Abstract
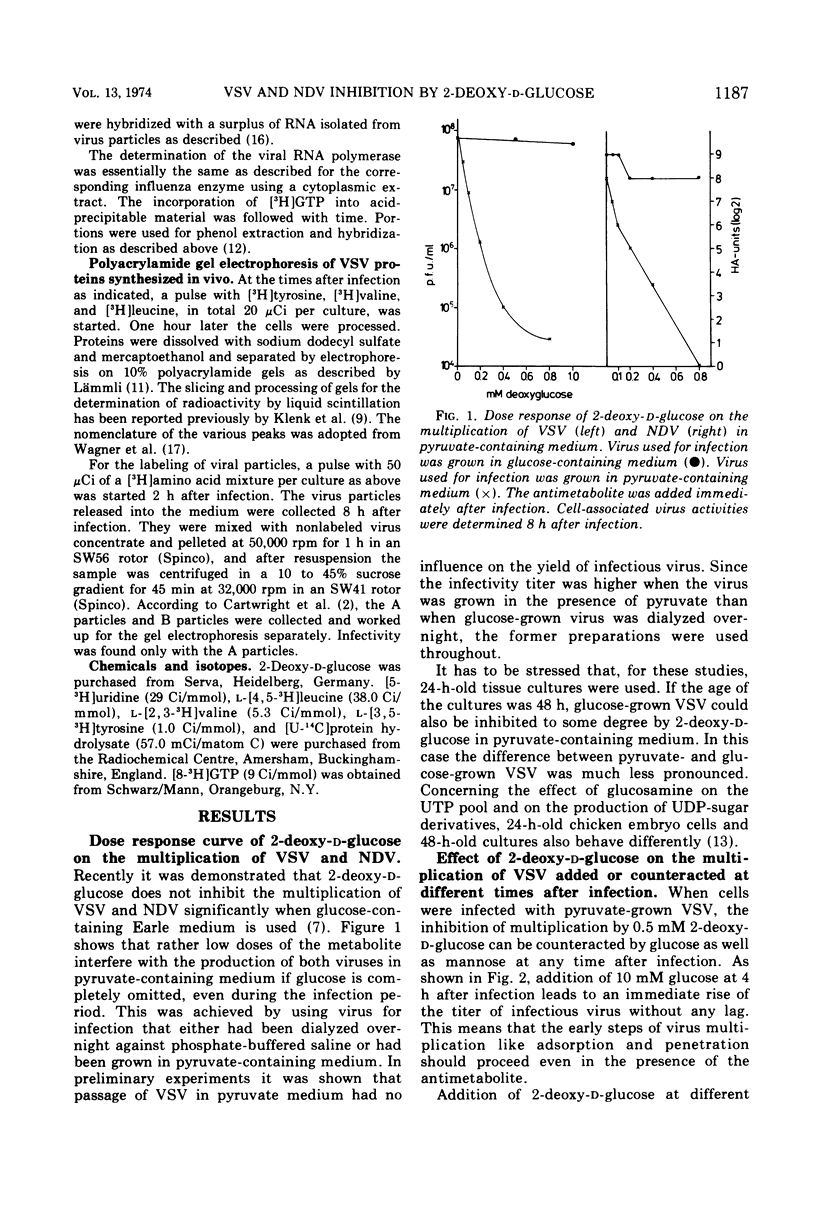
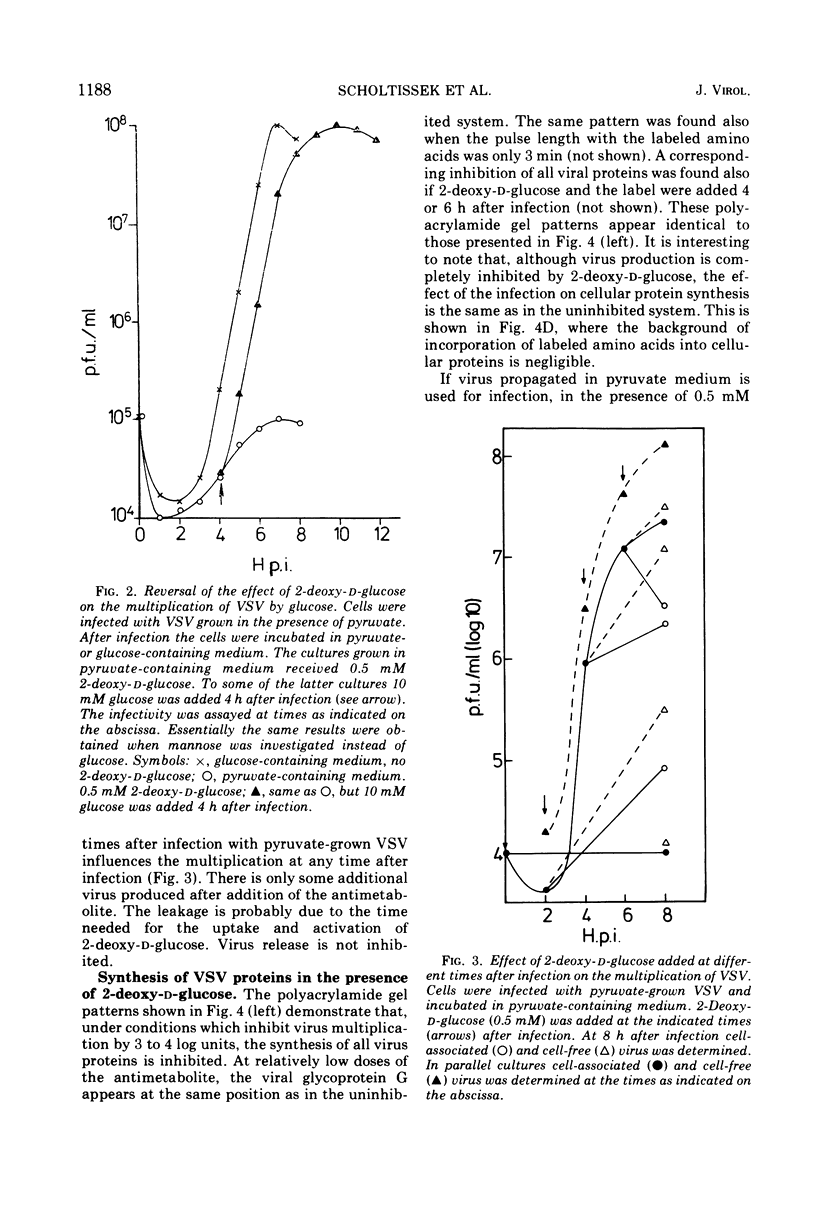
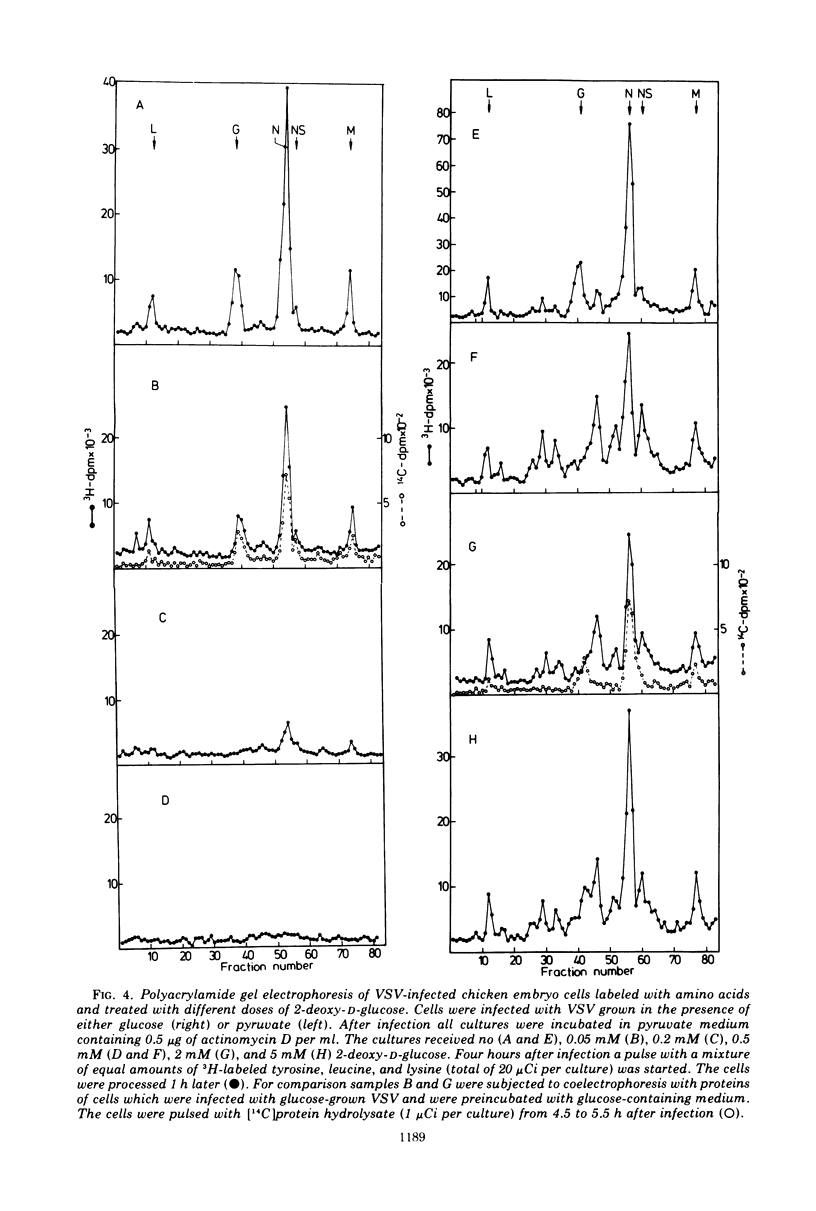
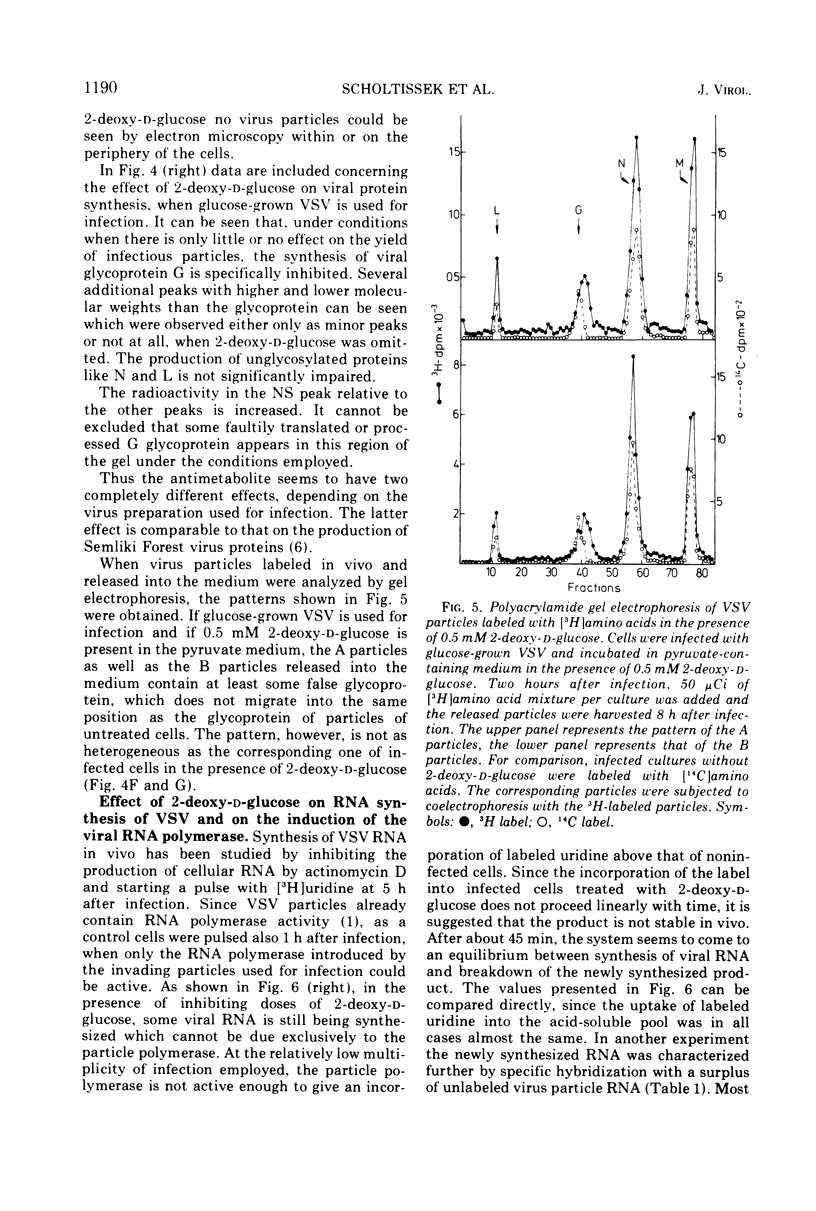
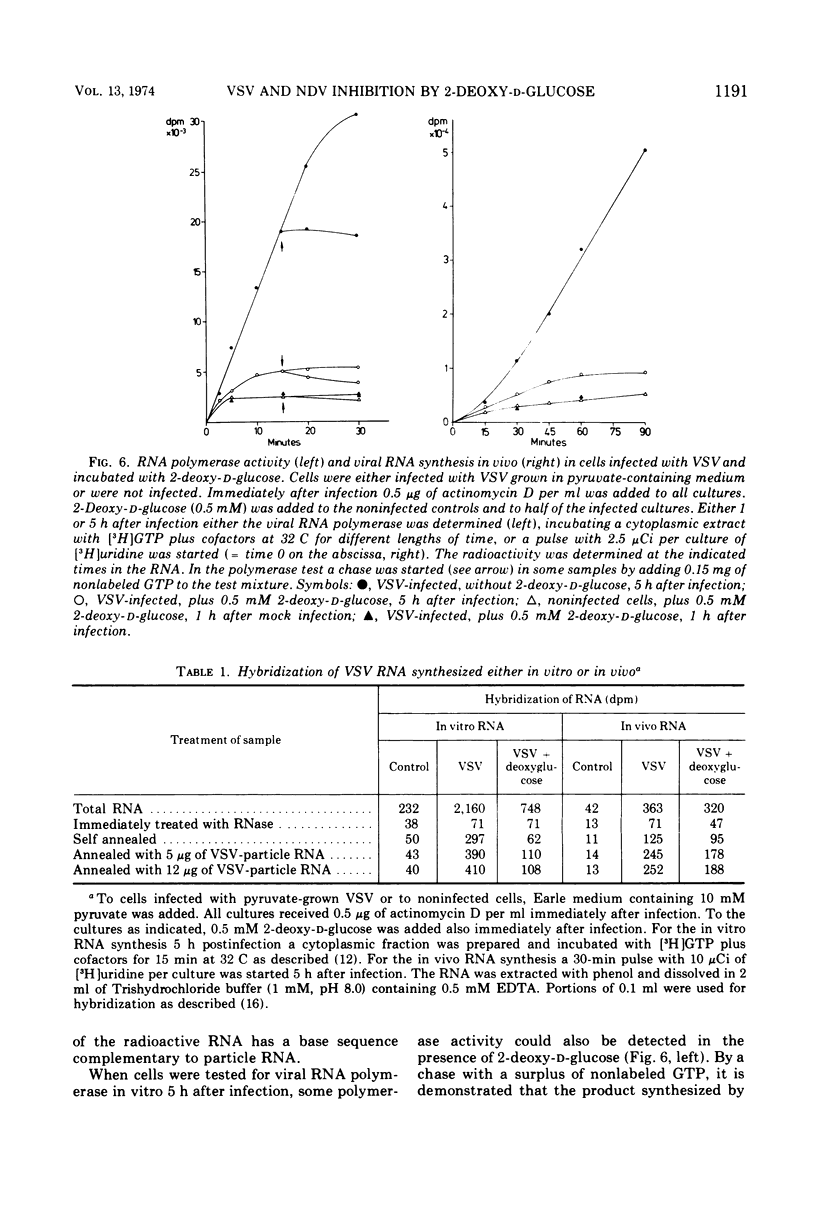
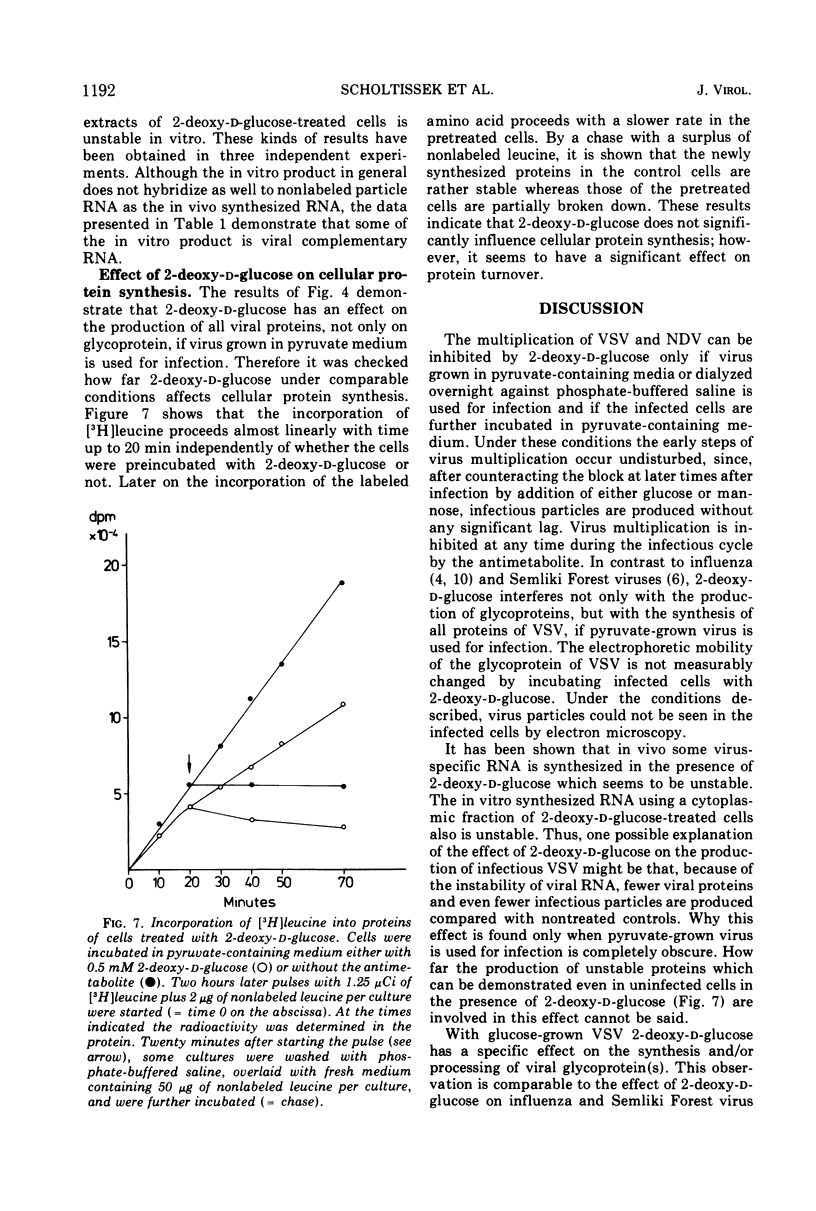
The production of infectious vesicular stomatitis (VSV) and Newcastle disease virus can be completely inhibited by 2-deoxy-d-glucose in pyruvate-containing medium, if virus either grown in pyruvate-containing medium or dialyzed against phosphate-buffered saline is used for infection. Under these conditions, the synthesis of all VSV proteins is