Abstract
Two-thirds of adults in the U.S. are overweight or obese, and another 26 million have type 2 diabetes (T2D). Patients with diabetes and/or the metabolic syndrome have a significantly increased risk of heart attack and stroke compared with people with normal insulin sensitivity. Decreased insulin sensitivity in cardiovascular tissues as well as in traditional targets of insulin metabolic signaling, such as skeletal muscle, is an underlying abnormality in obesity, hypertension, and T2D. In the vasculature, insulin signaling plays a critical role in normal vascular function via endothelial cell nitric oxide production and modulation of Ca2+ handling and sensitivity in vascular smooth muscle cells. Available evidence suggests that impaired vascular insulin sensitivity may be an early, perhaps principal, defect of vascular function and contributor to the pathogenesis of vascular disease in persons with obesity, hypertension, and T2D. In the overweight and obese individual, as well as in persons with hypertension, systemic and vascular insulin resistance often occur in concert with elevations in plasma aldosterone. Indeed, basic and clinical studies have demonstrated that elevated plasma aldosterone levels predict the development of insulin resistance and that aldosterone directly interferes with insulin signaling in vascular tissues. Furthermore, elevated plasma aldosterone levels are associated with increased heart attack and stroke risk. Conversely, renin–angiotensin–aldosterone system and mineralocorticoid receptor (MR) antagonism reduces cardiovascular risk in these patient populations. Recent and accumulating evidence in this area has implicated excessive Ser phosphorylation and proteosomal degradation of the docking protein, insulin receptor substrate, and enhanced signaling through hybrid insulin/IGF-1 receptor as important mechanisms underlying aldosterone-mediated interruption of downstream vascular insulin signaling. Prevention or restoration of these changes via blockade of aldosterone action in the vascular wall with MR antagonists (i.e., spironolactone, eplerenone) may therefore account for the clinical benefit of these compounds in obese and diabetic patients with cardiovascular disease. This review will highlight recent evidence supporting the hypothesis that aldosterone and MR signaling represent an ideal candidate pathway linking early promoters of diabetes, especially overnutrition and obesity, to vascular insulin resistance, dysfunction, and disease.
ALDOSTERONE AND CARDIOVASCULAR RISK
Epidemiological studies demonstrate a clear correlation between elevated aldosterone levels and increased rates of cardiovascular disease (CVD) in patients with and without primary hyperaldosteronism (1,2). Furthermore, it is well-established that inhibitors of the renin–angiotensin–aldosterone system (RAAS) reduce cardiovascular ischemic events (i.e., heart attack and stroke) and mortality (3–6). This is true of antagonists to angiotensin II (Ang II) (converting enzyme [ACE] and receptor blockade) in multiple patient populations including patients with diabetes (4). The Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) and Randomized Aldactone Evaluation Study (RALES) trials, among others, extended these beneficial findings to mineralocorticoid receptor (MR) antagonists with pronounced reductions of cardiovascular mortality reported in patients with acute myocardial infarction/heart failure and severe heart failure, respectively (5,6). These trials were the first to examine the effect of MR antagonism, in addition to standard therapy (including ACE inhibitors), on cardiovascular outcomes in these patient populations. Post hoc analysis of the EPHESUS trial suggested a greater absolute risk reduction in diabetic patients compared with nondiabetics treated with eplerenone (7). Additionally, failure of the clinical trial for the HDL-raising cholesteryl ester transfer protein inhibitor torcetrapid has recently been associated with off-target elevations of plasma aldosterone that correlated with increased atherosclerosis, ischemia, and death (8,9). Notable recent work has revealed that elevations of plasma aldosterone, within the normal range, are associated with increased ischemic events and CVD death in patients with coronary artery disease but normal cardiac function (2). In the context of diabetes, subset analysis revealed that diabetic patients with small elevations in plasma aldosterone within the normal range exhibited a 10% increase in CVD mortality relative to diabetic patients with low-normal aldosterone levels (2). Furthermore, MR blockade with eplerenone in diabetic patients already receiving ACE inhibitor therapy improved coronary flow reserve (10). Thus, clinical evidence supports substantial cardiovascular benefit of MR antagonism, particularly in patients with diabetes.
An important finding in many of these clinical studies and others is that the benefit of MR antagonism to reduce CVD outcomes occurs either independent of blood pressure changes or is disproportionately greater than would be expected by the reported changes in blood pressure (1,3,6,7,10). Clinically, MR antagonists are widely prescribed antihypertensives owing to their diuretic action at the distal tubule and collecting duct. Thus, the disparate relationship between the blood pressure- and CVD-lowering effects of MR antagonists suggests that these agents confer protection through renal- and blood pressure–independent mechanisms including direct effects in cardiovascular tissue. In the vasculature, functional aldosterone-sensitive MRs have been described in both endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) (11,12). Importantly, studies have recently revealed a role for vascular-specific MR in vascular cell gene expression, injury, and pathology (3,13,14). The remainder of this review will focus on recent studies examining the impact of aldosterone and MR signaling on vascular insulin metabolic signaling and the potential role of MR-mediated impairments in vascular insulin sensitivity as an early defect in vascular function in insulin-resistant states.
