Abstract
Receptor for advanced glycation end products (RAGE) has been shown to be involved in adiposity as well as atherosclerosis even in nondiabetic conditions. In this study, we examined mechanisms underlying how RAGE regulates adiposity and insulin sensitivity. RAGE overexpression in 3T3-L1 preadipocytes using adenoviral gene transfer accelerated adipocyte hypertrophy, whereas inhibitions of RAGE by small interfering RNA significantly decrease adipocyte hypertrophy. Furthermore, double knockdown of high mobility group box-1 and S100b, both of which are RAGE ligands endogenously expressed in 3T3-L1 cells, also canceled RAGE-medicated adipocyte hypertrophy, implicating a fundamental role of ligands–RAGE ligation. Adipocyte hypertrophy induced by RAGE overexpression is associated with suppression of glucose transporter type 4 and adiponectin mRNA expression, attenuated insulin-stimulated glucose uptake, and insulin-stimulated signaling. Toll-like receptor (Tlr)2 mRNA, but not Tlr4 mRNA, is rapidly upregulated by RAGE overexpression, and inhibition of Tlr2 almost completely abrogates RAGE-mediated adipocyte hypertrophy. Finally, RAGE−/− mice exhibited significantly less body weight, epididymal fat weight, epididymal adipocyte size, higher serum adiponectin levels, and higher insulin sensitivity than wild-type mice. RAGE deficiency is associated with early suppression of Tlr2 mRNA expression in adipose tissues. Thus, RAGE appears to be involved in mouse adipocyte hypertrophy and insulin sensitivity, whereas Tlr2 regulation may partly play a role.
Receptor for advanced glycation end products (RAGE), a pattern recognition receptor, is a multiligand cell-surface protein that was isolated from bovine lung in 1992 by the group of Schmidt and colleagues (1,2). RAGE is initially identified as a receptor for Nε-carboxymethyllysine (3), a major AGE in vivo, and has been implicated in both micro- and macro-diabetic vascular complications (4–7). Of note, RAGE also interacts with other endogenous nonglycated peptide ligands including S100 (8), high mobility group box-1 (HMGB1) (9,10), amyloid fibrils (11), and a leukocyte integrin, Mac-1 (12), many of which are important inflammatory regulators. Thus, potential significance in inflammation suggests that RAGE is not only involved in AGE-deteriorating action in diabetes, but also plays a central role in regulation of inflammation, which is now recognized as a core piece in the pathophysiology of obesity and metabolic syndrome (13,14).
It is becoming apparent that RAGE is involved in atherogenesis even in nondiabetic conditions in a mouse model of atherosclerosis, apolipoprotein E (apoE)−/− mice (7,15). We have recently shown that RAGE acceleration of atherogenesis is associated with increased adiposity in nondiabetic apoE−/− mice (16). Moreover, we have shown in human clinical studies that obesity is one of the strongest predictors of circulating endogenous secretory RAGE, an alternatively spliced form of RAGE, both in diabetic and nondiabetic conditions (17,18). Combined with the potential role of RAGE in inflammation, we sought to examine mechanisms underlying how RAGE can regulate adiposity using an in vitro mouse adipogenesis system.
RESEARCH DESIGN AND METHODS
3T3-L1 cell culture and differentiation.
3T3-L1 preadipocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Mediatech, Herndon, VA) supplemented with 10% FBS. Two days after reaching confluency (day 0), differentiation was initiated by the addition of DMEM containing 10% FBS, 1 μmol/L insulin (Sigma-Aldrich), 0.5 mmol/L isobutylmethyl xanthine (Sigma-Aldrich), and 0.25 μmol/L dexamethasone (Sigma-Aldrich) for 2 days (days 0–2). Then, the medium was removed, and the cells were cultured for 2 more days in DMEM plus 10% FBS and 1 μmol/L insulin (days 2–4). The cells were then maintained in DMEM plus 10% FBS medium for 2 days (days 4–6). Oil Red O (Sigma-Aldrich) staining of 3T3-L1 adipocytes was performed to determine adipogenesis. For histochemical determination of hypertrophic adipocytes, we measured ring-like lipid droplet (Fig. 1B, arrow) using five random fields (×20 power) scanned by BioZero fluorescent microscope (Keyence, Osaka, Japan). The numbers of ring-like lipid droplets were counted by two blinded observers, and the mean of the data was used for final count. Interobserver coefficient of variation in three different experimental conditions was 8.5%. To examine the effect of RAGE or Toll-like receptor (Tlr)2 ligands on adipogenesis, HMGB1 (1–10 ng/mL; Sigma-Aldrich), S100b (10–100 nmol/L; Calbiochem), or palmitate (0.5–1.0 mmol/L; Sigma-Aldrich) was added during differentiation step. Palmitate was solubilized in 10% BSA, which resulted in 10 mmol/L palmitate stock solution. BSA (10%) solution was used as vehicle.
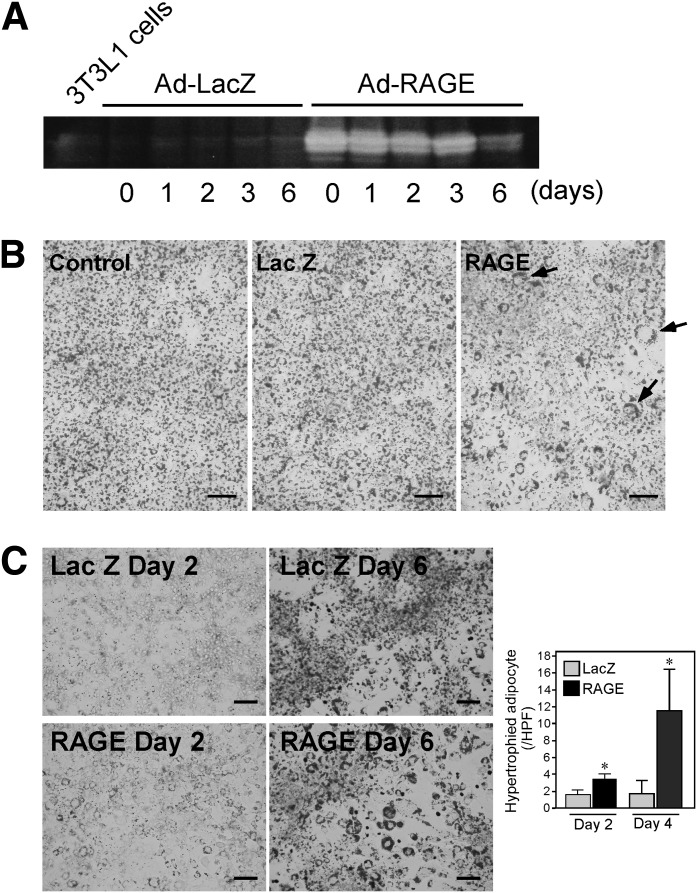
FIG. 1.
RAGE overexpression in 3T3L1 preadipocytes accelerates adipocyte hypertrophy. Confluent 3T3L1 cells were infected with human RAGE- or control β-galactosidase (LacZ)–expressing adenovirus. At day 0, differentiation was initiated by the addition of 10% FBS, 1 μmol/L insulin, and 0.5 mmol/L isobutylmethyl xanthine, and 0.25 μmol/L dexamethasone for 2 days. Then, the cells were cultured for 2 more days in 10% FBS and 1 μmol/L insulin (day 2–4). The cells were then maintained in 10% FBS medium for 2 days (day 4–6). A: At indicated days of differentiation, RAGE protein expression in 3T3L1 cells was determined by Western blot analyses using goat anti-human RAGE antibody. Adenoviral overexpression of RAGE was maintained up until day 6 during adipocyte differentiation. B: Oil Red O staining of 3T3-L1 adipocytes was performed to determine adipogenesis at day 6. More hypertrophic adipocytes with ring-like lipid staining (arrows) were observed in RAGE-overexpressed cells. Control represents 3T3L1 cells without adenoviral infection. C: Oil Red O staining was performed at days 2 or 6 during differentiation. Numbers of hypertrophic adipocytes, as defined as cells with ring-like lipid droplets, were counted in five random fields (×20 power) scanned by BioZero fluorescent microscope (Keyence). Each column represents mean ± SD. Scale bars, 100 μm. *P < 0.05, Student t test. HPF, high-power field.
Flow cytometry.
Adipogenically induced 3T3-L1 cells were also analyzed by flow cytometry (FACS Canto; BD Biosciences) as described previously (19). Cells were briefly rinsed twice with prewarmed 0.25% trypsin-EDTA and then incubated for 5 min at 37°C. Cells were then gently resuspended in PBS, washed twice with PBS, resuspended in cold PBS, and kept on ice prior to flow cytometric analysis. The dot plot of cytometric forward scatters (FSC, shown as the x-axis) and side scatters (SSC, shown as the y-axis) reflects the cell diameter and granular structures within the cell, respectively (Fig. 2A). It has been shown that after adipogenic induction, the cells containing greater granular structure were markedly increased, and this increase in granularity positively correlated with the time of the postadipogenic induction (19). In some experiments, cells were also stained for 10 min with 0.5 μmol/L dipyrromethene boron difluoride (BODIPY; Molecular Probes, Life Technology, NY) to analyze the proportion of lipid-laden cells.
