Abstract
The melanocortin-4 receptor (MC4R) is well recognized as an important mediator of body weight homeostasis. Activation of MC4R causes dramatic weight loss in rodent models, and mutations in human are associated with obesity. This makes MC4R a logical target for pharmacological therapy for the treatment of obesity. However, previous studies in rodents and humans have observed a broad array of side effects caused by acute treatment with MC4R agonists, including increased heart rate and blood pressure. We demonstrate that treatment with a highly-selective novel MC4R agonist (BIM-22493 or RM-493) resulted in transient decreases in food intake (35%), with persistent weight loss over 8 weeks of treatment (13.5%) in a diet-induced obese nonhuman primate model. Consistent with weight loss, these animals significantly decreased adiposity and improved glucose tolerance. Importantly, we observed no increases in blood pressure or heart rate with BIM-22493 treatment. In contrast, treatment with LY2112688, an MC4R agonist previously shown to increase blood pressure and heart rate in humans, caused increases in blood pressure and heart rate, while modestly decreasing food intake. These studies demonstrate that distinct melanocortin peptide drugs can have widely different efficacies and side effects.
Maintenance of body weight and energy homeostasis requires balance between energy intake and expenditure and is achieved via the interaction between central and peripheral signals. The central melanocortin system is one of the key neural circuits involved in mediating the integration of information from both sites. Proopiomelanocortin is a prohormone that is processed into multiple bioactive peptides, including α-melanocyte–stimulating hormone (MSH), β-MSH, γ-MSH, and the endogenous opioid β-endorphin (1). α-MSH, or its analogs, are potent inhibitors of food intake and increase energy expenditure to promote weight loss in rodent and rhesus macaque models (2–5). Central melanocortins are involved in many physiological functions, including stress responses; however, their actions on the regulation of food intake and energy expenditure have been a focus.
Melanocortin-4 receptor (MC4R) is the main melanocortin receptor involved in the regulation of food intake and energy expenditure, primarily through modulation of sympathetic outflow (6–8). MC4R has a broad distribution, including expression in several peripheral tissues, such as muscle, kidney, and lung (9). The importance of MC4R in the maintenance of body weight homeostasis is highlighted by genetic studies in humans and mice. MC4R−/− mice are hyperphagic, have increased adipose and lean mass, and develop insulin resistance (10). In humans, mutations in the proopiomelanocortin gene (11) and the MC4R gene have a similar phenotype (12–14).
Although the effects of MC4R agonists on energy/glucose homeostasis (15,16) make it an attractive target for a therapeutic agent, the potential side effects of increasing heart rate and blood pressure have been a major limitation (17). Indeed, recent studies reported by Greenfield et al. (18) showed that acute peripheral administration with a centrally acting MC4R selective agonist increased blood pressure and heart rate in moderately obese humans. There is an obvious concern in treating obese individuals with a high risk of hypertension and cardiovascular disease with a weight loss therapy that is exacerbating these same risks. In the current study, we use a diet-induced obesity (DIO) nonhuman primate model (NHP) to determine if long-term treatment with the MC4R-specific agonist BIM-22493 can reduce food intake and adiposity without adversely affecting cardiovascular function.
RESEARCH DESIGN AND METHODS
Animals.
All animal care and procedures were done according to the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee at Oregon Health & Science University. For all studies, food intake was carefully recorded daily, and water was provided ad libitum. Lights were on from 7:00 a.m. to 7:00 p.m.
Telemetry.
PhysioTel Multiplus Transmitters (model D70-PCT; DSI, St. Paul, MN) were implanted in the abdomen of each monkey by ONPRC veterinarians to measure blood pressure (diastolic, systolic, pulse, and mean arterial pressure), heart rate, core body temperature, activity, and electrocardiogram (ECG) 4 weeks before the start of baseline measurements. ECG electrodes were tunneled subcutaneously to the left thoracic region (positive) and the right clavicle (negative). The pressure sensor catheter was tunneled subcutaneously to the left femoral groove, where it was inserted into the femoral artery and secured. Measurements were collected for 48 h before and after implantation of drug or vehicle minipump. Data were reported for the 48 h before surgery, except in the case of 48 h after implantation of the first dose of BIM-22493 and the first vehicle implant. These data points are considered as week 1 and week 9 in the graph and represent some of the immediate effects of the compound and compound removal. The recordings were continuous and values were calculated for a.m. measurements (average for 11:00 a.m.–1:00 p.m.) and p.m. measurements (11:00 p.m.–1:00 a.m.), and values were averaged for the 48 h. For the experiments involving LY2112688 (Fig. 5) a longer interval (3 h) was chosen because these data were reported as a single day. Throughout the study, heart rate measurements were performed via the ECG leads. Blood pressure measurements were subject to catheter failures. In some instances, fewer animals were used to report blood pressure data due to these missing values.