VASCULAR INSULIN SIGNALING AND RESISTANCE
Insulin metabolic signaling is an important contributor to normal vascular function and homeostasis. Much effort has been focused on delineating the various pathways for and roles of insulin signaling in vascular cells, and this material has been reviewed elsewhere (15). Beyond its systemic metabolic actions, insulin signals in vascular cells via parallel pathways downstream of the insulin receptor (IR) that account for the well-described vasomotor effects of insulin (15).
Insulin effects in ECs.
Briefly, in vascular ECs, insulin stimulates production of the vasodilator nitric oxide (NO) via activation of IR substrate (IRS)-1/phosphatidylinositol 3-kinase (PI3K) signaling (Fig. 1) (15). In contrast, insulin also stimulates production of the vasoconstrictor endothelin-1 (ET-1) via Ras/mitogen-activated protein kinase (MAPK)–dependent signaling (15). Thus, vascular insulin signaling involves a balance between vasodilator/anti-inflammatory/antiatherogenic (IRS/PI3K/NO) signaling and vasoconstrictor/proinflammatory/proatherogenic growth (Ras/MAPK/ET-1) signaling pathways. Although some controversy remains, the majority of evidence in animal and human studies demonstrates that, in healthy subjects, insulin induces endothelium- and NO-dependent vasodilation in vivo and in vitro. Further, the beneficial actions of insulin-induced NO production appropriately limit the contractile, proliferative, and inflammatory actions of insulin-stimulated growth factor production (reviewed in Ref. 15).
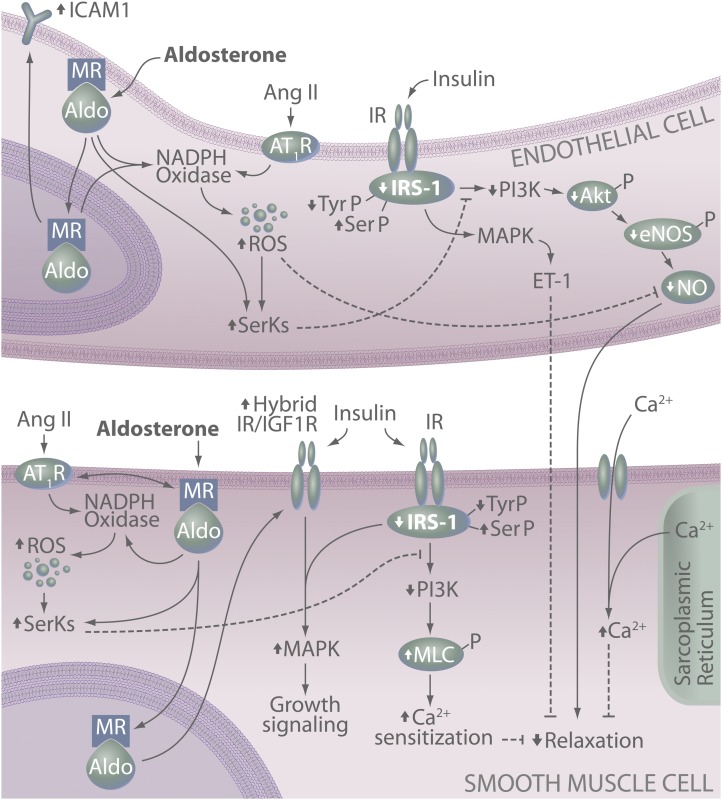
FIG. 1.
MR-dependent effects on endothelial and SMC function and insulin signaling. In vascular cells, MR activation leads to attenuated insulin signaling and ultimately reduced insulin-induced vasodilation. This involves activation of redox-sensitive and -insensitive serine kinases (SerKs), degradation of IRS-1, reduced IRS-1 signaling via PI3K, reduced production of NO, and increased smooth muscle Ca2+ levels and sensitization. MR activation also promotes a proatherogenic vascular phenotype via increased endothelial expression of ICAM-1 as well as increased insulin-mediated growth signaling in SMCs. Up (↑) and down (↓) arrows indicate effects of aldosterone/MR and/or Ang II signaling on specific molecules. Akt-P, phosphorylated protein kinase B; Aldo, aldosterone; eNOS-P, phosphorylated endothelial NO synthase; MLC-P, phosphorylated myosin light chain; SerP, serine phosphorylation; TyrP, tyrosine phosphorylation.
Insulin effects in VSMCs.