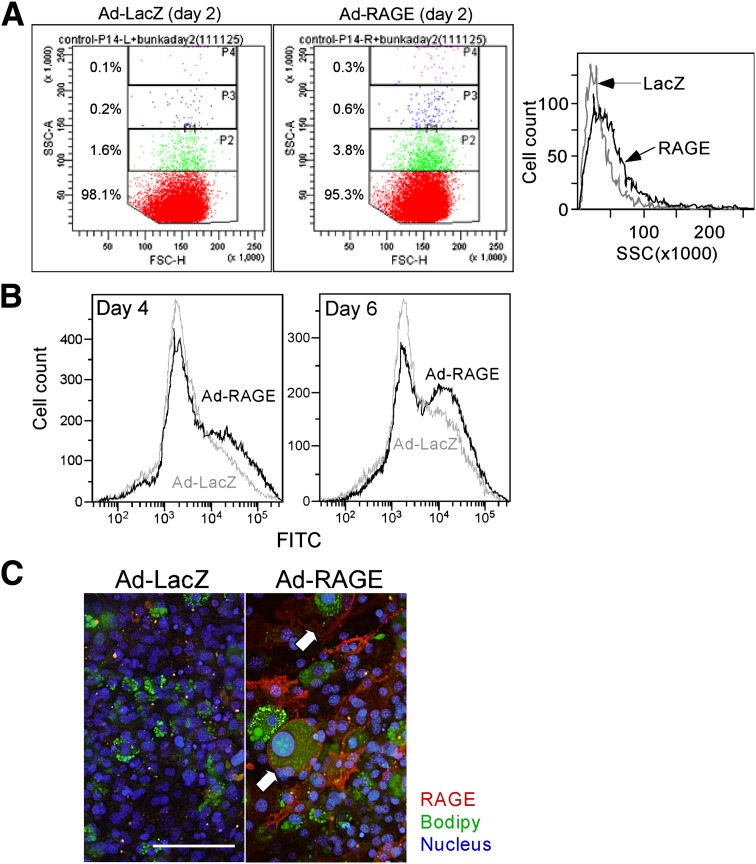
FIG. 2.
Flow cytometric analysis for adipogenesis. Adipogenic differentiation of 3T3-L1 cells was analyzed by flow cytometry (FACS Canto; BD Biosciences) as described previously (19). Cells were briefly rinsed twice with prewarmed 0.25% trypsin-EDTA and then incubated for 5 min at 37°C. Cells were then gently resuspended in PBS, washed twice with PBS, resuspended in cold PBS, and kept on ice prior to flow cytometric analysis. A: Following differentiation process, the Ad-RAGE–infected cells with greater SSC were increased as early as day 2 as compared with Ad-LacZ–infected cells. Distribution of SSC intensity at day 6 is shown in the right panel. B: At days 4 and 6 of differentiation, Ad-LacZ– or Ad-RAGE–infected 3T3-L1 cells were stained for 1 h with 0.5 μmol/L BODIPY to analyze the proportion of lipid-laden cells prior to flow cytometry. Fluorescein isothiocyanate (FITC) intensity was analyzed to determine lipid-rich differentiated adipocytes. C: Double-immunofluorescent analyses for RAGE expression and lipid accumulation. 3T3-L1 preadipocytes were infected with Ad-RAGE or LacZ and then cultured with differentiation media. Expression of human RAGE in the differentiated 3T3-L1 adipocytes was analyzed at day 6, and the cells were costained with 0.5 μmol BODIPY to visualize adipocytes and with DAPI to visualize the nuclei. Scale bar, 100 μm. (A high-quality digital representation of this figure is available in the online issue.)
Adenoviral infection to 3T3-L1 preadipocytes.
Human RAGE-expressing pAdHM15-RAGE (Ad-RAGE) was constructed using a full-length human RAGE cDNA (20) as previously described (21). The 3T3-L1 preadipocytes (2 days postconfluency) were infected with Ad-RAGE or Ad-LacZ at multiplicity of infection of 50 for 3 h with 0.5 μg/mL polylysine as described (22), washed, and cultured in fresh DMEM with 10% FBS for an additional 12 h prior to experimentation (day 0 for Fig. 1). Under these conditions, infection efficiency was typically >80% as determined by β-galactosidase staining following Ad-LacZ infection. RAGE protein expression was determined by Western blot analysis as previously described (21) using goat anti-human RAGE antibody (R&D Systems, Minneapolis, MN).
Gene knockdown with small interfering RNAs.
3T3-L1 preadipocytes were cultured in DMEM with 10% FBS for 2 days. The cells were harvested, suspended, and transfected with predesigned small interfering RNA (siRNA) for mouse RAGE, HMGB1, S100b, Tlr2, or Tlr4 (Ambion) using an Amaxa Nucleofector 1 electroporation system (Amaxa Biosystems), according to the manufacturer’s instructions. After 24 h, the cells were infected with Ad-RAGE or Ad-LacZ as described above. One day after adenoviral infection, differentiation was initiated and then maintained in DMEM plus 10% FBS as described above.
Fluorescent immunocytochemistry.
3T3-L1 preadipocytes were infected with Ad-RAGE or LacZ and then cultured with differentiation media for 3 days as described above. Expression of human RAGE in the differentiated 3T3-L1 adipocytes was analyzed by the fluorescent immunocytochemistry as described previously (23). Goat anti-human RAGE antibody (5 μg/mL; R&D Systems) and rhodamine-conjugated anti-goat IgG antibody (1:500 dilution; Cappel) were used as the first and second antibody, respectively. The cells were costained for 1 h with 0.5 μmol BODIPY to visualize adipocytes and with DAPI to visualize nuclei. Images were obtained by BioZero fluorescent microscope (Keyence).
Real-time quantitative RT-PCR.
cDNA was synthesized from total RNA by TaqMan reverse transcription reagents, and expressions of mouse RAGE, glucose transporter type 4 (Glut4), fatty acid binding protein 4 (FABP4), peroxisome proliferator-activated receptor (PPAR)γ, adiponectin, Tlr2, Tlr4, myeloid differentiation factor 88 (MyD88), S100b, and HMGB1, monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), and CD68 mRNA were measured quantitatively by the TaqMan Real-Time RT-PCR technique (Applied Biosystems, Foster City, CA). Ready-to-use primers and fluorescence probes for each of the mouse genes and 18S ribosomal RNA were purchased from Applied Biosystems (Taqman Gene Expression Assays) and used according to the manufacturer’s protocol. The threshold cycle (Ct) value for every sample was measured, and mRNA expression levels of each mouse gene were determined by a comparative Ct method using 18S ribosomal RNA as endogenous reference (Applied Biosystems).
Phosphorylation of insulin receptor and insulin receptor substrates.
Insulin-stimulated phosphorylation of insulin receptor and insulin receptor substrates (IRS) were determined as previously described (24). Differentiated adipocytes were serum-starved overnight and subsequently stimulated with 100 nmol/L insulin for 10 min. The monolayers were washed with ice-cold buffer (137 mmol/L NaCl, 1 mmol/L MgCl2, 1 mmol/L CaCl2, 0.1 mmol/L Na2VO2, 20 mmol/L Tris–HCl at pH 7.6) and lysed in the same buffer supplemented with 1% Nonidet P-40, 10% glycerol, 2 mmol/L EDTA, 10 mmol/L sodium pyrophosphate, 10 mmol/L NaF, 2 mmol/L Na2VO2, 2 mmol/L phenylmethylsulfonyl fluoride, and 8 μg/mL leupeptin. After removal of insoluble materials, protein concentration was determined by BCA assay. Lysates (50 μg protein) were used for Western blotting. Antibodies recognizing murine IRS1 and IRS2 (0.5 μg/mL; Upstate Biotechnology), insulin receptor β-chain (0.5 μg/mL; Santa Cruz Biotechnology), α-tubulin (1,000-fold dilution; Cell Signaling Technology), and phosphotyrosine (PY20, 2 μg/mL; Upstate Biotechnology) were used for analyses. For immunoprecipitation of insulin receptor β-chain or IRS1, 500 μg of the lysates were incubated with 2 μg of anti-insulin receptor β-chain or IRS1 antibody for 3 h at 4°C and precipitated with protein A-agarose (20 μL; Santa Cruz Biotechnology) for 1 h at 4°C.
Insulin-stimulated glucose uptake.
At day 6 of differentiation period, adipocytes were serum-starved overnight and subsequently stimulated with 100 nmol/L insulin for 18 min. Glucose uptake was determined by 2-deoxyglucose uptake with an enzymatic photometric assay by using 2-deoxyglucose uptake measurement kit (COSMO BIO Co. Ltd., Tokyo, Japan) (25).