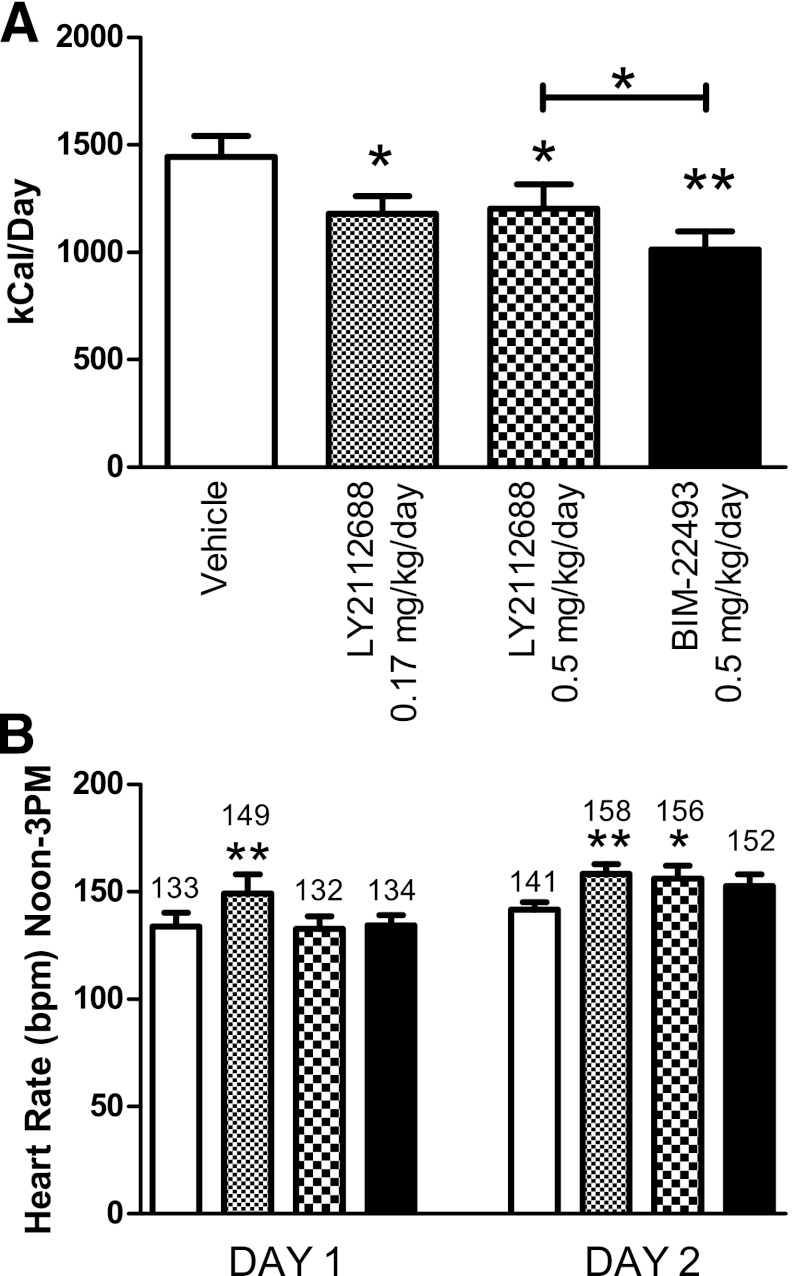
FIG. 5.
LY2112688 administration results in decreased food intake and increased heart rate. A: LY2112688 reduced average daily food intake similar to BIM-22493 measured over the week of treatment, although not as effectively (P < 0.0001). B: LY2112688 increased heart rate for 2 days after implantation of the minipump, whereas BIM-22493 did not alter heart rate (P < 0.001). All statistics were performed by repeated-measures ANOVA. *Individually significant values by Bonferroni post hoc test from vehicle period. *P < 0.05, **P < 0.01.
Experiment 1: Chronic treatment in DIO animals.
The study used 12 mature adult (age 9–11 years) male rhesus macaques, with body weights ranging from 9 to 19 kg. Monkeys were maintained in single-housing cages and fed a high-fat diet (HFD: 32% calories from fat; Custom Diet 5A1F; Test Diet, Richmond, IN) daily plus calorically dense enrichment. These animals had been maintained on the HFD for approximately 1.5 years before these studies. Nine animals were obese, insulin-resistant, and hypertensive, classified as diet-sensitive (Table 1). Three animals maintained normal body weight, adiposity, and blood pressure, and were classified as diet-resistant. Two-week minipumps (model 2ML2; Alzet, Cupertino, CA) were implanted subcutaneously in the scapular region under ketamine sedation (5 mg/kg). The animal received minipumps containing vehicle (0.9% saline, 2% heat-inactivated nonhuman primate [NHP] serum and 5% N,N-dimethylacetamide) for 4 weeks (pumps were exchanged after 2 weeks) to obtain baseline values. On study day 0, a 2-week minipump was implanted in all animals containing 0.5 mg/kg/day of BIM-22493 (dissolved in 0.9% saline, 2% heat-inactivated NHP serum, and 5% N,N-dimethylacetamide). For an overview of the study design, see Supplementary Fig. 1.
TABLE 1.
Telemetry and body composition by dual-energy X-ray absorptiometry measurements before and after treatment in diet-sensitive animals
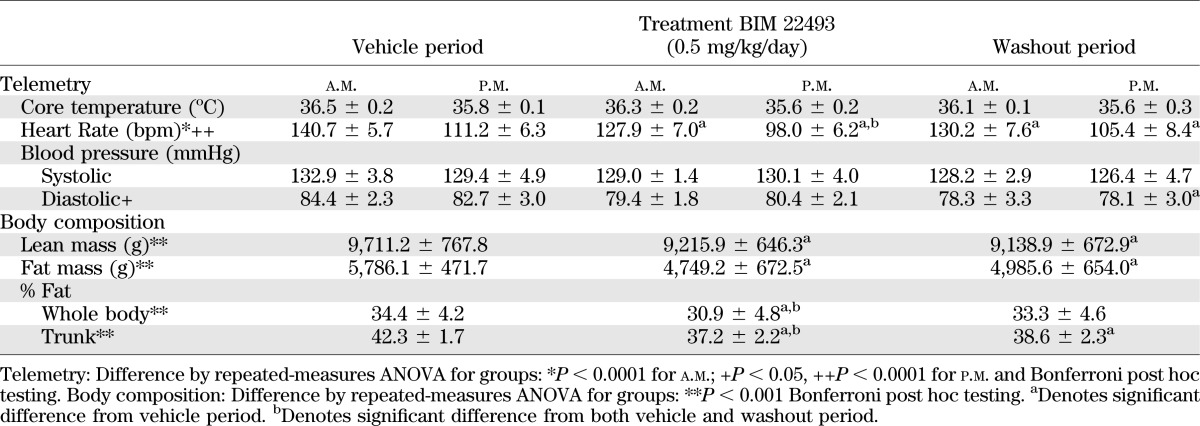
Food intake was measured daily and body weight was recorded during each minipump implantation. Minipumps were replaced biweekly for a total of 8 weeks of treatment. Dual-energy X-ray absorptiometry scanning, intravenous glucose tolerance test (IVGTT), and body weight measurement were performed (19) during the baseline period as well as 4 and 8 weeks into treatment with BIM-22493. Samples for energy expenditure using double-labeled water were collected during baseline and 8 weeks after treatment (20,21). The measurements were repeated after a 4-week washout period (through study week 12), during which period animals were kept on a vehicle minipump (0.9% saline, 2% heat-inactivated NHP serum and 5% N,N-dimethylacetamide). The purpose of this 4-week extension of the study was to determine the response of the animals to BIM-22493 washout in presence of vehicle. After 24 weeks, animals were reimplanted with new telemetry units, and the experiment was repeated with a lower dose of BIM-22493 (0.17 mg/kg/day). The results are described in the text and the Supplementary data.
