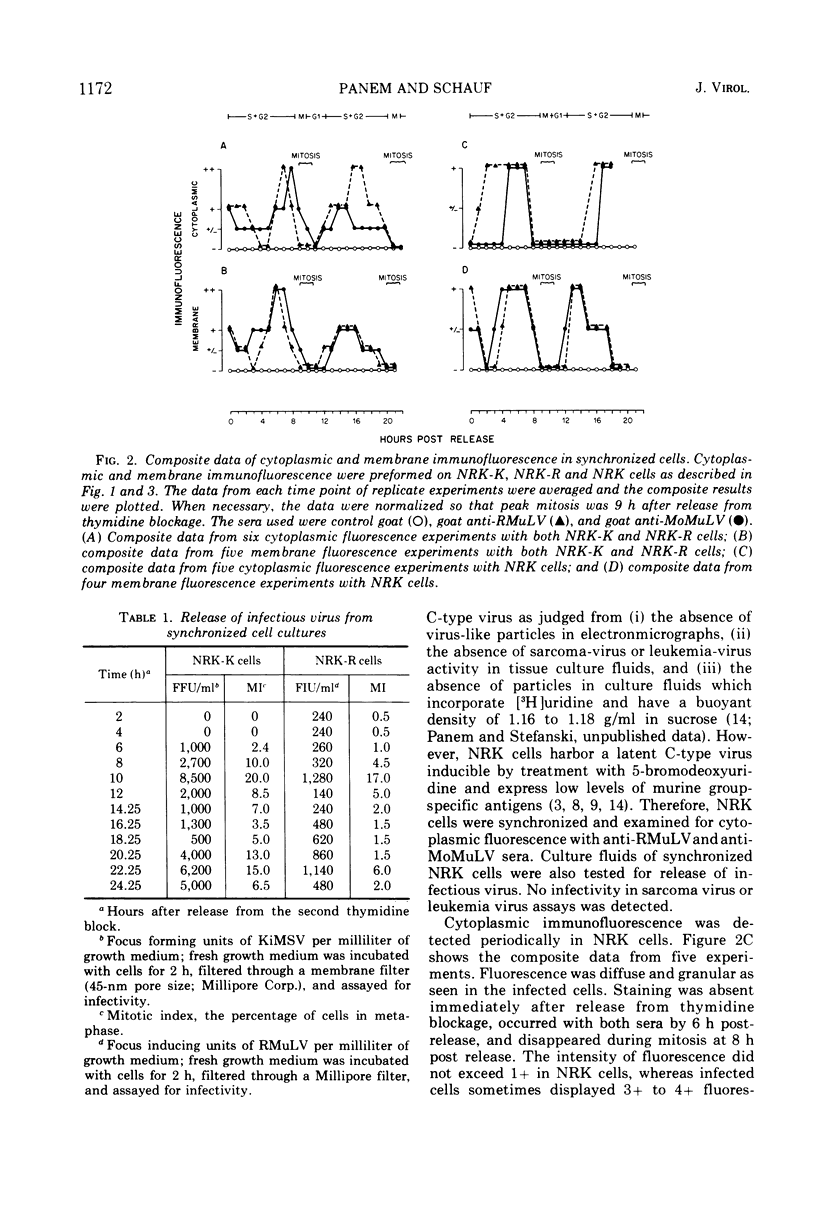
Normal rat kidney (NRK) cells, NRK cells infected with Rauscher murine leukemia virus, and NRK cells infected with Kirsten murine sarcoma-leukemia virus (NRK-K) were synchronized by a double thymidine block. At intervals after release from thymidine blockage, the cells were examined for the presence of viral antigens in the cytoplasm and on the cell surface by immunofluorescent microscopy by using goat anti-Rauscher murine leukemia virus and goat anti-Moloney leukemia virus (Tween-ether disrupted) sera. Detection of viral antigens in the cytoplasm was periodic during the cell cycle. Antigens were detected first during the S phase, increased during the G2 phase, and disappeared during the M and G1 phases. A similar pattern of surface immunofluorescence was observed. Infectious virus was detected in culture fluids from synchronized cells during the M phase. Surface immunofluorescence was detected in NRK-K cells with anti-Rauscher murine leukemia virus and may represent the presence of group-specific antigens on the cell surface. Control, uninfected NRK cells, which did not normally fluoresce, showed weak immunofluorescence during the S and G2 phases after synchronization. Synchronization can be used to amplify latent oncornavirus expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Oroszlan S. Group-specific antigens of RNA tumor viruses as markers for subinfectious expression of the RNA virus genome. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1021–1025. doi: 10.1073/pnas.69.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi A., Hampar B., Hsu K. C., Oroszlan S., Hornberger E., Kelloff G., Gilden R. V. Intracellular localization of mammalian type C virus species-specific (gs-1) and interspecies-specific (gs-3) antigenic determinants with the indirect immunoperoxidase technique and light microscopy. J Immunol. 1973 Jul;111(1):152–156. [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers J., Nowinski R. C., Geering G., Hardy W. Detection of avian and mammalian oncogenic RNA viruses (oncornaviruses) by immunofluorescence. Cancer Res. 1972 Jan;32(1):98–106. [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Gilden R. V., Oroszlan S., Sarma P. S., Rongey R. W., Gardner M. B. Rat