In addition to the endothelial actions of insulin leading to vasodilation, insulin promotes vasodilation via direct action in VSMCs to modulate Ca2+ handling and sensitivity (16). Specifically, insulin reduces agonist-mediated vasoconstriction in endothelium-denuded rat aorta and contraction of cultured VSMCs in vitro (16). Subsequent work revealed that insulin acts to limit Ca2+ influx through receptor- and voltage-gated Ca2+ channels and enhances Ca2+ efflux via Ca2+-ATPase (16). In addition, insulin acts to reduce Ca2+ sensitivity of VSMC contractile machinery via activation of myosin phosphatase and decreased myosin light chain phosphorylation (16). Similar to insulin signaling in ECs, evidence demonstrates that insulin actions in VSMCs require PI3K/inducible NO synthase/cyclic guanosine monophosphate signaling (16). Thus, insulin signals through the IRS/PI3K pathway in both ECs and VSMCs to induce vasodilation.
Vascular insulin resistance.
A primary feature of insulin resistance whether in skeletal muscle or vascular cells is the selective impairment of insulin-stimulated IRS/PI3K signaling with little change of insulin signaling via MAPK and other growth pathways (15). Consequently, the actions of insulin to modulate the contractile state of VSMCs directly (i.e., via Ca2+ handling) and via EC NO production are attenuated. Furthermore, downstream antioxidant, anti-inflammatory, and antiatherogenic effects of insulin signaling via IRS/PI3K are also reduced. Thus, in the face of insulin resistance-associated hyperinsulinemia, insulin-mediated MAPK signaling to oxidative, proliferative, inflammatory, and mitogenic pathways, including ET-1, is typically augmented. This scenario of reduced NO production and enhanced production of vasoconstrictor factors such as ET-1 is a characteristic feature of vascular dysfunction in insulin-resistant states (17,18). In vivo, this results in blunted insulin-induced vasodilation, capillary recruitment, and glucose disposal across skeletal muscle vascular beds (15,19).
IS VASCULAR INSULIN RESISTANCE AN EARLY CONTRIBUTOR TO VASCULAR DISEASE?
It has been accepted for many years that signs of systemic insulin resistance (e.g., hyperinsulinemia) are harbingers of more widespread and severe disease processes. Thus, it seems reasonable to inquire whether resistance to the vascular effects of insulin precedes other defects of vascular function commonly found in CVD states. There is a dearth of available information directly addressing this question, as such studies require, at a minimum, the use of animals with very early stage disease in which vasomotor responses to insulin and other vasomotor agents are examined simultaneously. A cross-sectional examination of available data, however, offers some evidence of such a scenario.
The most compelling evidence that vascular insulin resistance occurs prior to widespread vascular dysfunction comes from studies using the Zucker obese (ZO) rat model. In cremaster muscle arterioles from 14–16-week-old ZO rats, impaired insulin-induced vasodilation has been demonstrated in the absence of impaired dilation to acetylcholine (20). This is consistent with evidence from coronary arterioles in the ZO model in which insulin-induced vasodilation is attenuated as early as 12 weeks of age, whereas acetylcholine-induced vasodilation is impaired much later at 28–36 weeks of age (21,22). These studies are especially noteworthy as they demonstrate early vascular insulin resistance in the skeletal and cardiac muscle microcirculation, the primary site of tissue blood flow control. Early vascular insulin resistance has also been reported in rodent models of hypertension and aging. Specifically, aortic dilation to insulin, but not acetylcholine, is reduced in the spontaneously hypertensive rat prior to the onset of hypertension (23) and in aged (24-month-old) rats (24). Data from these studies remain equivocal regarding the potential involvement of increased reactive oxygen species (ROS) formation in reducing vascular insulin signaling, particularly in ECs. Thus, vascular insulin resistance appears to be an early vascular defect in multiple disease models and may serve as a unifying hypothesis for widespread vascular disease. Furthermore, a contribution of EC insulin resistance to the development of more global EC dysfunction (i.e., dysfunction involving other pathways regulating NO production) is suggested by the presence of endothelial dysfunction in mice with EC-specific insulin resistance and in human carriers of the IRS-1 Arg972 polymorphism (25,26). Thus, a better understanding of the precipitating factors and mechanisms underlying early defects in vascular insulin signaling is a critical area of research and an exciting target for future therapeutics.
ALDOSTERONE AND VASCULAR INSULIN RESISTANCE
Associations among aldosterone, obesity, and CVD.