Experiment 2: Effect of melanocortin agonist LY2112688 on food intake and heart rate.
After full recovery from the BIM-22493 treatment (more than 8 weeks after completion of 0.17 mg/kg/day treatment), 8 diet-sensitive animals with working telemetry devices were selected from the 12 animals described in experiment 1. These animals received three consecutive weekly therapies, each separated by 1 week of vehicle treatment, in the following order: LY2112688 (0.17 and 0.5 mg/kg/day), followed by BIM-22493 (0.5 mg/kg/day) for comparison. Food intake over the week of active treatment and cardiovascular measurements were recorded.
Statistical analysis.
Data are expressed as mean ± SEM. A repeated-measures ANOVA was used to test the significance of the outcomes using a Dunnett or Bonferroni post hoc analysis (GraphPad Prism, La Jolla, CA). A P value of ≤0.05 was considered significant. In some instances, a paired Student t test was performed and is noted as such in the figure legends. A significance of 0.05 or lower was considered significant.
RESULTS
Effects of BIM-22493 in DIO rhesus macaques.
A dose-response study (0.17, 0.5, and 1.5 mg/kg/day) in lean animals demonstrated that BIM-22493 at 0.5 mg/kg/day maximally reduced food intake; thus, this dose was chosen for chronic treatment in DIO rhesus macaques (Supplementary Fig. 2). Food intake significantly decreased by 35% during the first week of drug exposure compared with levels during the vehicle treatment period (Fig. 1A). However, this effect on food intake was transient, because food intake had normalized by weeks 4 through 7 and showed an increase during week 8 of drug treatment. It should be noted that the diet-sensitive (obese; 33% ± 5.6, n = 9) and resistant (lean; 29% ± 15, n = 3) animals displayed comparable decreases in food intake; therefore, the data were combined. Although food intake at the end of the treatment period was higher than during the vehicle treatment period, a significant increase in food intake was only observed after cessation of BIM-22493 treatment. Food intake remained elevated in these animals for several more weeks during the washout period and did not completely return to normal until 12 weeks after the cessation of BIM-22493 treatment (Supplementary Fig. 4A), when animals had returned to pretreatment body weight.
FIG. 1.
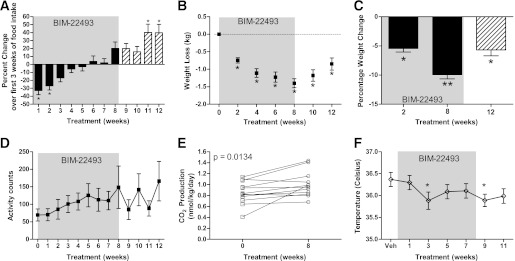
BIM-22493 treatment reduces food intake, body weight, and core body temperature and increases activity and energy expenditure in obese NHP. A: Administration of BIM-22493 (0.5 mg/kg/day) decreased food intake in the first 4 weeks. Upon termination of the treatment, a slight rebound hyperphagia can be observed during the washout period (weeks 9–12). The black bars are data from actively treated NHP, the cross-hatched bars are during vehicle treatment; the shaded area represents the 8-week treatment period. Body weight change in kilograms (B) and percentage (C) at specific time points during treatment with BIM-22493. Data (n = 12) were analyzed with repeated-measures ANOVA (P < 0.001). *Individually significant time points by Dunnett post hoc test (vs. vehicle). C: Data were analyzed by repeated measures ANOVA (P < 0.001) with Bonferroni post hoc test (weight loss at 8 weeks significantly different [**P < 0.01] vs. 2 and 12 weeks). D: Activity increases incrementally during treatment (P = 0.037 repeated-measures ANOVA over treatment period of 0–8 weeks). E: Energy expenditure as measured by double-labeled water is increased after treatment (P = 0.0134 paired Student t test compared with baseline). F: Core body temperature trended toward a decrease in response to BIM-22493. P = 0.0021 by repeated-measures ANOVA. *Individually significant time points by Dunnett post hoc test vs. vehicle.
Consistent with the decrease in food intake, the animals lost 1 kg of body weight on average (Fig. 1B and C and Supplementary Fig. 3 for individual animals) during the first 4 weeks of treatment; however, animals continued to lose weight through the 8 weeks of treatment, with a total average loss of 1.5 kg at 8 weeks of treatment (∼10% of total body weight, Fig. 1C). This continued weight loss continued during the last 4 weeks of treatment, despite the animals regaining normal consumption of a palatable HFD and calorically dense treats. The average peak weight loss achieved was 13.5% of total body weight, as 8 of the 12 animals continued to lose weight for 2 weeks after cessation of BIM-22493 treatment during the washout period. Animals had regained approximately 0.5 kg of body weight by the end of the 4-week vehicle washout period (Fig. 2B and C). The animals did not fully regain their initial body weight until 8 weeks after cessation of BIM-22493 treatment (Supplementary Fig. 4B).