Animals and experimental protocol.
C57BL/6J wild-type (WT) and RAGE−/− mice were produced and maintained as previously described (22,26). WT and RAGE−/− mice were weaned at age 4 to 5 weeks and housed in specific pathogen-free cages. Mice were maintained in a temperature-controlled (24°C) facility with a strict 12-h light/dark cycle and given free access to food and water. All procedures in this study were approved by the Animal Care and Use Committee at the Osaka City University Graduate School of Medicine, Osaka, Japan. Male WT or RAGE−/− mice were randomly divided into high-fat diet groups (20% of total calories from cocoa butter and 0.15% from cholesterol; Clea Japan Inc., Tokyo, Japan) and standard chow diet groups. Mice were fed with either of the diet from 6 weeks of age until 15 or 20 weeks of age. Body weight was monitored weekly. At 15 or 20 weeks of age, mice were overnight fasted, scarified under the anesthesia with pentobarbital, and tissues were collected.
To analyze insulin sensitivity, mice were injected with insulin (2.0 units/kg for mice) intraperitoneally after a 6-h fast. Blood samples were taken at various time points (0–120 min). Blood glucose levels were measured with a portable glucose meter (Glu-test Sensor; Sanwa Chemical, Aichi, Japan) after tail snipping. Serum adiponectin levels were determined with the mouse/rat adiponectin ELISA kit (Ohtsuka, Tokushima, Japan).
Adipose tissue preparations, histochemical analysis, and total RNA isolation.
To characterize the effect of RAGE on adiposity, epididymal fat weight, which has been typically analyzed for adiposity (27,28), was compared between WT and RAGE−/− mice fed with standard or high-fat diet as previously described (16). For comparisons of adipocyte size, epididymal adipose tissues were fixed with paraformaldehyde, paraffin-embedded, and stained with hematoxylin and eosin. For quantitation, five random fields (×20 power) were scanned by Coolscope (Nikon, Tokyo, Japan), and numbers of adipocyte were counted to calculate the average size of the cells. For real-time PCR analyses, total RNA was isolated by using RNAiso and RNeasy MinElute Spin Columns (QIAGEN, Venlo, the Netherlands) from whole epididymal adipose tissues.
RESULTS
RAGE accelerates adipocyte hypertrophy in 3T3-L1 preadipocytes.
First, we examined whether RAGE is directly involved in adipogenesis in vitro by using 3T3-L1 preadipocytes. RAGE mRNA levels were detectable in 3T3-L1 cells by real-time quantitative RT-PCR (Fig. 3B), suggesting its potentially direct role in adipogenesis. Adenoviral overexpression of RAGE in 3T3-L1 cells resulted in marked increase in RAGE protein expression at 24 h after infection when differentiation was initiated (day 0) (Fig. 1A). RAGE protein expression was still detectable at day 6 of the differentiation process. When 3T3-L1 cells were infected with Ad-RAGE, and the cells were induced to differentiate for 6 days, significant numbers of adipocytes become hypertrophic as compared with Ad-LacZ–infected cells (Fig. 1B). As shown in Fig. 1C, larger adipocytes were already present at 2 days of differentiation protocol, implicating the role of RAGE in adipocyte hypertrophy since the early phase of adipogenesis.
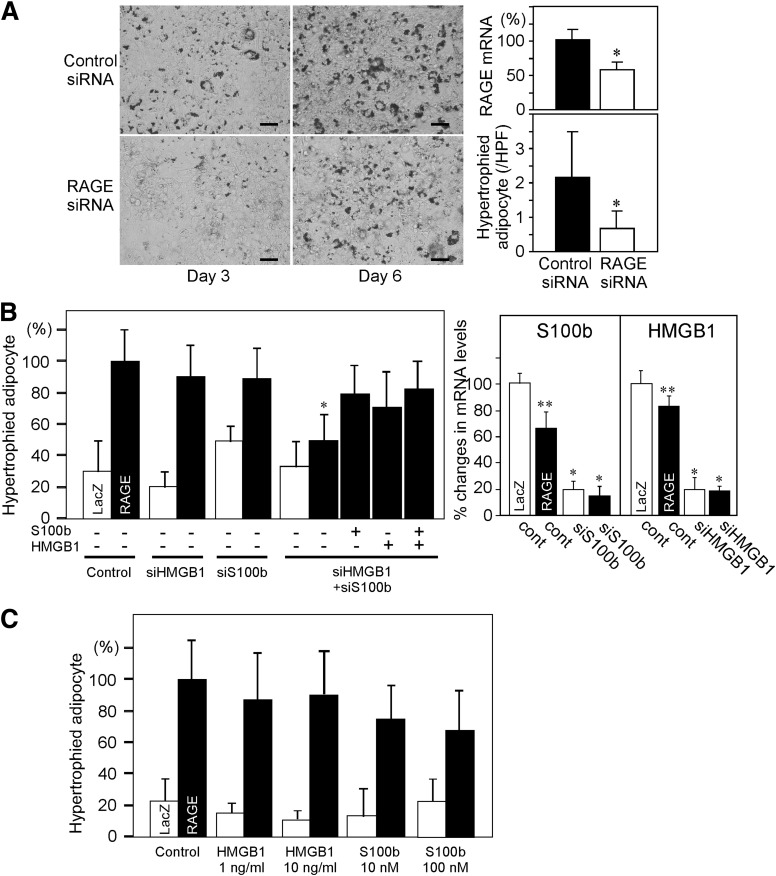
FIG. 3.
A: Endogenous RAGE expression is involved in adipocyte hypertrophy. 3T3-L1 preadipocytes were cultured in DMEM with 10% FBS for 2 days. The cells were harvested, suspended, and transfected with predesigned siRNA for mouse RAGE using electroporation system. Twenty-four hours after siRNA transfection, the cells were infected with Ad-RAGE or Ad-LacZ. One day after adenoviral infection, differentiation was induced. Adipocyte differentiation was determined by Oil Red O staining 3 and 6 days after differentiation. Scale bars, 100 μm. *P < 0.05, Student t test. B: RAGE ligation with endogenous ligand(s) is indispensable for RAGE-stimulated adipocyte hypertrophy. The cells were transfected with siRNAs for control (cont), S100b alone, HMGB1 alone, or S100b + HMGB1, infected with Ad-LacZ or Ad-RAGE, and differentiated as described in A. In S100b and HMGB1 double-knockdown condition, addition of S100b (100 nmol/L), HMGB1 (10 ng/mL) alone, or both ligands during differentiation step negates the effect of siRNAs. Right panel shows the effect of RAGE overexpression or the effects of siRNAs for S100b and HMGB1 on mRNA levels of respective ligands. *P < 0.05 vs. control, **P < 0.05 vs. LacZ, Student t test. C: Effects of RAGE ligands on adipocyte hypertrophy in the presence or absence of RAGE overexpression. Indicated concentrations of HMGB1 or S100b were added during differentiation step following adenoviral infection as described in A. HPF, high-power field.
The effect of RAGE on adipocytic differentiation was also examined by flow cytometric analysis (Fig. 2). The cells with greater SSC were already increased 2 days after RAGE overexpression (Fig. 2A), and significant proportions of RAGE-overexpressed cells were also detected with greater SSC at day 6 (Fig. 2A, right panel). Distribution of FSC appears similar between LacZ– and RAGE-overexpressing cells. Analysis of intracellular lipid by using BODIPY staining confirmed that RAGE-overexpressing cells exhibited as more lipid-laden cell population than LacZ–overexpressing cells both at days 4 and 6 (Fig. 2B). Thus, in addition to the effect on adipocyte hypertrophy, RAGE overexpression appears to accelerate adipogenic differentiation. Importantly, double-immunofluorescent analyses reveal that many of the massively hypertrophic adipocytes as determined by BODIPY staining are associated with high RAGE expression (Fig. 2C).
RAGE knockdown in 3T3-L1 preadipocytes to ∼60% expression level resulted in less numbers of hypertrophic adipocytes at both days 3 and 6 of adipogenic differentiation (Fig. 3A). We noted that both HMGB1 and S100b were endogenously expressed in this experimental system by using real-time RT-PCR, even though both of the mRNAs were not increased, rather decreased, by RAGE overexpression (Fig. 3B, right panel). Gene knockdown by using siRNAs for HMGB1 and S100b successfully suppressed mRNA levels of the respective RAGE ligands both in the presence and absence of RAGE overexpression (Fig. 3B, right panel). Of importance, although knockdown of neither of the genes canceled RAGE-mediated adipocyte hypertrophy, simultaneous knockdown of both of the genes significantly suppressed RAGE-mediated adipocyte hypertrophy at day 6 of the differentiation process, implicating ligand engagement is indispensable for RAGE-medicated function in preadipocytes (Fig. 3B). The addition of either HMGB1 or S100b negated the effect of simultaneous knockdown of HMGB1 and S100b on adipocyte hypertrophy (Fig. 3B). We next examined if either of the endogenous RAGE ligand plays an important role in RAGE-mediated adipocyte hypertrophy. Addition of neither of the RAGE ligands significantly upregulates RAGE mRNA levels in 3T3-L1 preadipocytes (data not shown). Addition of neither HMGB1 (1–10 ng/mL) nor S100b (10–100 nmol/L) significantly increased adipocyte hypertrophy in both LacZ– and RAGE-overexpressing 3T3-L1 cells (Fig. 3C).