Based on recent and accumulating reports, we posit that aldosterone and MR signaling represents an ideal candidate pathway linking early promoters of diabetes, especially overnutrition and obesity, to vascular insulin resistance, dysfunction, and disease (Fig. 2). This is supported by several parallel lines of evidence. First, strong associations of aldosterone, MR signaling, and systemic insulin resistance have been reported and reviewed elsewhere (27,28). Importantly, plasma aldosterone is correlated with BMI and insulin resistance in normotensive subjects (29). A link between progressive elevations of plasma aldosterone and BMI is further suggested by recent evidence that the novel adipokine complement-C1q tumor necrosis factor–related protein 1 stimulates aldosterone production and is elevated in adipose tissue of obese rodents (30). Secondly, dietary salt restriction, which increases aldosterone production, also induces vascular insulin resistance, as reflected in reduced insulin-induced vasodilation in healthy subjects (28). Finally, mild elevations of plasma aldosterone within the normal range increase cardiovascular mortality in patients with coronary artery disease (2). This evidence supports the notion that aldosterone production is related to adiposity and that even mild elevations in plasma aldosterone confer significant effects on vascular insulin sensitivity and cardiovascular outcomes. Although we believe MR signaling to be a primary player in outcomes related to overnutrition and obesity, we recognize that other adipokines also likely contribute to vascular insulin resistance in vivo. Thus, aldosterone and MR signaling likely converge with other systemic (i.e., adipokines) and local factors related to overnutrition. One example is activation of the nutrient-sensing mammalian target of rapamycin pathway to impair insulin signaling in vascular tissues. With regard to the pathogenesis of diabetes and CVD, however, we believe available evidence supports a principal role for progressive elevations in aldosterone production and vascular MR activation, related to increases in adiposity, in the development of systemic and vascular insulin resistance.
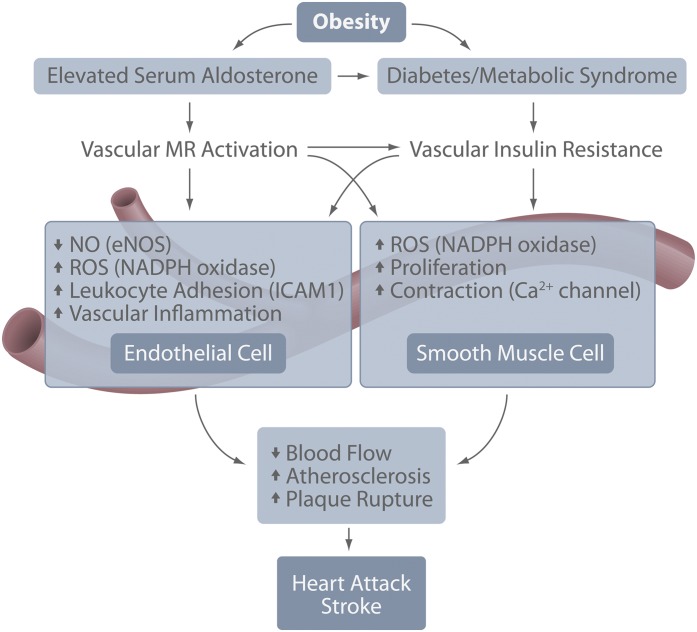
FIG. 2.
Schematic model representing the relationships and interactions among obesity, diabetes, vascular aldosterone/MR, and insulin signaling that contribute to heart attack and stroke. eNOS, endothelial NO synthase.
Aldosterone-induced insulin resistance in VSMCs.
Although aldosterone has been linked to impaired insulin-induced vasodilation, few studies have examined underlying mechanism(s) in vascular cells. Recent seminal work in VSMCs, however, demonstrates pronounced effects of aldosterone to limit insulin signaling and action in the vasculature (Fig. 1) (31,32). These reports demonstrate effects of aldosterone at the level of both IRS-1 and via IGF-1 receptor (IGF-1R) (31,32). Regarding effects on IRS-1, aldosterone treatment of cultured VSMCs increases proteosomal degradation of IRS-1 and attenuates insulin-induced Akt phosphorylation and glucose uptake (31). This effect was prevented by MR blockade, Src inhibition, and antioxidants, indicating a role for MR-dependent activation of Src and ROS generation. These results are in line with evidence implicating excessive Ser phosphorylation of IRS-1 as a key mechanism underlying cellular insulin resistance (33). Aldosterone-induced activation of Src and ROS production can also activate both redox-sensitive and -insensitive Ser kinases, including p70 S6 kinase 1, Rho kinase, and protein kinase C, among others (34). Aldosterone has been linked to ROS-dependent activation of Rho kinase in VSMCs (35) and p70 S6 kinase activation in proximal tubule and glomerular mesangial cells (36). Thus, it is likely that MR signaling activates multiple kinases directly or indirectly via ROS production and changes in cellular redox state leading to increased IRS-1 Ser phosphorylation, reduced IRS-1 tyrosine phosphorylation, and subsequent attenuation of vascular insulin signaling. Future work is necessary to elucidate the specific kinases involved in aldosterone-induced IRS-1 degradation in VSMCs.