FIG. 2.
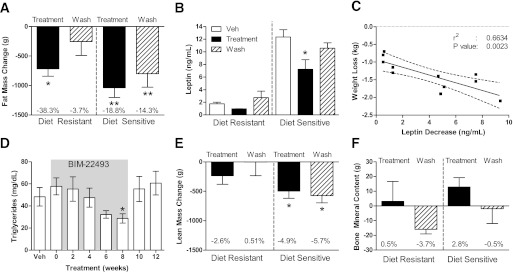
BIM-22493 treatment reduced fat mass, leptin levels, lean mass, and triglycerides in obese NHP. A: Dual-energy X-ray absorptiometry scanning determined that total fat mass was reduced in diet-resistant (n = 3) and diet-sensitive animals (n = 9) after 8 weeks of BIM-22493 treatment compared with the baseline fat mass. This loss was maintained the 4-week washout (Wash) period of saline treatment. *P < 0.05, **P < 0.005 by paired Student t test compared with starting weight. B: Leptin levels drop accordingly with body fat loss after treatment (*P < 0.001) compared with vehicle (Veh) and rebounded slightly after 4 weeks of washout. C: The decrease in leptin shown in B after treatment correlates with the amount of body weight loss (r2 = 0.6634, P = 0.0023). D: Triglyceride levels in serum drop during the drug treatment (shaded area). P = 0.0238 by ANOVA. *Individually significant by Dunnett post hoc test from vehicle. E: Lean mass was slightly reduced only in diet-sensitive animals after 8 weeks of treatment and maintained after 4 weeks of saline treatment (Wash). *P < 0.002 by paired Student t test compared with starting weight. F: Bone mineral content was not significantly altered from baseline at the end of the 8-week BIM 22493 treatment.
The decreased food intake likely contributed to the initial weight loss but does not explain the persistent weight loss. Interestingly, daytime activity gradually increased during the treatment period and nearly doubled by the end of BIM-22493 treatment (Fig. 1D; P = 0.037 repeated-measures ANOVA over the treatment period). It is difficult to determine from these studies if the change in activity was in response to BIM-22493 treatment or was secondary to the weight loss. However, regression analysis demonstrated that there was no significant relationship between the amount of weight lost and the increase in activity (data not shown). There was no difference in nighttime activity, indicating that there was no disturbance in sleep patterns. Energy expenditure, as indicated by increased CO2 production from double-labeled water studies, was also increased by 14% at the end of the treatment period compared with the vehicle period (Fig. 1E). Core body temperature was decreased by approximately 0.5°C with BIM-22493 treatment (Fig. 1F; P = 0.0161 repeated-measures ANOVA, *P < 0.05 with Dunnett post hoc test compared with vehicle).
BIM-22493 reduced fat mass in diet-sensitive (obese) and diet-resistant (lean) animals (Fig. 2A and Table 1). After 8 weeks of BIM-22493 treatment, the diet-sensitive animals lost an average of 1.04 ± 0.17 kg of fat mass (or 18.8 ± 3.5% of their original fat mass), whereas diet-resistant animals lost almost 0.75 ± 0.13 kg of fat mass (38.3 ± 7.2%; Table 1). Diet-sensitive animals retained significantly lower fat mass compared with the period of vehicle treatment (P < 0.05; Table 1). As expected, leptin levels were also decreased with BIM-22493 treatment; however, this decrease was only significant in the diet-sensitive animals (Fig. 2B). As a group, the magnitude of the decrease in leptin was significantly correlated with weight loss (Fig. 2C), and removal of the drug resulted in a rebound in leptin levels. Fasting serum triglyceride levels displayed a gradual decrease through the BIM-22493 treatment but were only significantly different at the end of 8 weeks (Fig. 2D). As expected given the profound levels of body weight loss, lean mass was also significantly decreased in diet-sensitive animals with BIM-22493 treatment (Fig. 2E). Bone mineral content and bone mineral density were not altered (Fig. 2F and data not shown). No differences in serum chemistry were observed in levels of blood urea nitrogen, alanine transaminase, bilirubin, creatinine, albumin, and creatinine kinase (Supplementary Table 2).
Diet-sensitive animals displayed significant insulin resistance before drug treatment, as indicated by increased fasting hyperinsulinemia and increased glucose-stimulated insulin secretion. Some animals also displayed impaired glucose clearance during the IVGTT; however, none of the animals displayed fasting hyperglycemia, indicating they were not diabetic. After 8 weeks of BIM-22493 treatment, fasting insulin levels and homeostasis model assessment insulin resistance improved in the diet-sensitive animals (Fig. 3A and B for diet-sensitive animals, data not shown; and Supplementary Fig. 4C for diet-resistant animals). Surprisingly, even though animals had regained some of their body fat by 4 weeks after cessation of drug treatment, fasting insulin levels remained improved in diet-sensitive animals.
FIG. 3.
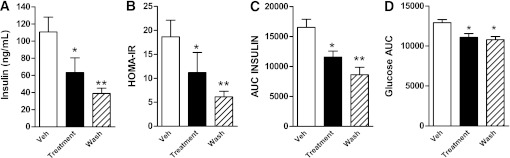
BIM-22493 ameliorates insulin resistance in obese NHP. Fasting insulin levels (A) and homeostasis model assessment insulin resistance (HOMA-IR) (B) in diet-sensitive animals is dramatically decreased during BIM-22493 treatment and is maintained for 4 weeks after cessation of treatment compared with vehicle (Veh). Wash, washout. *P < 0.05, **P < 0.005 compared with control by repeated-measures ANOVA with Dunnett post hoc testing. C: Insulin release in response to an IVGTT is reduced in obese NHP treated with BIM-22493, as measured by area under the curve (AUC). *P < 0.01, **P < 0.0001 by repeated-measures ANOVA with Dunnett post hoc testing. D: Glucose clearance as measured by AUC was significantly improved in response to an IVGTT. *P < 0.05 by repeated-measures ANOVA with Dunnett post hoc testing.