RAGE-mediated hypertrophy of adipocytes is associated with lower expression of Glut4 and adiponectin and attenuated insulin-stimulated signaling.
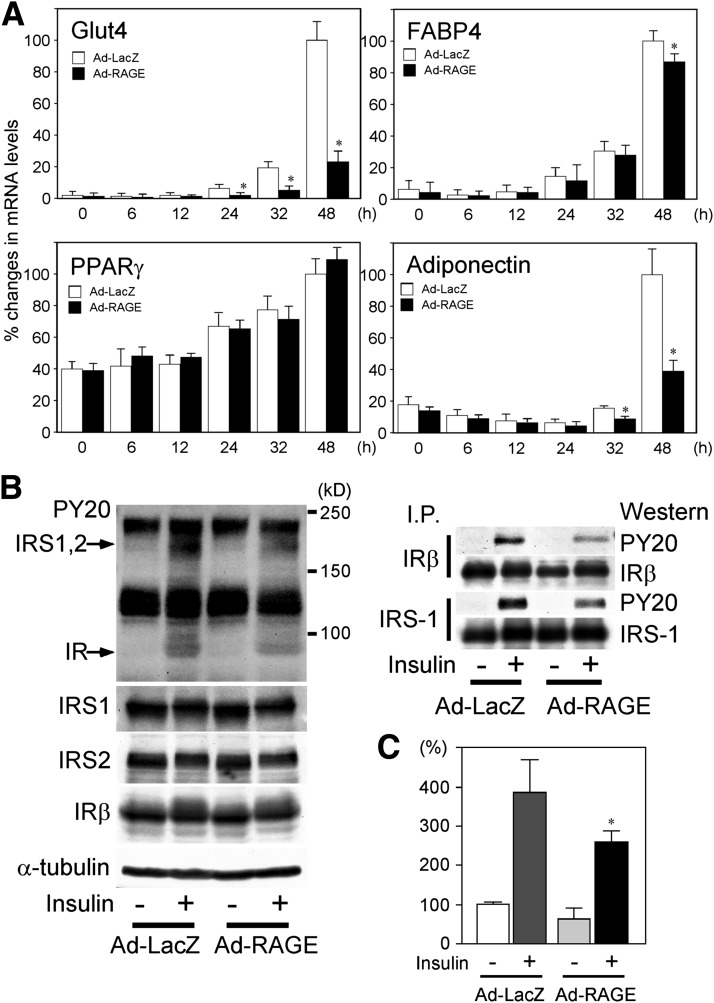
We next examine whether the effect of RAGE on adipocyte hypertrophy is associated with altered function. Analyses of gene expression in the course of differentiation showed that mRNA levels of Glut4 and adiponectin, but not for FABP4 and PPARγ, were markedly inhibited in cells with RAGE overexpression as compared with those with LacZ overexpression (Fig. 4A). As shown in Fig. 4B, insulin-stimulated phosphorylation of insulin receptor and IRS was less in adipocytes (at day 6) following overexpression of RAGE than those of LacZ. Protein levels of insulin receptor β-chain, IRS1, and IRS2 were comparable between RAGE- and LacZ–overexpressing cells. Attenuated insulin signaling in RAGE-overexpressing cells was further confirmed by immunoprecipitation of insulin receptor or IRS1 and immunoblotting with antiphosphotyrosine (Fig. 4B, right panel). Moreover, insulin-stimulated glucose uptake was also significantly blunted in adipocytes overexpressing RAGE (Fig. 4C). Thus, RAGE-mediated adipocyte hypertrophy is associated with attenuated insulin signaling, decreased expression of genes involved in insulin sensitivity, and blunted insulin function.
FIG. 4.
A: mRNA expression of Glut4, FABP4, PPARγ, and adiponectin following adenoviral infection and differentiation induction. Confluent 3T3-L1 cells were infected with human RAGE- (Ad-RAGE) or control LacZ–expressing adenovirus (Ad-LacZ) (−24 h). At 0 h, differentiation was initiated, and total RNA was isolated at indicated hours of differentiation. mRNA expression of each of the genes was determined by quantitative real-time RT-PCR. mRNA expression levels of each mouse gene were determined by a comparative Ct method using 18S ribosomal RNA as endogenous reference, and mRNA level of Ad-LacZ at −24 h was expressed as 100%. *P < 0.05 vs. Ad-LacZ. B: Attenuated phosphorylation of insulin receptor (IR) or IRS in RAGE-overexpressing adipocytes. At day 6 of differentiation period, adipocytes were serum-starved overnight and subsequently stimulated with 100 nmol/L insulin for 10 min. Phosphorylation of IR and IRS was determined by Western blot analyses using anti-phosphotyrosine (PY20, 1 μg/mL) antibody. After stripping, the blot was reprobed with antibodies recognizing murine IRS1 and IRS2 (1 μg/mL) and IR β-chain (1 μg/mL) to determine respective protein levels. Equal protein loading of the blot was evaluated by reprobing with anti–α-tubulin antibody. Right panel represents immunoblot following immunoprecipitation of IR or IRS1. Samples (500 μg) incubated with 2 μg of anti-IRβ or IRS1 antibody were immunoprecipitated with protein A-agarose. The protein was recovered and analyzed by immunoblotting as described above. I.P., precipitation. kD, kilodalton. C: Attenuated insulin-stimulated glucose uptake in RAGE-overexpressing adipocytes. At day 6 of differentiation period, adipocytes were serum-starved overnight and subsequently stimulated with 0.1 μmol/L insulin for 18 min. Glucose uptake was determined by 2-deoxyglucose uptake by an enzymatic photometric assay. *P < 0.05 vs. Ad-LacZ.
Tlr2 potentially mediates RAGE regulation of adipocyte hypertrophy in vitro.
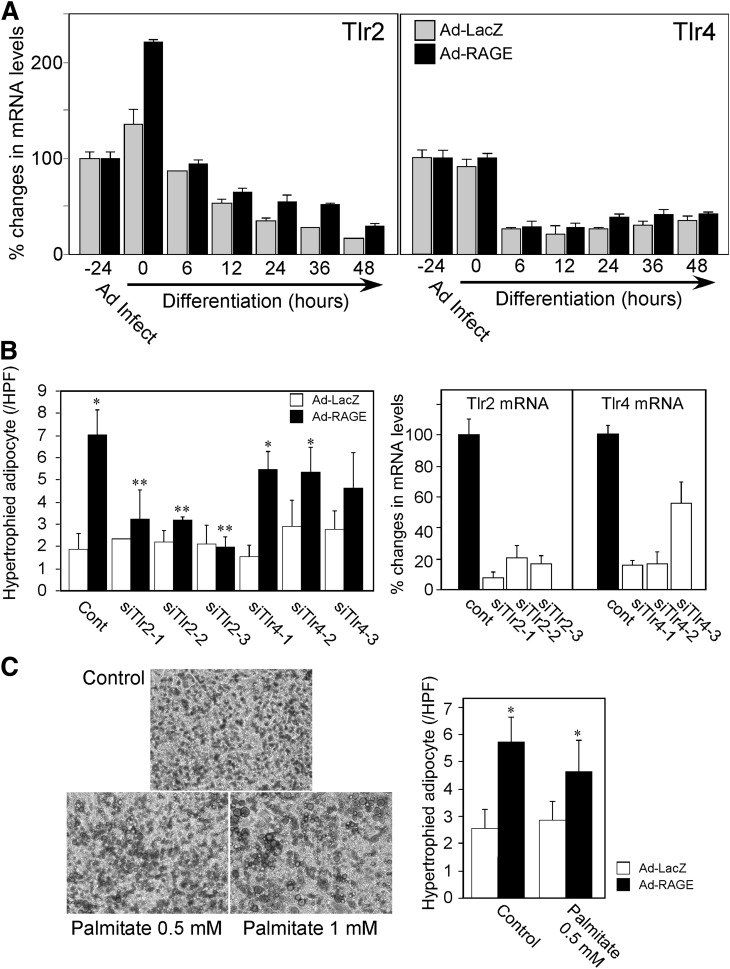
To explore potential mechanisms underlying RAGE regulation of adipocyte hypertrophy, we examined involvement of the Tlr system, because interactions between RAGE and Tlr systems have been implicated in multiple pathophysiological conditions (29–31). As shown in Fig. 5A, Tlr2 mRNA expression was induced by more than twofold following RAGE overexpression, and its level was gradually decreased during adipogenic differentiation. In contrast, Tlr4 mRNA expression was not changed by RAGE overexpression. Tlr4 mRNA expression was suppressed following induction of differentiation in both RAGE- and LacZ–overexpressing 3T3-L1 cells.
