Type 2 diabetes (T2DM) is characterized by hyperglycemia, and the underlying pathophysiology includes insulin resistance and pancreatic β-cell failure. The precise cellular and molecular regulators of insulin resistance and impaired insulin secretion are under intense investigation, but a unifying theory remains elusive. The role of lipids in insulin resistance and β-cell failure has been the subject of considerable empirical investigation, and intermediates of lipid metabolism including diacylglycerols, long-chain fatty acyl-CoAs, and ceramides have all been examined to varying degrees and with varying levels of success. There is good evidence that diacylglycerols and fatty acyl-CoAs directly contribute to dysregulation of cellular insulin signaling through incomplete fatty acid oxidation and/or phosphorylation/dephosphorylation of specific serine and threonine sites on insulin receptor substrate-1 (1,2). Evidence regarding the role of ceramides in insulin resistance has been less consistent. Ceramides are members of the sphingolipid family of lipids and are integral to the structure of the lipid bilayer that makes up all cell membranes (3). They also exert biological effects through cellular proliferation, differentiation, and cell death and interact with several pathways involved in insulin resistance, oxidative stress, inflammation, and apoptosis, all of which are linked to T2DM (4–6). Several lines of evidence suggest that the liver is the major source of plasma ceramides in animals and humans (7,8). In a hamster model, de novo synthesis of ceramides in the liver is induced in response to stress and inflammation, and this is paralleled by the increased appearance of ceramides in circulating lipoproteins (9). Further, Wiesner et al. (10) have performed a very detailed lipid species analysis of lipoprotein fractions in which they found that LDL and VLDL are the main ceramide carriers in plasma. However, knowledge of the role of ceramides in the pathogenesis of T2DM is limited, due in part to their ubiquitous nature, low concentrations in tissue and plasma, and the complexity associated with quantification of the wide range of ceramide species found in biological samples.
Emerging data now support a regulatory role for ceramides in glucose homeostasis and even glucose-stimulated insulin secretion. New data in this current issue of Diabetes add to the momentum and move the field forward in a substantial way. The studies described by Boon et al. (11) are elegant and extensive and provide numerous new insights into the role of plasma ceramides using in vivo and novel in vitro approaches. The work is focused on ceramides complexed to LDL, i.e., LDL-ceramide, and for the most part examines the role of circulating C24 ceramide, one of the most abundant of the ceramide subspecies. First, clinical data are presented to show that plasma LDL-ceramide is elevated in patients with T2DM compared with lean control subjects, and these elevated levels are inversely correlated with insulin sensitivity assessed by homeostasis model assessment of insulin resistance. Although these observations alone are not unique (12), the data are important in establishing the conditions for subsequent experiments in mouse and cell models that show how circulating LDL-ceramide specifically targets skeletal muscle and induces insulin resistance.
Ceramide secretion from myocytes, 3T3-L1 adipocytes, and hepatocytes isolated from mice fed a high- or low-fat diet revealed increased ceramide secretion specifically in the cultured hepatocytes from the obese mice, supporting the view that liver is the primary source of circulating ceramide. In order to demonstrate that LDL-ceramide did indeed cause insulin resistance, the investigators cleverly reconstituted an LDL-C24:0 ceramide complex using a previously established procedure in which ceramide was dissolved in a human LDL and potato starch mix and then extracted by polar hydration (13). When this LDL-C24:0 ceramide was infused into lean mice, the mice became insulin resistant and exhibited impaired skeletal muscle insulin signaling through Akt and reduced insulin-mediated glucose uptake. It is noteworthy that the infused ceramide did not accumulate in the muscle in vivo, but instead appeared to remain for the most part in the muscle plasma membrane. In contrast, LDL-ceramide did accumulate in C2C12 myotubes; the effect was independent of de novo synthesis, and cellular uptake did not appear to occur through the LDL receptor. One of the limitations of this study is that there is no good explanation for the internalization process in vitro, and the absence of LDL-ceramide accumulation in muscle is inconsistent with other published data showing increased ceramide in skeletal muscle in obesity and T2DM (14–16). Overall however, the authors do provide novel and substantive evidence that plasma ceramides can induce insulin resistance in skeletal muscle via downregulation of insulin signaling, primarily through Akt (Fig. 1).
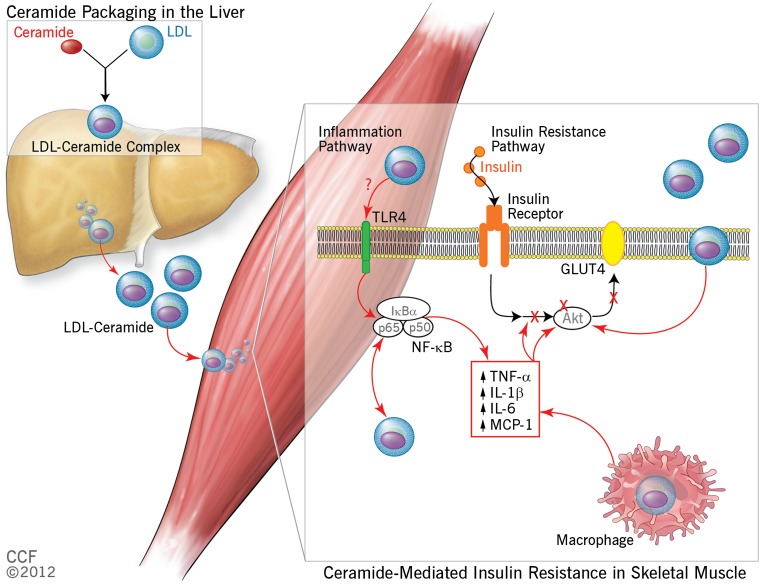
FIG. 1.