To gain a better understanding of the insulin sensitivity and glucose tolerance we performed an IVGTT. Consistent with the improved insulin sensitivity, insulin secretion and glucose clearance were improved in diet-sensitive animals after 8 weeks of BIM-22493 treatment (Fig. 3C and D). Again, insulin secretion and glucose tolerance remained improved even 4 weeks after cessation of BIM-22493 treatment. By 12 weeks of drug washout, insulin sensitivity and glucose tolerance returned to predrug treatment levels in diet-sensitive animals (Supplementary Fig. 4). It is important to note that a similar treatment paradigm with a threefold lower dose of BIM-22493 (0.17 mg/kg/day) resulted in a similar improvement in insulin sensitivity while having less of an effect on food intake and body weight (Supplementary Fig. 5). This lower dose also did not result in changes to blood pressure or heart rate (Supplementary Fig. 5D and E).
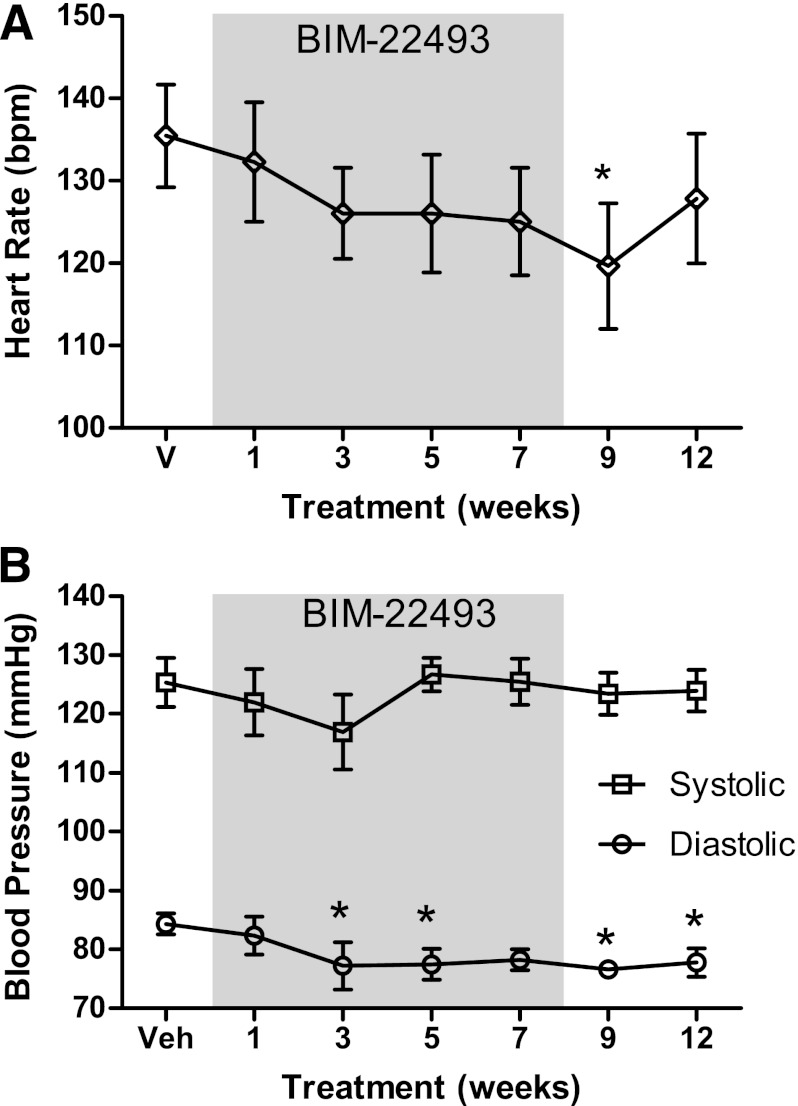
There is substantial evidence in the literature that agonists of the MC4R pathway can result in increased sympathetic outflow and increased heart rate and blood pressure (18,22–24). To characterize the cardiac events during drug treatment, the animals were implanted with telemetry devices to measure blood pressure and heart rate. No acute increase in heart rate occurred on the first day of drug exposure (Supplementary Fig. 6). Throughout the treatment period, increases in heart rate and blood pressure were not observed with BIM-22493 treatment. In fact, as a whole, there was a significant decrease in heart rate in response to the BIM-22493 treatment (P = 0.008 repeated-measures ANOVA); however, the only individual time point that was significant by post hoc analysis (Dunnett multiple comparison test) was the week after cessation of drug treatment (Fig. 4A). The decreases in heart rate were noted at a time of significant weight loss. The decrease in heart rate was primarily observed in diet-sensitive animals (Table 1), where there was a 13-bpm decrease with drug treatment. Paralleling the decreased heart rate, there was also a significant reduction in diastolic blood pressure (Fig. 4B), which was observed in both diet-sensitive and diet-resistant animals (Table 1). Systolic pressure was not altered during treatment.
FIG. 4.
BIM-22493 reduces heart rate and diastolic blood pressure over the course of the treatment. A: Measurements of heart rate after administration of BIM-22493. Heart rate measurements during the normal daytime hours demonstrated that a trend toward reduction in heart rate was seen over the whole period (P = 0.0035). B: Diastolic blood pressure, but not systolic, was reduced in response to the BIM-22493 treatment (P = 0.02). All statistics were performed by repeated-measures ANOVA. *Individually significant by Dunnett post hoc test vs. vehicle. All data points are recorded before surgery, except in cases of week 1 of treatment and week 9 of washout, where data were collected for 48 h postimplantation of BIM-22493 and vehicle respectively.