FIG. 5.
A: Tlr2 and Tlr4 mRNA expression following adenoviral infection and differentiation induction. Confluent 3T3-L1 cells were infected with human RAGE- (Ad-RAGE) or control LacZ–expressing adenovirus (Ad-LacZ) (−24 h). At 0 h, differentiation was initiated, and total RNA was isolated at indicated hours of differentiation. Tlr2 and Tlr4 mRNA expression was determined by quantitative real-time RT-PCR. mRNA expression levels of each mouse gene were determined by a comparative Ct method using 18S ribosomal RNA as endogenous reference, and mRNA level of Ad-LacZ–infected (Ad Infect) cells at −24 h was expressed as 100%. B: Knockdown of Tlr2, but not Tlr4, ameliorates adipocyte hypertrophy induced by RAGE overexpression. 3T3-L1 preadipocytes were cultured in DMEM with 10% FBS for 2 days. The cells were harvested, suspended, and transfected with predesigned siRNA for mouse Tlr2 (siTlr2-1–3; corresponding to three distinct regions of Tlr2) or Tlr4 (siTlr4-1–3; corresponding to three distinct regions of Tlr4) using electroporation system. Twenty-four hours after siRNA transfection, the cells were infected with Ad-RAGE or Ad-LacZ. One day after adenoviral infection, differentiation was induced. Hypertrophied adipocyte was determined by Oil Red O staining 6 days after differentiation. *P < 0.05 vs. Ad-LacZ, **P < 0.05 vs. control (Cont), Student t test. Right panel: Tlr2 and Tlr4 mRNA levels 24 h after respective siRNA transfection. C: Effects of palmitate, a Tlr2 ligand, on adipogenesis in 3T3-L1 preadipocytes. As shown in the left panel, addition of 0.5 or 1.0 mmol/L palmitate increased lipid droplet in adipocytes, but did not increase adipocytes with ring-like lipid staining. Right panel represents the effects of 1.0 mmol/L palmitate on adipocyte hypertrophy in the presence or absence of RAGE overexpression. HPF, high-power field. *P < 0.05 vs. Ad-LacZ.
To directly examine the role of Tlr2 in RAGE-mediated adipocyte hypertrophy, gene knockdown experiments were performed. Both Tlr2 and Tlr4 siRNAs (three distinct regions) successfully suppressed respective endogenous mRNAs (Fig. 5B). In this system, inhibition of Tlr2 significantly suppressed RAGE-mediated adipocyte hypertrophy at day 6 of differentiation, whereas inhibition of Tlr4 did not show significant effects (Fig. 5B). We also examined the effect of palmitate, a fatty acid recognized by Tlr2 (32), on adipogenic differentiation of 3T3-L1 cells. Although palmitate (0.5–1.0 mmol/L) did not increase the numbers of hypertrophic adipocyte defined as ring-like lipid staining, it increased the size of individual lipid droplet inside the adipocytes (Fig. 5C, left panel). Further, palmitate did not accelerate RAGE-mediated adipocyte hypertrophy (Fig. 5C, right panel). Palmitate did not affect the levels of RAGE mRNA in this system (data not shown). Thus, it appears likely that Tlr2 is essential, but not sufficient, for RAGE-mediated generation of hypertrophic adipocytes.
RAGE deficiency is associated with resistant to obesity, higher serum adiponectin, greater insulin sensitivity, and early decrease in Tlr2 mRNA in epididymal adipose tissue in mice.
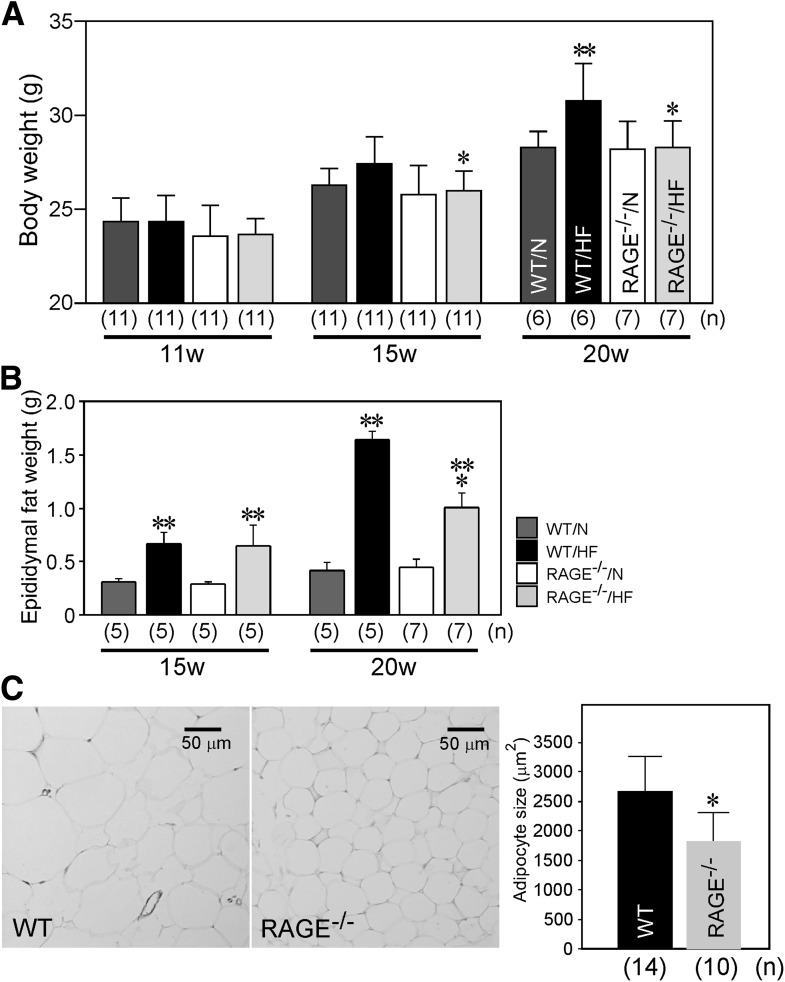
To define the role of RAGE in adiposity and in Tlr2 mRNA regulation in vivo, we fed WT or RAGE−/− C57BL/6J mice with a normal or high-fat diet until 20 weeks of age. As observed in the apoE-deficient genetic background (16), RAGE−/− mice exhibited significant resistance to obesity induced by high-fat diet at 15 and 20 weeks of age (Fig. 6A). Epididymal fat weight at 20 weeks of age was also significantly less in RAGE−/− mice than WT mice when mice were fed with a high-fat diet (Fig. 6B). Adipocyte size was also significantly less in RAGE−/− mice at 20 weeks fed with a high-fat diet (Fig. 6C).
FIG. 6.
RAGE deficiency is associated with decrease in body weight (A), adipose tissue weight (B), and adipocyte size (C) in mice. A: Increase in body weight induced by high-fat diet (HF) is suppressed in RAGE−/− mice at 15 and 20 weeks of age. B: Epididymal adipose tissues were isolated from WT and RAGE−/− mice fed either with normal (N) or HF diet at 15 and 20 weeks of age, and tissue weight was determined. All columns represent mean ± SD. C: Epididymal adipose tissues were isolated from WT or RAGE−/− mice at 20 weeks fed with HF diet, and adipocyte size was determined histochemically. Columns represent mean ± SD. *P < 0.05 vs. WT mice. **P < 0.05 vs. normal diet, Student t test. Numbers of animals are shown in parentheses.