reduced. VSV RNA, which is synthesized at reduced rates, seems to be unstable. The effect is completely reversible. If virus grown in glucose-containing medium is used for infection, the production of both viruses is not significantly inhibited by 2-deoxy-d-glucose. Under these conditions the production of the VSV glycoprotein is specifically impaired, but does not lead to a marked reduction of the yield of infectious virus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J., Steiner S. M., Benyesh-Melnick M. Effects of 2-deoxy-D-glucose on herpes simplex virus replication. Virology. 1973 Apr;52(2):447–455. doi: 10.1016/0042-6822(73)90340-1. [DOI] [PubMed] [Google Scholar]
- Gandhi S. S., Stanley P., Taylor J. M., White D. O. Inhibition of influenza viral glycoprotein synthesis by sugars. Microbios. 1972 Jan;5(17):41–50. [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- KILBOURNE E. D. Inhibition of influenza virus multiplication with a glucose antimetabolite (2-deoxy-D-glucose). Nature. 1959 Jan 24;183(4656):271–272. doi: 10.1038/183271b0. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Schmidt M. F., Scholtissek C. Effect of 2-deoxy-D-glucose on the multiplication of Semliki Forest virus and the reversal of the block by mannose. Virology. 1973 Jul;54(1):179–189. doi: 10.1016/0042-6822(73)90127-x. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Scholtissek C., Rott R. Inhibition of the multiplication of enveloped RNA-viruses by glucosamine and 2-deoxy-D-glucose. J Gen Virol. 1972 Mar;14(3):251–259. doi: 10.1099/0022-1317-14-3-251. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Caliguiri L. A., Choppin P. W. The proteins of the parainfluenza virus SV5. II. The carbohydrate content and glycoproteins of the virion. Virology. 1970 Oct;42(2):473–481. doi: 10.1016/0042-6822(70)90290-4. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Scholtissek C., Rott R. Inhibition of glycoprotein biosynthesis of influenza virus by D-glucosamine and 2-deoxy-D-glucose. Virology. 1972 Sep;49(3):723–734. doi: 10.1016/0042-6822(72)90529-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. Detection of an unstable RNA in chick fibroblasts after reduction of the UTP pool by glucosamine. Eur J Biochem. 1971 Dec;24(2):358–365. doi: 10.1111/j.1432-1033.1971.tb19694.x. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Effect of temperature on the multiplication of an Influenza virus. J Gen Virol. 1969 Sep;5(2):283–290. doi: 10.1099/0022-1317-5-2-283. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Synthesis in vivo of influenza virus plus and minus strand RNA and its preferential inhibition by antibiotics. Virology. 1970 Apr;40(4):989–996. doi: 10.1016/0042-6822(70)90145-5. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. Synthesis in vitro of RNA complementary to parental viral RNA by RNA polymerase induced by influenza virus. Biochim Biophys Acta. 1969 Apr 22;179(2):389–397. doi: 10.1016/0005-2787(69)90047-1. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