It is important to note that during these studies no changes in animal behavior were noted before, during, or after administration with BIM-22493 (Supplementary Data).
Effects of LY2112688 in DIO rhesus macaques.
Previous studies performed in rodents and humans have shown increases in blood pressure in response to MC4R agonists (18,22–24). These anticipated changes were not observed in our chronic BIM-22493 treatment with osmotic minipumps. To determine whether a distinct compound, which has been tested in humans and adversely affected heart rate and blood pressure, while showing marginal efficacy, could cause similar effects in the DIO NHP model, we synthesized the compound LY2112688 used by Greenfield et al. (18). We compared a BIM-22493 dose of 0.5 mg/kg/day with several concentrations of LY2112688 (up to 0.5 mg/kg/day) and observed that although LY2112688 significantly reduced food intake (Fig. 5A), the decrease was significantly smaller than a comparable dose of BIM-22493. Despite its smaller effect on food intake, indicative of a smaller pharmacodynamic effect on the MC4R compared with BIM-22493, LY2112688 significantly increased heart rate for up to 2 days when administered via implanted minipumps (n = 8, Fig. 5B). Similar findings were reported by Greenfield et al. (18). Significant mean increases of 15.5 and 16.5 bpm for LY2112688 at 0.17 mg/kg/day for day 1 and 2, respectively, and 14.4 bpm for LY2112688 at 0.5 mg/kg/day on day 2 were observed. Furthermore, three of five animals with functioning blood pressure catheters displayed an increase in blood pressure (Supplementary Fig. 6). Importantly, the same dose of BIM-22493 did not significantly raise heart rate (Fig. 5B) or affect blood pressure (Supplementary Fig. 6).
DISCUSSION
This study describes a MC4R agonist that reduced body weight and adiposity through inhibition of food intake and increasing energy expenditure in a NHP model of DIO. On average, the animals displayed a peak weight loss of 13.5%. Most of the weight loss was due to loss of body fat, with smaller losses of lean mass. Impressively, this weight loss occurred in animals being maintained ad libitum on a palatable high-fat and high-calorie diet. Although all animals lost weight, the more obese animals lost a greater absolute amount of fat mass and lean mass. One striking observation was the effect of BIM-22493 on food intake, which was significantly reduced during the first 2 weeks of treatment but increased back to its original starting levels by weeks 4 and 5 of treatment. Although this transient reduction in food intake might signal a resistance to the function of the drug, the animals continued to lose weight during the whole 8-week treatment period. It was not until the cessation of BIM-22493 treatment that the body weight started returning to pretreatment levels. Therefore, it is likely that the drop in food intake caused the initial decrease in body weight in the earlier weeks but that the small increase in activity and energy expenditure observed in the late stages of treatment maintained continued weight loss despite increased food intake.
This proposed mechanism is supported by studies in female rhesus macaques that demonstrated restricting caloric intake has no effect on body weight because the animals simply reduced activity to maintain body weight homeostasis. Indeed, an increase in physical activity, provided by exercising the animals was needed to induce weight loss (25). It should also be noted that while the effects on food intake did appear transient, stopping BIM-22493 treatment did cause significant rebound hyperphagia. This could suggest that BIM-22493 may suppress food intake throughout the treatment period; however, the set point of food intake may be shifting as the animals lose weight.
Consistent with the significant weight loss with BIM-22493 treatment is the dramatic improvement of glucose homeostasis. Diet-sensitive animals showed a highly significant decrease in fasting insulin levels and in insulin secretion during an IVGTT, suggesting improvement in overall insulin sensitivity in these animals persisted well beyond the cessation of drug treatment. The improvement in insulin sensitivity is likely due to several effects of BIM-22493. Studies in genetically obese mice demonstrated that BIM-22493 could have direct effects on glucose homeostasis (26), consistent with other studies demonstrating direct regulation by central melanocortins (8,27). Therefore, treatment with BIM-22493 in the obese NHP likely improved glucose homeostasis initially via direct effects of the drug on the central melanocortin system. It is surprising that improved insulin sensitivity persisted for more than 4 weeks after BIM-22493 treatment was completed and did not return to pretreatment levels until 12 weeks of washout. During this time, the animals were consuming substantially more fat calories, and circulating triglyceride levels returned to pretreatment levels. These data suggest that insulin sensitivity was more closely linked to adiposity than circulating triglycerides and argue that sustained improvement in insulin sensitivity is likely secondary to the loss of body fat rather than a direct effect of the MC4R agonist.
Although it is well accepted that MC4R agonists can cause a suppression of food intake and weight loss, the main concern limiting them as therapeutic agents is the possible adverse effect on cardiovascular function. Humans with MC4R mutations display lower blood pressure compared with weight-matched controls (18), and conversely, several rodent studies have demonstrated that MC4R agonists can increase blood pressure and heart rate, likely through activation of the sympathetic nervous system (22,28–33). The concerns over the therapeutic value of MC4R agonists for the treatment of obesity in humans were recently highlighted by a study by Greenfield et al. (18) demonstrating that a specific MC4R agonist caused a significant 9.1 mmHg increase in blood pressure in patients. These patients also experienced a 2.85-bpm increase in heart rate for the highest dose tested. These studies raised the concerns that chronic treatment with any MC4R agonist may increase risks of cardiovascular disease in patients already at high risk. To address this, we used telemetry to measure changes in blood pressure and heart rate in our animals. However, we observed no changes in blood pressure or heart rate with BIM-22493 during the initial drug exposure period when BIM-22493 was administered by the slow-release osmotic minipump. Instead, we observed a steady decline in heart rate and diastolic blood pressure throughout the treatment period. Like the changes in glucose homeostasis, the improvement in cardiovascular function is likely secondary to weight loss because there was no acute increase in these parameters with cessation of BIM-22493 treatment.