A more recent investigation demonstrated, using both in vivo and in vitro techniques, that aldosterone-induced vascular insulin resistance involves upregulation of IGF-1R and the hybrid insulin/IGF-1R receptor in VSMCs (Fig. 1) (32). Specifically, these data showed that MR- and ROS-dependent upregulation of these receptors aggravates in vivo glucose metabolism while augmenting insulin-stimulated extracellular signal-regulated kinase 1/2 phosphorylation and hypertrophic signaling in VSMCs (i.e., protein synthesis) (32). This is consistent with previous work demonstrating aldosterone-mediated enhancement of IGF-1 signaling in VSMCs (37). With regard to insulin signaling, however, these results suggest that high levels of insulin, as occurs in insulin resistance, may signal through IGF-1R or hybrid receptors that are upregulated by aldosterone. Expression of the IR was not changed by aldosterone treatment, suggesting that the increase in hybrid receptor occurred via increased IGF-1R and subsequent association with IR (32). Although the role of IGF-1R and hybrid receptor signaling remains unclear in VSMCs, particularly with regard to vascular protective versus pathological effects, this study has improved our understanding of the potential mechanisms underlying the increased cardiovascular risk associated with elevations in aldosterone and MR signaling. However, important questions remain regarding the specific signaling cascades activated by aldosterone leading to degradation of IRS-1 and upregulation of IGF-1R and whether these are genomic or nongenomic effects of MR activation.
Aldosterone and insulin resistance in ECs.
Aldosterone and MR signaling is also known to contribute to vascular EC function (3,11); however, no study has directly examined MR-dependent effects on EC insulin signaling. This is an intriguing area of future research given multiple reports of improved endothelial function in various disease models following treatment with MR antagonists (38). Furthermore, available data demonstrate activation of various Ser kinases and reductions in EC IRS-1 protein insulin signaling in models of insulin resistance (39). Although interactions between EC MR and insulin signaling have not been described, insight regarding putative mechanisms underlying MR-insulin cross talk may be garnered from studies that have examined Ang II-dependent attenuation of EC insulin signaling. This is particularly relevant given evidence of substantial cross-activation between vascular MR and angiotensin type I receptors (AT1Rs) by their respective ligands and that MR blockade attenuates Ang II-induced vascular damage (12,14). Attenuation of insulin action in ECs by Ang II involves at least two primary mechanisms. First, in cultured ECs, Ang II induces insulin resistance via activation of numerous Ser kinases, including Jun NH2-terminal kinase, MAPK, and p70 S6 kinase 1, leading to increased Ser phosphorylation of IRS-1 and impaired insulin signaling via PI3K (33). Second, Ang II, like aldosterone, is a potent stimulator of ROS production and elevations in EC oxidative stress act indirectly to reduce insulin action by reducing insulin-stimulated NO bioavailability and vasodilation (33). These data suggest that parallels may exist between MR-mediated insulin resistance in ECs and VSMCs, particularly as they relate to the role of Ser kinases. Further studies are necessary, however, to directly examine the role of aldosterone and MR signaling in the modulation of EC insulin signaling and sensitivity and whether the benefit of MR blockade on EC function in diabetes involves restoration of EC insulin signaling.
ALDOSTERONE, INSULIN, AND ATHEROSCLEROSIS
Although the role of aldosterone-induced vascular insulin resistance in atherosclerosis has not been directly explored, overlapping pathways are dysregulated in the setting of vascular insulin resistance and vascular MR activation, thus supporting a potential molecular link (Fig. 2). Atherosclerosis is a chronic inflammatory condition of the vasculature characterized by the formation of lipid-laden plaques in the arterial circulation. Unstable plaques, characterized by increased inflammatory cells and a necrotic core with a decreased VSMC cap, are prone to rupture, resulting in acute vascular thrombosis and occlusion, the root cause of most heart attacks and strokes.
Available evidence demonstrates that MR activation promotes a proatherogenic vascular phenotype, particularly with regard to the early stages of atherosclerosis. Atherogenesis initiates with EC dysfunction characterized by decreased NO production, increased vascular ROS, and enhanced EC adhesion molecule expression with subsequent inflammatory cell recruitment and VSMC hypercontractility and proliferation. In ECs, aldosterone-induced MR activation increases expression of intracellular cell adhesion molecule 1 (ICAM-1) and the NADPH oxidase Nox4, thereby promoting oxidative stress and decreased NO bioavailability (3,11). In addition, specific deletion of SMC MR was recently shown to decrease ROS production and agonist-mediated vasoconstriction in aged mice (14). In animal models of atherosclerosis, aldosterone enhances plaque burden via increased ROS generation due, in part, to macrophage NADPH oxidase (reviewed in Ref. 3). In addition, in a mouse model with genetic deletion of the cortisol-inactivating enzyme 11-β-hydroxysteroid dehydrogenase type 2, chronic MR activation by cortisol resulted in increased atherosclerotic lesion size with increased plaque inflammation, although this mouse model is complicated by associated hypertension (40). Overall, MR activation promotes vascular endothelial dysfunction, oxidative stress and inflammation, and the formation of unstable atherosclerotic plaques that are prone to rupture. Inhibition of these processes may be responsible for the cardiovascular benefits observed with MR antagonism (41).