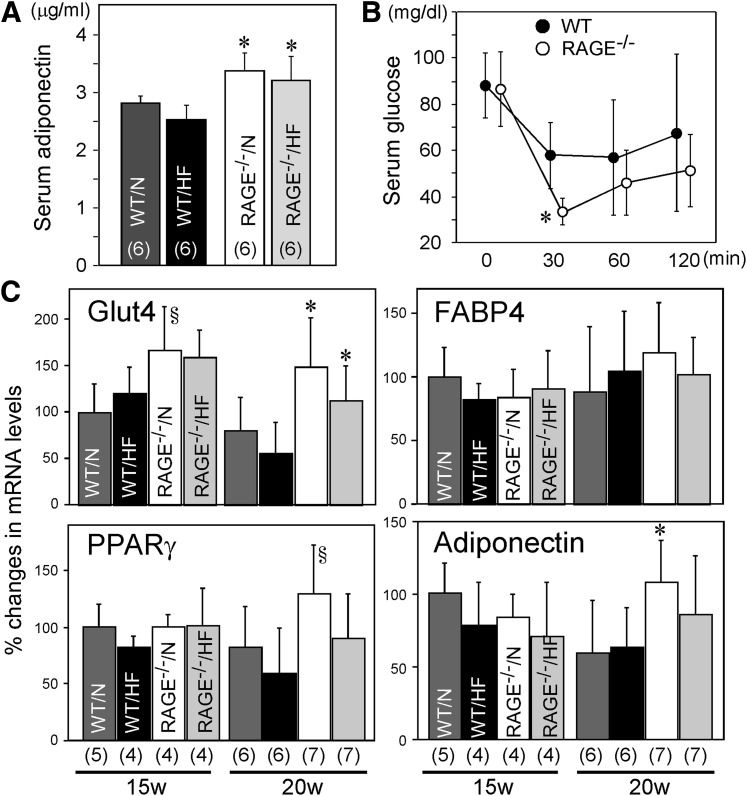
Fasting plasma glucose levels were not statistically different between WT and RAGE−/− mice at 20 weeks (WT/normal 105.8 ± 17.5 mg/dL [n = 6], RAGE−/−/normal 108.0 ± 16.0 mg/dL [n = 7], P = 0.82, Student t test; WT/high fat 118.6 ± 15.3 mg/dL [n = 7], RAGE−/−/high fat 110.2 ± 14.9 mg/dL [n = 7], P = 0.34). Plasma insulin levels were not also statistically different between WT and RAGE−/− mice at 20 weeks (WT/normal 0.95 ± 0.28 ng/mL [n = 5], RAGE−/−/normal 1.09 ± 0.21 ng/mL [n = 5], P = 0.50; WT/high fat 2.67 ± 0.39 ng/mL (n = 4), RAGE−/−/high fat 3.07 ± 0.97 ng/mL [n = 4], P = 0.48). RAGE−/− mice at 20 weeks also exhibited significantly higher serum adiponectin levels than WT mice fed both with a normal and high-fat diet (Fig. 7A). RAGE−/− mice also exhibited greater insulin sensitivity even in conditions fed with a normal diet as determined by insulin tolerance test (Fig. 7B). These altered metabolic phenotypes of RAGE−/− mice were associated with significantly higher mRNA expression in epididymal fat of Glut4 and adiponectin at 20 weeks fed with a normal diet (Fig. 7C). Glut4 mRNA expression tended to be higher in RAGE−/− than WT mice at 15 weeks in normal diet and significantly higher at 20 weeks even in conditions fed with a high-fat diet. PPARγ mRNA level tended to be higher in RAGE−/− than WT mice at 20 weeks fed with a normal diet. FABP4 mRNA levels were comparable in all experimental conditions.
FIG. 7.
A: Serum adiponectin levels at 20 weeks in WT or RAGE−/− mice fed either with normal (N) or high-fat (HF) diet. B: Insulin tolerance test was done in WT (n = 9; 18.0 ± 1.3 weeks of age) or RAGE−/− mice (n = 8; 18.1 ± 1.4 weeks of age) fed with normal diet as described in Research Design and Methods. Mice were injected with insulin (2.0 units/kg for mice) intraperitoneally after a 6-h fast. Blood samples were taken at indicated time points, and blood glucose levels were determined. Reproducible results were obtained in another set of experiments. C: Total RNA was isolated from epididymal adipose tissues obtained from WT or RAGE−/− mice fed either with N or HF diet at 15 or 20 weeks of age. mRNA levels of corresponding Glut4, FABP4, PPARγ, and adiponectin were determined by quantitative real-time RT-PCR. mRNA expression levels of each mouse gene were determined by a comparative Ct method using 18S ribosomal RNA as endogenous reference, and mRNA level of WT/N at 15 weeks was expressed as 100%. Columns represent mean ± SD. Numbers of animals are shown in parentheses. *P < 0.05 vs. WT mice, §P < 0.1 vs. WT, Student t test.
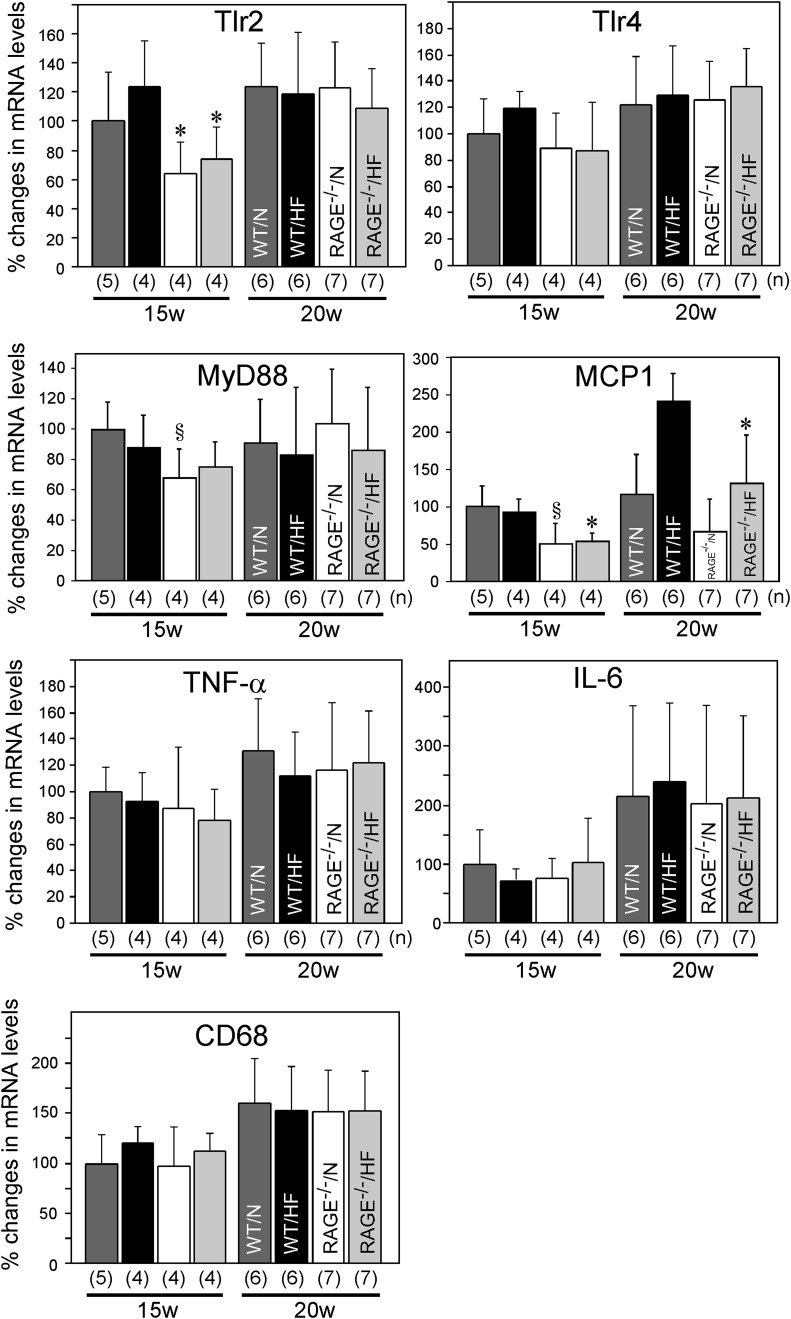
Of interest, Tlr2 mRNA levels in epididymal adipose tissue were already decreased at 15 weeks in RAGE−/− mice than WT mice in both normal and high-fat diet–feeding conditions (Fig. 8). Tlr2 mRNA levels were comparable between WT and RAGE−/− mice at 20 weeks of age. On the contrary, Tlr4 mRNA levels in epididymal adipose tissues were not significantly different in any of the conditions both at 15 and 20 weeks. MyD88 mRNA levels, a downstream mediator for Tlr signaling, tended to be decreased in RAGE−/− mice at 15 weeks fed with a normal diet as compared with WT mice. Gene expression of MCP-1, a representative inflammatory mediator expressed in adipose tissue (33), was decreased in RAGE−/− mice at 15 weeks of age, as compared with WT mice. The difference in MCP-1 mRNA was still significant at 20 weeks in mice fed with a high-fat diet. mRNA levels of TNF-α, IL-6, and a maker for macrophage lineage (CD68) were not significantly different between WT and RAGE−/− mice both at 15 and 20 weeks of age.
FIG. 8.
Tlr2, Tlr4, MyD88, MCP-1, TNF-α, IL-6, and CD68 mRNA levels in RAGE-deficient mice. Total RNA was isolated from epididymal adipose tissues obtained from WT or RAGE−/− mice fed either with normal (N) or high-fat (HF) diet at 15 or 20 weeks of age. Each mRNA level was determined by quantitative real-time RT-PCR. mRNA expression levels of each mouse gene were determined by a comparative Ct method using 18S ribosomal RNA as endogenous reference, and mRNA level of WT/N at 15 weeks was expressed as 100%. Columns represent mean ± SD. *P < 0.05 vs. WT mice, §P < 0.1 vs. WT, Student t test. Numbers of animals are shown in parentheses.