Considering the published evidence (18,30,34,35), the lack of activation of the cardiovascular system by BIM-22493 was surprising. To compare the performance of the DIO NHPs treated with BIM-22493 and the data published with other MC4R agonists, we synthesized a compound previously known to increase blood pressure and heart rate in humans (LY2112688 (18)). Indeed, this compound increased heart rates and blood pressure in our NHP model even when administered using the same subcutaneous continuous infusion previously used for BIM-22493. The difference was not due to the dose of LY2112688, because LY2112688 also suppressed food intake in our model but to a lower extent than BIM-22493. This differential response between the two compounds underscores the importance of performing cardiovascular testing of potential MC4R agonists in NHP but also provides evidence that not all MC4R agonists act in a similar manner. The reason for the differential effect is unknown, but possible explanations include:
Difference in receptor pharmacology and the mechanism and specificity by which both compounds activate the MC4R; however, BIM-22493 and LY2112688 have similar binding affinities for the MC4R, with LY2112688 being more effective in activating the receptor.
Variability in selectivity at different receptors: BIM-22493 has a higher affinity for the MC3R than the LY2112688 and perhaps modest activation of this proposed autoinhibitory receptor can change the final sympathetic output (36) and lower blood pressure. Recent studies using MC3R-antagonists demonstrated that inhibition of this receptor raises blood pressure (37).
Differences in the brain penetration of the two agonists may contribute to the variable effects on the cardiovascular system. For instance, intracerebroventricular administration of α-MSH, but not peripheral administration, results in increases in mean arterial blood pressure and heart rate in mice (24).
This would suggest that the agonist used by Greenfield et al. (18) may have a more complete brain penetration resulting in broad activation of all of the sites involved in the regulation of sympathetic outflow to the cardiovascular system. By contrast, BIM-22493 may have limited brain penetration, possibly only in areas with reduced blood–brain barrier and thus incomplete activation of the sympathetic nervous system (38). Skibicka and Grill (39) recently demonstrated that fourth ventricle administration of melanotan-II decreases food intake and increases heart rate. Furthermore, this group showed that the effect was much greater in decerebrate animals, where the response is strictly limited to actions by the brainstem. In fact, several groups have shown that activation of MC4Rs specifically in the brainstem can inhibit food intake and stimulate energy expenditure (40–43). Finally, several studies have demonstrated MC4Rs are expressed in peripheral tissues, such as skeletal muscle (9), that α-MSH increases fatty acid oxidation in isolated muscle cells (44), and that peripheral administration of NDP-αMSH, a stable analog agonist, increased resting metabolic rate (45). All of these studies suggest that deep brain penetration is not required for MC4R agonists to suppress food intake and increase energy expenditure.
In conclusion, we have described a MC4R agonist, BIM-22493 (also known as RM-493), which, after chronic subcutaneous infusion, reduced food intake and adiposity and resulted in an impressive 13.5% decrease in overall body weight. An associated improvement was noted in glucose homeostasis, insulin sensitivity, and leptin level results. Further, we report that activation of the melanocortin system using BIM-22493 does not result in immediate changes to the blood pressure or heart rate when the agonist is administered via a minipump, and in fact, that there is an overall chronic improvement of cardiovascular function secondary to weight loss. Future studies in human subjects will be needed to define the effectiveness of BIM-22493 as an antiobesity therapeutic.
ACKNOWLEDGMENTS
These studies were supported by a sponsored research agreement from Ipsen-Biomeasure Inc. and a P51 (OD011092-53) grant to the Oregon National Primate Research Center and K.L.G.
K.L.G. has received consulting fees from Ipsen-Biomeasure Inc. and Rhythm Pharmaceuticals. H.H., J.Z.D., and M.D.C. are employees of Ipsen-Biomeasure. No other potential conflicts of interest relevant to this article were reported.
P.K. researched data and wrote and reviewed the manuscript. H.H. and J.Z.D. designed and researched the data on the preclinical pharmacology and reviewed the manuscript. D.L.M. designed and researched the data on the rodent experiments and reviewed the manuscript. M.M.G., P.S., and L.P. researched data and reviewed the manuscript. M.A.C., K.L.G., and M.D.C. designed the study, reviewed the data, and reviewed the manuscript. K.L.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Lex van der Ploeg and Keith Gottesdiener, Rhythm Pharmaceuticals, reviewed and provided feedback on the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0598/-/DC1.