Impaired insulin signaling also promotes atherosclerosis by overlapping mechanisms; however, hyperinsulinemia alone is not sufficient to drive plaque formation (Figs. 1 and 2) (42). Thus, aldosterone-mediated systemic insulin resistance and the resultant hyperinsulinemia may act in concert to promote atherogenesis. Accordingly, impaired insulin signaling combined with severe dyslipidemia increases atherosclerosis by inhibiting endothelial NO synthase activation and increasing EC expression of adhesion molecules (43). Overlapping effects of insulin and MR on several vascular signaling cascades further support a link in the pathogenesis of atherosclerosis. In human VSMCs, insulin increases AT1R expression similar to reported effects of MR activation in disease states (44). Insulin-induced upregulation of the AT1R is more pronounced in cells isolated from patients with type 2 diabetes and precedes diabetes-induced plaque formation and inflammation (44,45), implicating RAAS activation in the early stages of insulin resistance-associated atherosclerosis. In addition, as discussed above, aldosterone enhances hypertrophic insulin signaling, in part, via upregulation of the hybrid insulin/IGF-1R (32), suggesting a shift in the predominant cascades activated by insulin from anti- to proatherogenic. This is consistent, in large part, with reports of proatherogenic IGF-1 signaling in disease states. Cholesterol-fed rabbits administered IGF-1 develop larger and more inflamed atherosclerotic plaques (46), and circulating IGF-1 levels are elevated in patients with complex, advanced atherosclerotic lesions (47). Conversely, overexpression of IGF-1 specifically in VSMCs in a hyperlipidemic mouse did not alter plaque size but produced plaques with increased VSMCs and decreased necrotic core, consistent with resistance to rupture (48). However, the relevance of this overexpression model requires further investigation. Further work is necessary given recent data that IGF-1/IGF-1R signaling prevents pathologic VSMC dedifferentiation, thereby increasing plaque stability (49). Overall, the proatherogenic effects of IGF-1R signaling (whether activated by insulin or IGF-1) appear to proceed predominantly through reduced NO bioavailability and increased inflammatory cell adhesion in ECs (50). As both diabetes and hyperaldosteronism predispose to cardiovascular ischemia, it seems that in the setting of advanced EC dysfunction, such as with insulin resistance and MR activation, the proatherosclerotic effects of insulin/IGF-1/IGF-1R signaling dominate to promote lesion development, inflammation, and plaque instability. Thus, although a direct link among vascular MR signaling, insulin resistance, and atherosclerosis has yet to be established, vascular insulin resistance and vascular MR regulate overlapping pathways that promote inflammatory atherosclerosis with unstable plaque morphology that would predispose to plaque rupture, heart attack, and stroke (Fig. 2).
SUMMARY
It is now clear that MR antagonists are protective in multiple CVD states. Moreover, the relationship between obesity and plasma aldosterone supports a role for MR activation as an early insult in obesity and overnutrition-related vascular dysfunction and disease. Specifically, MR activation in VSMCs induces vascular insulin resistance and downstream signaling defects characteristic of obesity-related vascular dysfunction. Importantly, evidence suggests that vascular insulin resistance may be an early and primary defect in vascular dysfunction. The role of MR activation to induce EC insulin resistance remains largely unstudied but warrants further investigation, as does the role of MR-induced vascular insulin resistance in long-term vascular complications such as atherosclerosis. Promising avenues of future investigation regarding the interaction between MR and insulin signaling in vascular tissues include MR-dependent activation of Ser kinases and IRS-1 Ser phosphorylation, as well as the delineation of MR effects on insulin–IGF-1 signaling. Furthermore, future studies should address the temporal relationship between vascular insulin resistance and overt vascular dysfunction in CVD states. Further exploration of these areas has the potential to yield novel treatment targets to prevent vascular dysfunction and CVD in the growing population of high-risk individuals with obesity and diabetes.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (HL-073101 and HL-107910 to J.R.S. and HL-095590 to I.Z.J.) and the Veterans Affairs Merit System (0018 to J.R.S.).
No potential conflicts of interest relevant to this article were reported.
S.B.B., A.P.M., I.Z.J., and J.R.S. researched, wrote, and edited the manuscript.
The authors are grateful for the assistance of Brenda Hunter and Stacy Turpin at the University of Missouri in manuscript and figure preparation, respectively.
