Abstract
Adult non–insulin requiring diabetes includes latent autoimmune diabetes of adults (LADA), distinguished from type 2 diabetes by the presence of islet autoantibodies. LADA China determined the characteristics of Chinese LADA. This nationwide, multicenter, clinic-based cross-sectional study was conducted in 46 university-affiliated hospitals in 25 Chinese cities. All 4,880 ketosis-free diabetic patients (<1 year postdiagnosis, without insulin therapy for >6 months, aged ≥30 years) had GAD antibody (GADA) and HLA-DQ genotype measured centrally with clinical data collected locally. GADA-positive subjects were classified as LADA. Of the patients, 5.9% were GADA positive with LADA. LADA showed a north-south gradient. Compared with GADA-negative type 2 diabetes, LADA patients were leaner, with lower fasting C-peptide and less metabolic syndrome. Patients with high GADA titers are phenotypically different from those with low GADA titers, while only a higher HDL distinguished the latter from those with type 2 diabetes. HLA diabetes–susceptible haplotypes were more frequent in LADA, even in those with low-titer GADA. HLA diabetes-protective haplotypes were less frequent in LADA. Our study implicates universal immunogenetic effects, with some ethnic differences, in adult-onset autoimmune diabetes. Autoantibody positivity and titer could be important for LADA risk stratification and accurate therapeutic choice in clinical practice.
Diabetes is prevalent worldwide, and the epidemic of diabetes is global, not least in China (1–4). Among children, autoimmune diabetes, known as type 1 diabetes, is the most prevalent form of the disease (2,5). Much of our knowledge about type 1 diabetes comes from studies of children in Europe and North America (6–10). Childhood-onset type 1 diabetes in China is infrequent (8). However, autoimmune diabetes also occurs in adults (11,12), where it is characterized by an association with the same HLA genes linked to childhood-onset type 1 diabetes and by serum islet autoantibodies, most notably GAD autoantibodies (GADAs) (11). Latent autoimmune diabetes in adults (LADA) describes a form of adult-onset autoimmune diabetes that, at least initially, does not require insulin treatment (5,12,13). The immunogenetic and clinical characteristics of LADA have been extensively studied in Caucasians (11–15), although the relationship between LADA and the other two major forms of diabetes, type 1 and type 2 diabetes, remains controversial (5,13). LADA could be distinct from type 1 and type 2 diabetes, while incorporating certain features of each or be part of a spectrum of autoimmune diabetes (16,17).
Although LADA is prevalent and potentially more prevalent than childhood-onset type 1 diabetes, that frequency depends on the defining autoantibody assay (usually GADA), ethnic group, age at diagnosis, and mode of ascertainment. Using GADA, the frequency of LADA in adult-onset diabetes has ranged from 4 to 12% in Caucasian populations, with higher frequencies in those younger at diagnosis or insulin treated (11,18). In China as well, GADA positivity was prevalent in hospital-based adult-onset diabetic patients from the Hunan province (7.1%) (19) and in a local small population-based study in Tianjin (9.2% [46 of 498]) (20). Therefore, we established a large multicenter clinical study (LADA China) to determine the prevalence, immunogenotype, and clinical characteristics of this form of diabetes in China. Since China has one-fifth of the world’s population, of whom ~92 million adults have diabetes, within 56 ethnic groups spread over 9.6 million square kilometers with remarkably varied climates, diets, and patterns of infectious diseases, data from China should inform our general knowledge of the nature of diabetes (1). We now report, for the first time, that GADA positivity and LADA are prevalent among adult-onset non–insulin-requiring diabetic patients, with apparent type 2 diabetes, throughout China, with a geographic difference between north and south, and clinical and immunogenetic features implying both similarities and differences in relation to Caucasian LADA patients but consistent with LADA in China being part of an autoimmune spectrum.