REFERENCES
- 1.Castro MG, Morrison E. Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Crit Rev Neurobiol 1997;11:35–57 [DOI] [PubMed] [Google Scholar]
- 2.Poggioli R, Vergoni AV, Bertolini A. ACTH-(1-24) and alpha-MSH antagonize feeding behavior stimulated by kappa opiate agonists. Peptides 1986;7:843–848 [DOI] [PubMed] [Google Scholar]
- 3.McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW. Effect of intracerebroventricular alpha-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. Am J Physiol Regul Integr Comp Physiol 2000;279:R695–R703 [DOI] [PubMed] [Google Scholar]
- 4.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 1999;24:155–163 [DOI] [PubMed] [Google Scholar]
- 5.Koegler FH, Grove KL, Schiffmacher A, Smith MS, Cameron JL. Central melanocortin receptors mediate changes in food intake in the rhesus macaque. Endocrinology 2001;142:2586–2592 [DOI] [PubMed] [Google Scholar]
- 6.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertens 2001;14:103S–115S [DOI] [PubMed] [Google Scholar]
- 7.Nogueiras R, Wiedmer P, Perez-Tilve D, et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 2007;117:3475–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest 2001;108:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mountjoy KG, Jenny Wu CS, Dumont LM, Wild JM. Melanocortin-4 receptor messenger ribonucleic acid expression in rat cardiorespiratory, musculoskeletal, and integumentary systems. Endocrinology 2003;144:5488–5496 [DOI] [PubMed] [Google Scholar]
- 10.Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997;88:131–141 [DOI] [PubMed] [Google Scholar]
- 11.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 1998;19:155–157 [DOI] [PubMed] [Google Scholar]
- 12.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet 1998;20:113–114 [DOI] [PubMed] [Google Scholar]
- 13.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet 1998;20:111–112 [DOI] [PubMed] [Google Scholar]
- 14.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 2003;348:1085–1095 [DOI] [PubMed] [Google Scholar]
- 15.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 1997;385:165–168 [DOI] [PubMed] [Google Scholar]
- 16.Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci 1998;18:10128–10135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva AA, Kuo JJ, Tallam LS, Liu J, Hall JE. Does obesity induce resistance to the long-term cardiovascular and metabolic actions of melanocortin 3/4 receptor activation? Hypertension 2006;47:259–264 [DOI] [PubMed] [Google Scholar]
- 18.Greenfield JR, Miller JW, Keogh JM, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med 2009;360:44–52 [DOI] [PubMed] [Google Scholar]
- 19.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 2009;119:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoeller DA, Kushner RF, Jones PJ. Validation of doubly labeled water for measuring energy expenditure during parenteral nutrition. Am J Clin Nutr 1986;44:291–298 [DOI] [PubMed] [Google Scholar]
- 21.Blanc S, Schoeller D, Kemnitz J, et al. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab 2003;88:16–23 [DOI] [PubMed] [Google Scholar]
- 22.Kuo JJ, da Silva AA, Tallam LS, Hall JE. Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension 2004;43:370–375 [DOI] [PubMed] [Google Scholar]
- 23.Kuo JJ, Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension 2003;41:768–774 [DOI] [PubMed] [Google Scholar]
- 24.Ni X-P, Butler AA, Cone RD, Humphreys MH. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J Hypertens 2006;24:2239–2246 [DOI] [PubMed] [Google Scholar]
- 25.Sullivan EL, Cameron JL. A rapidly occurring compensatory decrease in physical activity counteracts diet-induced weight loss in female monkeys. Am J Physiol Regul Integr Comp Physiol 2010;298:R1068–R1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar KG, Sutton GM, Dong JZ, et al. Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R- and MC4R-deficient C57BL/6J mice. Peptides 2009;30:1892–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 2005;54:3182–3189 [DOI] [PubMed] [Google Scholar]
- 28.da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, Hall JE. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension 2008;51:884–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Silva AA, Kuo JJ, Hall JE. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension 2004;43:1312–1317 [DOI] [PubMed] [Google Scholar]
- 30.Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension 2005;46:326–332 [DOI] [PubMed] [Google Scholar]
- 31.Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology 2009;150:5351–5361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voss-Andreae A, Murphy JG, Ellacott KL, et al. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 2007;148:1550–1560 [DOI] [PubMed] [Google Scholar]
- 33.Fan W, Voss-Andreae A, Cao WH, Morrison SF. Regulation of thermogenesis by the central melanocortin system. Peptides 2005;26:1800–1813 [DOI] [PubMed] [Google Scholar]
- 34.Pavia JM, Schiöth HB, Morris MJ. Role of MC4 receptors in the depressor and bradycardic effects of alpha-MSH in the nucleus tractus solitarii of the rat. Neuroreport 2003;14:703–707 [DOI] [PubMed] [Google Scholar]
- 35.Nordheim U, Nicholson JR, Dokladny K, Dunant P, Hofbauer KG. Cardiovascular responses to melanocortin 4-receptor stimulation in conscious unrestrained normotensive rats. Peptides 2006;27:438–443 [DOI] [PubMed] [Google Scholar]
- 36.Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001;411:480–484 [DOI] [PubMed] [Google Scholar]
- 37.Peter JC, Zipfel G, Lecourt AC, Bekel A, Hofbauer KG. Antibodies raised against different extracellular loops of the melanocortin-3 receptor affect energy balance and autonomic function in rats. J Recept Signal Transduct Res 2010;30:444–453 [DOI] [PubMed] [Google Scholar]
- 38.Trivedi P, Jiang M, Tamvakopoulos CC, et al. Exploring the site of anorectic action of peripherally administered synthetic melanocortin peptide MT-II in rats. Brain Res 2003;977:221–230 [DOI] [PubMed] [Google Scholar]
- 39.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology 2009;150:1705–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li G, Zhang Y, Cheng KY, Scarpace PJ. Lean rats with hypothalamic pro-opiomelanocortin overexpression exhibit greater diet-induced obesity and impaired central melanocortin responsiveness. Diabetologia 2007;50:1490–1499 [DOI] [PubMed] [Google Scholar]
- 41.Sutton GM, Duos B, Patterson LM, Berthoud H-R. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology 2005;146:3739–3747 [DOI] [PubMed] [Google Scholar]
- 42.Williams DL, Bowers RR, Bartness TJ, Kaplan JM, Grill HJ. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology 2003;144:4692–4697 [DOI] [PubMed] [Google Scholar]
- 43.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol 2005;289:R247–R258 [DOI] [PubMed]
- 44.An JJ, Rhee Y, Kim SH, et al. Peripheral effect of alpha-melanocyte-stimulating hormone on fatty acid oxidation in skeletal muscle. J Biol Chem 2007;282:2862–2870 [DOI] [PubMed] [Google Scholar]
- 45.Hoggard N, Rayner DV, Johnston SL, Speakman JR. Peripherally administered [Nle4,D-Phe7]-alpha-melanocyte stimulating hormone increases resting metabolic rate, while peripheral agouti-related protein has no effect, in wild type C57BL/6 and ob/ob mice. J Mol Endocrinol 2004;33:693–703 [DOI] [PubMed] [Google Scholar]