REFERENCES
- 1.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 2005;45:1243–1248 [DOI] [PubMed] [Google Scholar]
- 2.Ivanes F, Susen S, Mouquet F, et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J 2012;33:191–202 [DOI] [PubMed] [Google Scholar]
- 3.McCurley A, Jaffe IZ. Mineralocorticoid receptors in vascular function and disease. Mol Cell Endocrinol 2012;350:256–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heart Outcomes Prevention Evaluation Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–259 [PubMed] [Google Scholar]
- 5.Pitt B, Remme W, Zannad F, et al. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–1321 [DOI] [PubMed] [Google Scholar]
- 6.Pitt B, Zannad F, Remme WJ, et al. Randomized Aldactone Evaluation Study Investigators The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–717 [DOI] [PubMed] [Google Scholar]
- 7.O’Keefe JH, Abuissa H, Pitt B. Eplerenone improves prognosis in postmyocardial infarction diabetic patients with heart failure: results from EPHESUS. Diabetes Obes Metab 2008;10:492–497 [DOI] [PubMed] [Google Scholar]
- 8.Vergeer M, Bots ML, van Leuven SI, et al. Cholesteryl ester transfer protein inhibitor torcetrapib and off-target toxicity: pooled analysis of the rating atherosclerotic disease change by imaging with a new CETP inhibitor (RADIANCE) trials. Circulation 2008;118:2515–2522 [DOI] [PubMed] [Google Scholar]
- 9.Nicholls SJ, Tuzcu EM, Brennan DM, Tardif J-C, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation 2008;118:2506–2514 [DOI] [PubMed] [Google Scholar]
- 10.Joffe HV, Kwong RY, Gerhard-Herman MD, Rice C, Feldman K, Adler GK. Beneficial effects of eplerenone versus hydrochlorothiazide on coronary circulatory function in patients with diabetes mellitus. J Clin Endocrinol Metab 2007;92:2552–2558 [DOI] [PubMed] [Google Scholar]
- 11.Caprio M, Newfell BG, la Sala A, et al. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res 2008;102:1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res 2005;96:643–650 [DOI] [PubMed] [Google Scholar]
- 13.Newfell BG, Iyer LK, Mohammad NN, et al. Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways. Arterioscler Thromb Vasc Biol 2011;31:1871–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCurley A, Pires PW, Bender SB, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 2012;18:1429–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 2006;113:1888–1904 [DOI] [PubMed] [Google Scholar]
- 16.Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol 2004;286:H1597–H1602 [DOI] [PubMed] [Google Scholar]
- 17.Bender SB, Klabunde RE. Altered role of smooth muscle endothelin receptors in coronary endothelin-1 and alpha1-adrenoceptor-mediated vasoconstriction in Type 2 diabetes. Am J Physiol Heart Circ Physiol 2007;293:H2281–H2288 [DOI] [PubMed] [Google Scholar]
- 18.Bender SB, Newcomer SC, Laughlin MH. Differential vulnerability of skeletal muscle feed arteries to dysfunction in insulin resistance: impact of fiber type and daily activity. Am J Physiol Heart Circ Physiol 2011;300:H1434–H1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubota T, Kubota N, Kumagai H, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 2011;13:294–307 [DOI] [PubMed] [Google Scholar]
- 20.Eringa EC, Stehouwer CDA, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Metab 2007;293:E1134–E1139 [DOI] [PubMed] [Google Scholar]
- 21.Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 2006;291:H1780–H1787 [DOI] [PubMed] [Google Scholar]
- 22.Katakam PV, Tulbert CD, Snipes JA, Erdös B, Miller AW, Busija DW. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. Am J Physiol Heart Circ Physiol 2005;288:H854–H860 [DOI] [PubMed] [Google Scholar]
- 23.Li R, Zhang H, Wang W, et al. Vascular insulin resistance in prehypertensive rats: role of PI3-kinase/Akt/eNOS signaling. Eur J Pharmacol 2010;628:140–147 [DOI] [PubMed] [Google Scholar]
- 24.Schulman IH, Zhou M-S, Jaimes EA, Raij L. Dissociation between metabolic and vascular insulin resistance in aging. Am J Physiol Heart Circ Physiol 2007;293:H853–H859 [DOI] [PubMed] [Google Scholar]
- 25.Perticone F, Sciacqua A, Scozzafava A, et al. Impaired endothelial function in never-treated hypertensive subjects carrying the Arg972 polymorphism in the insulin receptor substrate-1 gene. J Clin Endocrinol Metab 2004;89:3606–3609 [DOI] [PubMed] [Google Scholar]
- 26.Duncan ER, Crossey PA, Walker S, et al. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes 2008;57:3307–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briet M, Schiffrin EL. The role of aldosterone in the metabolic syndrome. Curr Hypertens Rep 2011;13:163–172 [DOI] [PubMed] [Google Scholar]
- 28.Feldman RD, Schmidt ND. Moderate dietary salt restriction increases vascular and systemic insulin resistance. Am J Hypertens 1999;12:643–647 [DOI] [PubMed] [Google Scholar]