DISCUSSION
This study is the first to demonstrate direct role of RAGE in adipocyte hypertrophy and insulin sensitivity, whereas Tlr2 potentially and at least partly, plays a fundamental role.
RAGE and adiposity.
Recent reports suggest that RAGE could be involved in progression of obesity. Our human clinical studies suggest obesity is closely associated with circulating endogenous secretory RAGE, an alternatively spliced form of RAGE, both in diabetic and nondiabetic conditions (17,18). Recent study in humans shows RAGE mRNA expression in subcutaneous adipose tissues (34). Although this study does not delineate which cells in adipose tissue express RAGE, our current animal study shows RAGE expression in adipocyte as well as endothelial cells in adipose tissues (16). In apoE/RAGE double-knockout mice, progression of atherosclerosis is closely associated with RAGE-regulated adiposity in nondiabetic conditions (16). We also add evidence in this study that RAGE is involved in adiposity even in the apoE+/+ genetic background. At 20 weeks of age, body weight, epididymal adipose tissue weight, and epididymal adipocyte size of RAGE−/− mice were significantly smaller than that of WT mice.
Only limited reports showed a direct role of RAGE in adipocyte function in vitro. Unoki et al. (35) showed that AGEs inhibit the glucose uptake in 3T3-L1 adipocytes, which was completely prevented by antibody against AGE or RAGE. Our current findings are the first to demonstrate RAGE directly regulate adipogenesis and hypertrophic process of adipocyte differentiation in vitro. Adenoviral overexpression of RAGE markedly increases generation of hypertrophic adipocytes. More importantly, RAGE knockdown by using siRNA system significantly suppresses generation of hypertrophic adipocytes, strongly implicating that endogenously expressed RAGE is indeed involved in adipocyte hypertrophy. Morphological changes of adipocytes induced by RAGE overexpression were associated with decrease in expression of genes involved in insulin sensitivity, Glut4, and adiponectin and attenuated insulin signaling and function. We further extended our in vitro observations to in vivo ones using RAGE−/− mice. RAGE deficiency is associated with less body weight, less epididymal fat weight, less adipocyte size, higher serum adiponectin, higher expressions of Glut4 and adiponectin in epididymal fat, and greater insulin sensitivity in insulin tolerance tests; many of these phenotypes are mirror images of RAGE overexpression in adipocyte in vitro.
At present, our data do not delineate which types of RAGE ligands play fundamental roles in RAGE-medicated adipocyte hypertrophy. Involvement of AGEs in this system is unlikely, although we could not negate the possibility that the impact of small amounts of AGEs potentially present in culture media might be augmented by overexpression of RAGE. RAGE also interacts with other endogenous nonglycated peptide ligands including S100/calgranulin (8) and HMGB1 (9,10), both of which are important inflammatory regulators. Early studies show expression of S100b protein in pre- and mature adipocytes, and its expression is induced during adipogenesis (36,37). Physiological S100b levels appear to closely reflect adipose tissue mass or insulin resistance in humans (38–40). HMGB1 is also found to be expressed in human adipose tissue with the expression levels associated with the fat mass and obesity-associated gene (41). We observed that both HMGB1 and S100b mRNAs are endogenously expressed in this experimental system. Neither of the mRNAs was induced by RAGE overexpression, and addition of neither of the ligands significantly increased adipocyte hypertrophy both in the presence and absence of RAGE overexpression. Of particular importance, however, simultaneous knockdown of both of the genes canceled RAGE-mediated adipocyte hypertrophy, although suppression of neither of the genes alone is effective. Thus, it appears likely that ligation of ligands to RAGE play fundamental roles in RAGE function in adipocyte, and each RAGE ligand may play in a redundant fashion.
RAGE and Tlr.
Infection of cells by microorganisms activates the inflammatory response, and the initial sensing of infection is mediated by innate pattern recognition receptors, including Tlr (42). Interactions between RAGE and Tlr systems have been implicated in multiple pathophysiological conditions including obesity (29–31). HMGB1 may signal through RAGE, and via Tlr2 and Tlr4, with the activation resulting in induction of adhesion molecules and proinflammatory cytokines in endothelial cells (31). HMGB1 induces release of cytokines and activation of coagulation, and neutrophil recruitment in mice was shown via a mechanism that at least in part depends on Tlr4 and RAGE (30). Cytokine induction by glycated LDL in mouse macrophages was also shown to be dependent on Tlr4 and RAGE (29). Tlr4 appears to be fundamental for diet-induced obesity, vascular inflammation, and insulin resistance (43–46).
In our current study, Tlr2 signaling, but not Tlr4, could be an important mediator of RAGE function in mouse adipocyte. We found that RAGE overexpression increases Tlr2 mRNA levels, and knockdown of Tlr2 mRNA cancels RAGE-medicated adipocyte hypertrophy. Tlr2 also recognizes a large number of lipid-containing molecules and transduces inflammatory signaling in a variety of cell types, including insulin-responsive cells. The absence of Tlr2 is shown to attenuate local inflammatory cytokine expression and related signaling and increases insulin action specifically in the liver (47). In adipose tissues, apoCIII may activate inflammatory signaling through Tlr2 and induce proinflammatory adipokine expression in vitro and in vivo (48). Adipocytes derived from stromal vascular cells isolated from Tlr2-deficient adipose tissue are shown to have considerably greater basal and insulin-stimulated glucose uptake as compared with those obtained from WT mice (49). These previous findings suggest Tlr2 is also an important regulator of adipocyte function and may play fundamental roles in RAGE-mediated generation of hypertrophic adipocyte. In our system, palmitate, a saturated fatty acid recognized by Tlr2, accelerates accumulation of lipid droplets during 3T3-L1 adipogenesis.
Of particular importance, morphology of palmitate-induced adipocytes with larger lipid droplets is completely distinct from that of hypertrophic adipocytes induced by RAGE overexpression. Palmitate does homogenously increase the size of lipid droplets, but does not induce formation of hypertrophic adipocytes defined as ring-like lipid droplets (Fig. 1B versus Fig. 5C), suggesting that Tlr2 activation is essential but not sufficient for RAGE-mediated generation of hypertrophic adipocytes. RAGE overexpression results in only isolated cells (<10%) becoming hypertrophic, whereas the others remain not hypertrophic as determined by cytomorphometric analyses, which may result in distribution of FSC in flow cytometry being indistinguishable between LacZ– and RAGE-overexpressing cells. Of importance, however, these RAGE-mediated sporadic changes are associated with detectable and significant functional changes of total adipocytes, including gene expression (i.e., marked suppression of Glut4 and adiponectin), attenuated insulin-mediated signaling, and insulin sensitivity (attenuated glucose uptake). It appears unlikely that isolated hypertrophy occurs only in the cells that were infected with the Ad-RAGE, because overall infection efficiency was >80% as determined by β-galactosidase staining after Ad-LacZ infection. Fig. 2C also showed that not all RAGE-positive cells became hypertrophic. At present, no evidence was available to explain this sporadic effect of RAGE overexpression. In our preliminary observations, adenoviral overexpression of RAGE retards 3T3-L1 cell growth (data not shown), which may strongly affect adipogenic differentiation. Because expression of typical adipogenic factors including PPARγ or FABP4 was not markedly altered by RAGE overexpression, unidentified factors directly involved in hypertrophic process, might be an important target of RAGE signaling.
The particular importance of our study is that the potential RAGE–Tlr2 axis observed in 3T3-L1 culture system has been at least partly extended to the in vivo mouse model; Tlr2 mRNA expression is significantly lower in RAGE-deficient than that in WT adipose tissue. Of interest, suppression of Tlr2 mRNA expression in RAGE-deficient mice is observed only in a relatively earlier time point (15 weeks of age). This observation may be in good agreement that the effect of RAGE overexpression on increase in Tlr2 mRNA in vitro is also confined to an earlier time point, with the difference gradually disappearing during the differentiation process. Thus, only transient regulation of Tlr2 may be involved in RAGE-mediated regulation of adiposity.
Limitations.
In the current study, we observed potential RAGE regulation of Tlr2 in adipose tissue in vivo at an earlier age (15 weeks). Because the Tlr2 expression is not different in RAGE-deficient adipose tissue at the time when adipose tissue mass is affected (20 weeks), the data could be interpreted as suggesting that Tlr2 expression is affected by RAGE, but may not be a causative factor controlling its effect on adipose mass. Because RAGE–Tlr2 linkage could also be important in other cell components in adipose tissue, such as inflammatory cells or endothelial cells, it would not be easy to establish a simple model system to look at RAGE–Tlr2 system in adipocyte in vivo. Another important point to be unveiled in a future study is the distinct morphological phenotype between adipocytes induced by RAGE overexpression and Tlr2 ligands. Although the current study only focused on the functional phenotype induced by RAGE overexpression, future extension of this study to discriminate RAGE signaling from Tlr2 signaling will be of great importance in further understanding of adipocyte differentiation and function. Nevertheless, RAGE–Tlr2 linkage in adipocyte in vitro, however, is entirely a novel finding and could open up a new research area in the field of obesity and metabolic syndrome.