C-type virus induced in rat sarcoma cells by 5-bromodeoxyuridine. Nat New Biol. 1972 Aug 23;238(86):234–237. doi: 10.1038/newbio238234a0. [DOI] [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Huebner R. J. Rescue of the genome of focus forming virus from rat non-productive lines by 5'-bromodeoxyruidine. Nat New Biol. 1971 Nov 3;234(44):12–14. doi: 10.1038/newbio234012a0. [DOI] [PubMed] [Google Scholar]
- Nadler H. L., Gerbie A. B. Role of amniocentesis in the intrauterine detection of genetic disorders. N Engl J Med. 1970 Mar 12;282(11):596–599. doi: 10.1056/NEJM197003122821105. [DOI] [PubMed] [Google Scholar]
- PETERSEN D. F., ANDERSON E. C. QUANTITY PRODUCTION OF SYNCHRONIZED MAMMALIAN CELLS IN SUSPENSION CULTURE. Nature. 1964 Aug 8;203:642–643. doi: 10.1038/203642a0. [DOI] [PubMed] [Google Scholar]
- Panem S., Kirsten W. H. Cell-associated virions and nucleoids of a murine leukemia virus. J Natl Cancer Inst. 1973 Sep;51(3):865–874. doi: 10.1093/jnci/51.3.865. [DOI] [PubMed] [Google Scholar]
- Panem S., Kirsten W. H. Release of mouse leukemia-sarcoma virus from synchronized cells. J Natl Cancer Inst. 1973 Feb;50(2):563–565. doi: 10.1093/jnci/50.2.563. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Livingston D. M., Todaro G. J., Benveniste R. E., Scolnick E. M. Radioimmunoassay of mammalian type C viral proteins. 3. Detection of viral antigen in normal murine cells and tissues. J Exp Med. 1973 Mar 1;137(3):622–635. doi: 10.1084/jem.137.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ J., DEINHARDT F. Preparation of a semipermanent mounting medium for fluorescent antibody studies. Virology. 1960 Oct;12:316–317. doi: 10.1016/0042-6822(60)90205-1. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Wilsnack R. E., Aaronson S. A. Radioimmunoassay for avian C-type virus group-specific antigen: detection in normal and virus-transformed cells. J Virol. 1973 Jun;11(6):893–899. doi: 10.1128/jvi.11.6.893-899.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]
- Yoshiki T., Mellors R. C., Hardy W. D., Jr Common cell-surface antigen associated with murine and feline C-type RNA leukemia viruses. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1878–1882. doi: 10.1073/pnas.70.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]