- 29.Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab 2010;95:1986–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon JH, Kim KY, Kim JH, et al. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J 2008;22:1502–1511 [DOI] [PubMed] [Google Scholar]
- 31.Hitomi H, Kiyomoto H, Nishiyama A, et al. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension 2007;50:750–755 [DOI] [PubMed] [Google Scholar]
- 32.Sherajee SJ, Fujita Y, Rafiq K, et al. Aldosterone induces vascular insulin resistance by increasing insulin-like growth factor-1 receptor and hybrid receptor. Arterioscler Thromb Vasc Biol 2012;32:257–263 [DOI] [PubMed] [Google Scholar]
- 33.Olivares-Reyes JA, Arellano-Plancarte A, Castillo-Hernandez JR. Angiotensin II and the development of insulin resistance: implications for diabetes. Mol Cell Endocrinol 2009;302:128–139 [DOI] [PubMed] [Google Scholar]
- 34.Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85α: the two sides of a coin. Diabetes 2006;55:2392–2397 [DOI] [PubMed] [Google Scholar]
- 35.Montezano AC, Callera GE, Yogi A, et al. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol 2008;28:1511–1518 [DOI] [PubMed] [Google Scholar]
- 36.Huang S, Zhang A, Ding G, Chen R. Aldosterone-induced mesangial cell proliferation is mediated by EGF receptor transactivation. Am J Physiol Renal Physiol 2009;296:F1323–F1333 [DOI] [PubMed] [Google Scholar]
- 37.Cascella T, Radhakrishnan Y, Maile LA, et al. Aldosterone enhances IGF-I-mediated signaling and biological function in vascular smooth muscle cells. Endocrinology 2010;151:5851–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maron BA, Leopold JA. Mineralocorticoid receptor antagonists and endothelial function. Curr Opin Investig Drugs 2008;9:963–969 [PMC free article] [PubMed] [Google Scholar]
- 39.Gogg S, Smith U, Jansson P-A. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes 2009;58:2238–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deuchar GA, McLean D, Hadoke PWF, et al. 11β-hydroxysteroid dehydrogenase type 2 deficiency accelerates atherogenesis and causes proinflammatory changes in the endothelium in apoe-/- mice. Endocrinology 2011;152:236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki J, Iwai M, Mogi M, et al. Eplerenone with valsartan effectively reduces atherosclerotic lesion by attenuation of oxidative stress and inflammation. Arterioscler Thromb Vasc Biol 2006;26:917–921 [DOI] [PubMed] [Google Scholar]
- 42.Rask-Madsen C, Buonomo E, Li Q, et al. Hyperinsulinemia does not change atherosclerosis development in apolipoprotein E null mice. Arterioscler Thromb Vasc Biol 2012;32:1124–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galkina EV, Butcher M, Keller SR, et al. Accelerated atherosclerosis in Apoe-/- mice heterozygous for the insulin receptor and the insulin receptor substrate-1. Arterioscler Thromb Vasc Biol 2012;32:247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodroj W, Legedz L, Foudi N, et al. Increased insulin-stimulated expression of arterial angiotensinogen and angiotensin type 1 receptor in patients with type 2 diabetes mellitus and atheroma. Arterioscler Thromb Vasc Biol 2007;27:525–531 [DOI] [PubMed] [Google Scholar]
- 45.Ihara Y, Egashira K, Nakano K, et al. Upregulation of the ligand-RAGE pathway via the angiotensin II type I receptor is essential in the pathogenesis of diabetic atherosclerosis. J Mol Cell Cardiol 2007;43:455–464 [DOI] [PubMed] [Google Scholar]
- 46.Hirai H, Kanaya R, Maeda M, Qungfang D, Ina K, Hayashi T. The role of insulin growth factor on atherosclerosis and endothelial function: the effect on hyperlipidemia and aging. Life Sci 2011;88:425–431 [DOI] [PubMed] [Google Scholar]
- 47.Burchardt P, Gozdzicka-Jozefiak A, Zurawski J, et al. Are elevated levels of IGF-1 caused by coronary arteriesoclerosis?: Molecular and clinical analysis. Protein J 2010;29:538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shai S-Y, Sukhanov S, Higashi Y, Vaughn C, Kelly J, Delafontaine P. Smooth muscle cell-specific insulin-like growth factor-1 overexpression in Apoe-/- mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arterioscler Thromb Vasc Biol 2010;30:1916–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von der Thüsen JH, Borensztajn KS, Moimas S, et al. IGF-1 has plaque-stabilizing effects in atherosclerosis by altering vascular smooth muscle cell phenotype. Am J Pathol 2011;178:924–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li G, Barrett EJ, Ko S-H, Cao W, Liu Z. Insulin and insulin-like growth factor-I receptors differentially mediate insulin-stimulated adhesion molecule production by endothelial cells. Endocrinology 2009;150:3475–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]