In conclusion, RAGE appears to be involved in mouse adipocyte hypertrophy and insulin sensitivity, whereas Tlr2 regulation may at least partly play a role.
ACKNOWLEDGMENTS
This study was supported by a Grant-in-Aid for Scientific Research (23591329 to H.K.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by a Grant-in-Aid for Promotion of Technological Seeds in Advanced Medicine, Hyogo College of Medicine (to T.Y.).
No potential conflicts of interest relevant to this article were reported.
M.M., Y.O., T.M., K.Mor., T.S., Y.M., K.Mot., S.F., and M.K. researched data. H.K. wrote the manuscript and researched data. A.S., M.E., Y.Y., H.Y., Y.N., T.Y., and M.I. contributed to the discussion and reviewed and edited the manuscript. H.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Schmidt AM, Vianna M, Gerlach M, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem 1992;267:14987–14997 [PubMed] [Google Scholar]
- 2.Neeper M, Schmidt AM, Brett J, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem 1992;267:14998–15004 [PubMed] [Google Scholar]
- 3.Kislinger T, Fu C, Huber B, et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem 1999;274:31740–31749 [DOI] [PubMed] [Google Scholar]
- 4.Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 1998;4:1025–1031 [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Kato I, Doi T, et al. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest 2001;108:261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bierhaus A, Haslbeck KM, Humpert PM, et al. Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. J Clin Invest 2004;114:1741–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soro-Paavonen A, Watson AM, Li J, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 2008;57:2461–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 1999;97:889–901 [DOI] [PubMed] [Google Scholar]
- 9.Hori O, Brett J, Slattery T, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem 1995;270:25752–25761 [DOI] [PubMed] [Google Scholar]
- 10.Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 2000;405:354–360 [DOI] [PubMed] [Google Scholar]
- 11.Yan SD, Chen X, Fu J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 1996;382:685–691 [DOI] [PubMed] [Google Scholar]
- 12.Chavakis T, Bierhaus A, Al-Fakhri N, et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med 2003;198:1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001;293:1673–1677 [DOI] [PubMed] [Google Scholar]
- 14.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harja E, Bu DX, Hudson BI, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice. J Clin Invest 2008;118:183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueno H, Koyama H, Shoji T, et al. Receptor for advanced glycation end-products (RAGE) regulation of adiposity and adiponectin is associated with atherogenesis in apoE-deficient mouse. Atherosclerosis 2010;211:431–436 [DOI] [PubMed] [Google Scholar]
- 17.Koyama H, Shoji T, Yokoyama H, et al. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 2005;25:2587–2593 [DOI] [PubMed] [Google Scholar]
- 18.Koyama H, Yamamoto H, Nishizawa Y. RAGE and soluble RAGE: potential therapeutic targets for cardiovascular diseases. Mol Med 2007;13:625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YH, Chen SY, Wiesner RJ, Huang YF. Simple flow cytometric method used to assess lipid accumulation in fat cells. J Lipid Res 2004;45:1162–1167 [DOI] [PubMed] [Google Scholar]
- 20.Yonekura H, Yamamoto Y, Sakurai S, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J 2003;370:1097–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoji T, Koyama H, Morioka T, et al. Receptor for advanced glycation end products is involved in impaired angiogenic response in diabetes. Diabetes 2006;55:2245–2255 [DOI] [PubMed] [Google Scholar]
- 22.Orlicky DJ, Schaack J. Adenovirus transduction of 3T3-L1 cells. J Lipid Res 2001;42:460–466 [PubMed] [Google Scholar]
- 23.Morioka T, Koyama H, Yamamura H, et al. Role of H1-calponin in pancreatic AR42J cell differentiation into insulin-producing cells. Diabetes 2003;52:760–766 [DOI] [PubMed] [Google Scholar]
- 24.Mori K, Giovannone B, Smith RJ. Distinct Grb10 domain requirements for effects on glucose uptake and insulin signaling. Mol Cell Endocrinol 2005;230:39–50 [DOI] [PubMed] [Google Scholar]
- 25.Saito K, Lee S, Shiuchi T, et al. An enzymatic photometric assay for 2-deoxyglucose uptake in insulin-responsive tissues and 3T3-L1 adipocytes. Anal Biochem 2011;412:9–17 [DOI] [PubMed] [Google Scholar]
- 26.Myint KM, Yamamoto Y, Doi T, et al. RAGE control of diabetic nephropathy in a mouse model: effects of RAGE gene disruption and administration of low-molecular weight heparin. Diabetes 2006;55:2510–2522 [DOI] [PubMed] [Google Scholar]
- 27.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodgkinson CP, Laxton RC, Patel K, Ye S. Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arterioscler Thromb Vasc Biol 2008;28:2275–2281 [DOI] [PubMed] [Google Scholar]
- 30.van Zoelen MA, Yang H, Florquin S, et al. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock 2009;31:280–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 2008;11:91–99 [DOI] [PubMed] [Google Scholar]
- 32.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem 2006;281:26865–26875 [DOI] [PubMed] [Google Scholar]
- 33.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006;116:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodiño-Janeiro BK, Salgado-Somoza A, Teijeira-Fernández E, González-Juanatey JR, Alvarez E, Eiras S. Receptor for advanced glycation end-products expression in subcutaneous adipose tissue is related to coronary artery disease. Eur J Endocrinol 2011;164:529–537 [DOI] [PubMed] [Google Scholar]
- 35.Unoki H, Bujo H, Yamagishi S, Takeuchi M, Imaizumi T, Saito Y. Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes Res Clin Pract 2007;76:236–244 [DOI] [PubMed] [Google Scholar]
- 36.Haimoto H, Hosoda S, Kato K. Differential distribution of immunoreactive S100-alpha and S100-beta proteins in normal nonnervous human tissues. Lab Invest 1987;57:489–498 [PubMed] [Google Scholar]
- 37.Kato K, Suzuki F, Ogasawara N. Induction of S100 protein in 3T3-L1 cells during differentiation to adipocytes and its liberating by lipolytic hormones. Eur J Biochem 1988;177:461–466 [DOI] [PubMed] [Google Scholar]
- 38.Braga CW, Martinez D, Wofchuk S, Portela LV, Souza DO. S100B and NSE serum levels in obstructive sleep apnea syndrome. Sleep Med 2006;7:431–435 [DOI] [PubMed] [Google Scholar]
- 39.Steiner J, Schiltz K, Walter M, et al. S100B serum levels are closely correlated with body mass index: an important caveat in neuropsychiatric research. Psychoneuroendocrinology 2010;35:321–324 [DOI] [PubMed] [Google Scholar]
- 40.Steiner J, Walter M, Guest P, et al. Elevated S100B levels in schizophrenia are associated with insulin resistance. Mol Psychiatry 2010;15:3–4 [DOI] [PubMed] [Google Scholar]
- 41.Lappalainen T, Kolehmainen M, Schwab U, et al. Gene expression of FTO in human subcutaneous adipose tissue, peripheral blood mononuclear cells and adipocyte cell line. J Nutrigenet Nutrigenomics 2010;3:37–45 [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805–820 [DOI] [PubMed] [Google Scholar]
- 43.Suganami T, Yuan X, Shimoda Y, et al. Activating transcription factor 3 constitutes a negative feedback mechanism that attenuates saturated Fatty acid/toll-like receptor 4 signaling and macrophage activation in obese adipose tissue. Circ Res 2009;105:25–32 [DOI] [PubMed] [Google Scholar]
- 44.Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol 2007;27:84–91 [DOI] [PubMed] [Google Scholar]
- 45.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007;56:1986–1998 [DOI] [PubMed] [Google Scholar]
- 46.Kim F, Pham M, Luttrell I, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 2007;100:1589–1596 [DOI] [PubMed] [Google Scholar]
- 47.Kuo LH, Tsai PJ, Jiang MJ, et al. Toll-like receptor 2 deficiency improves insulin sensitivity and hepatic insulin signalling in the mouse. Diabetologia 2011;54:168–179 [DOI] [PubMed] [Google Scholar]
- 48.Abe Y, Kawakami A, Osaka M, et al. Apolipoprotein CIII induces monocyte chemoattractant protein-1 and interleukin 6 expression via Toll-like receptor 2 pathway in mouse adipocytes. Arterioscler Thromb Vasc Biol 2010;30:2242–2248 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Davis JE, Braucher DR, Walker-Daniels J, Spurlock ME. Absence of Tlr2 protects against high-fat diet-induced inflammation and results in greater insulin-stimulated glucose transport in cultured adipocytes. J Nutr Biochem 2011;22:136–141 [DOI] [PubMed] [Google Scholar]