Schematic view of the proposed role of plasma ceramide in the development of skeletal muscle insulin resistance. In this model, ceramides are packaged with LDL in the liver and released into the circulation where they target skeletal muscle in two specific ways. First, LDL-ceramide is internalized in the plasma membrane where it downregulates Akt signaling and subsequent insulin-mediated glucose uptake by the tissue, leading ultimately to hyperglycemia and T2DM. Second, LDL-ceramides activate nuclear factor-κB and initiate increased cytokine production. These cytokines also target insulin signaling and impair glucose uptake, further exacerbating hyperglycemia and the likelihood of developing diabetes. IκBα, nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor, α; IL-1β, interleukin-1β; IL-6, interleukin-6; MCP-1, monocyte chemotactic protein-1; NF-κB, nuclear factor-κB; TLR4, toll-like receptor-4; TNF-α, tumor necrosis factor-α. Reprinted with permission from the Cleveland Clinic Foundation (CCF).
This study also provides important data linking plasma ceramides with macrophage-induced inflammation and insulin resistance. Previous studies have shown that inflammatory cytokines, specifically tumor necrosis factor-α, correlate with several plasma ceramide subspecies including C24:0 ceramide (12,17). Further, tumor necrosis factor-α is a primary mediator in the inflammation-diabetes hypothesis (18). The correlation between tumor necrosis factor-α and ceramide was confirmed in the current study, and the authors went a step further to show that LDL-ceramide infusion could increase plasma cytokines in mice. Although this effect was not statistically significant, subsequent isolated cell studies revealed that LDL-ceramide activated nuclear factor-κB signaling and initiated proinflammatory gene expression in RAW264.7 macrophages. Further, these macrophages accumulated LDL-ceramide intracellularly suggesting that they could act as a ceramide sink, which might have important biological relevance for skeletal muscle in obese and T2DM patients.
This extensive body of work by Boon et al. is timely, especially given the recent interest in oxidized LDL and cardiovascular disease (19–21). Data reported in this article substantially increase our understanding of ceramides and their role in diabetes and metabolism. The authors have opened a new door in the house that is insulin resistance, and in so doing have discovered several important clues that can help to explain the complex interaction that links lipids and diabetes. Although there are many more doors that remain to be opened, these findings have both diagnostic and therapeutic implications for the treatment of T2DM.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 401.
REFERENCES
- 1.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 2.Rui L, Aguirre V, Kim JK, et al. Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest 2001;107:181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008;9:139–150 [DOI] [PubMed] [Google Scholar]
- 4.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab 2012;15:585–594 [DOI] [PubMed] [Google Scholar]
- 5.Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 2007;5:167–179 [DOI] [PubMed] [Google Scholar]
- 6.Pagadala M, Kasumov T, McCullough AJ, Zein NN, Kirwan JP. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol Metab 2012;23:365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lightle S, Tosheva R, Lee A, et al. Elevation of ceramide in serum lipoproteins during acute phase response in humans and mice: role of serine-palmitoyl transferase. Arch Biochem Biophys 2003;419:120–128 [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi M, Miyashita Y, Kumagai Y, Kojo S. Change in liver and plasma ceramides during D-galactosamine-induced acute hepatic injury by LC-MS/MS. Bioorg Med Chem Lett 2004;14:4061–4064 [DOI] [PubMed] [Google Scholar]
- 9.Memon RA, Holleran WM, Moser AH, et al. Endotoxin and cytokines increase hepatic sphingolipid biosynthesis and produce lipoproteins enriched in ceramides and sphingomyelin. Arterioscler Thromb Vasc Biol 1998;18:1257–1265 [DOI] [PubMed] [Google Scholar]
- 10.Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res 2009;50:574–585 [DOI] [PubMed] [Google Scholar]
- 11.Boon J, Hoy AJ, Stark R, et al. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 2013;62:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haus JM, Kashyap SR, Kasumov T, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009;58:337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieger M, Brown MS, Faust JR, Goldstein JL. Replacement of endogenous cholesteryl esters of low density lipoprotein with exogenous cholesteryl linoleate. Reconstitution of a biologically active lipoprotein particle. J Biol Chem 1978;253:4093–4101 [PubMed] [Google Scholar]
- 14.Adams JM, 2nd, Pratipanawatr T, Berria R, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 2004;53:25–31 [DOI] [PubMed] [Google Scholar]
- 15.Straczkowski M, Kowalska I, Baranowski M, et al. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia 2007;50:2366–2373 [DOI] [PubMed] [Google Scholar]
- 16.Coen PM, Dubé JJ, Amati F, et al. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 2010;59:80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H, Kasumov T, Gatmaitan P, et al. Gastric bypass surgery reduces plasma ceramide subspecies and improves insulin sensitivity in severely obese patients. Obesity (Silver Spring) 2011;19:2235–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor α: a key component of the obesity-diabetes link. Diabetes 1994;43:1271–1278 [DOI] [PubMed] [Google Scholar]
- 19.Ishigaki Y, Oka Y, Katagiri H. Circulating oxidized LDL: a biomarker and a pathogenic factor. Curr Opin Lipidol 2009;20:363–369 [DOI] [PubMed] [Google Scholar]
- 20.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev 2004;84:1381–1478 [DOI] [PubMed] [Google Scholar]
- 21.Podrez EA, Febbraio M, Sheibani N, et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest 2000;105:1095–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]