RESEARCH DESIGN AND METHODS
This cross-sectional study was conducted from June 2006 to January 2010. Patients were recruited consecutively from 46 centers (university-affiliated teaching hospitals) in 25 major cities, representing 53% of the total Chinese population aged ≥30 years. The patient population includes 15 major ethnic groups of which the majority (in excess of 98%) is Han. The ethics review committee/institutional review board of each study center approved the study protocol. The study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent. Inclusion criteria were as follows: 1) diagnosis of diabetes (World Health Organization [WHO] 99 criteria) at age ≥30.0 years, 2) disease duration of <1 year, 3) no ketoacidosis in the first 6 months after the diagnosis of diabetes, and 4) insulin independence (usage of insulin <1 month) for 6 months after diagnosis. Exclusion criteria were as follows: 1) secondary diabetes, 2) diabetes in pregnancy and gestational diabetes mellitus, and 3) malignancy. LADA was defined according to the Immunology of Diabetes Society (IDS) criteria: GADA-positive patients, initially non–insulin requiring for at least 6 months, diagnosed over the age of 30 years (12). GADA-negative subjects were diagnosed with type 2 diabetes. Detailed medical history was obtained by study physicians before collection of fasting blood samples. A 6-month follow-up stage was carried out to confirm the diabetic ketoacidosis status and insulin therapy for all subjects. We recruited 5,324 patients and eliminated 444 patients (8.3%) because disease duration at ascertainment was >1.0 year, age was under 30 years, or they developed diabetic ketoacidosis or insulin dependency within 6 months postdiagnosis. By the IDS criteria (12), 287 had LADA, representing 5.9% of those 4,880 subjects meeting the study criteria. Among all 287 LADA patients, 180 subjects consented to the genetic study. An approximately equal number (174 subjects) of age-, sex-, and center-matched GADA-negative type 2 diabetes samples for genetic analysis were collected as well. The oral glucose tolerance test was used to verify normal glucose tolerance among healthy control subjects from a community-based study for reference HLA-DQ genotyping (Fig. 1). Serum samples for antibody assays and whole blood samples for genotyping were shipped to the Diabetes Center of Central South University, Changsha, China, with transportation on ice within 1 day. Blood samples were stored at −80°C before analysis.
FIG. 1.
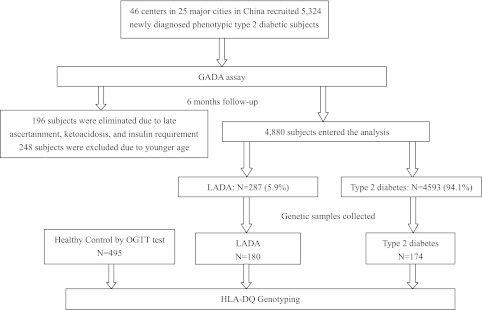
Flowchart of LADA China Study. OGTT, oral glucose tolerance test.
Clinical characteristics and biochemical measurements.
Body height, weight, waist circumference, hip circumference, and blood pressure were recorded by study physicians/coordinates. Overweight and obesity was defined as BMI ≥25 kg/m2 (WHO criteria). Hypertension was defined as systolic and diastolic blood pressure ≥140 or ≥90 mmHg, respectively, or current use of antihypertensive medications. Dyslipidemia was defined as serum triglycerides ≥1.7 mmol/L, HDL cholesterol <1.04 mmol/L for men and <1.30 mmol/L for women, or currently taking medication for hyperlipidemia. Metabolic syndrome was defined using advised National Cholesterol Education Program–Adult Treatment Panel III criteria, involving achievement of three or more of the following: 1) waist circumference ≥90 cm (Asian male) or ≥80 cm (Asian female); 2) triglyceride ≥1.7 mmol/L, 3) HDL cholesterol <1.04 mmol/L (male) or <1.30 mmol/L (female), 4) systolic/diastolic blood pressure ≥130/85 mmHg, and 5) fasting plasma glucose ≥5.6 mmol/L. Lipids, HbA1c, and fasting C-peptide (FCP) were assayed at the study sites by standard methods. GADA was analyzed in the core laboratory (Central South University) by radioligand assay as previously described (21,22); all samples were measured in duplicate. Positive samples were repeated to confirm their positivity. GADA positivity was determined as the 99.5th percentile of 188 healthy control subjects, i.e., as ≥18 units/mL (WHO unit) and revalidated when positive. The sensitivity and specificity of GADA assays were 82 and 98% respectively, as evaluated in the 4th Diabetes Autoantibody Standardization Program (unpublished data). Intra- and interassay coefficients of variation were 8.9 and 11.2%, respectively. Genomic DNA was extracted from anticoagulated peripheral blood using a phenol-chloroform method. HLA-DQA1 and -DQB1 genotypes were defined using PCR to amplify exon 2 of both DQA1 and DQB1 genes followed by standard DNA sequencing-based typing (17).
Statistical analysis.
Statistical analysis was performed using SPSS statistical software (version 13; SPSS, Chicago, IL). Normally distributed data were expressed as means ± SD. Variables with a skewed distribution were reported as median (range) and log transformed to approximate normality before analysis. Frequency differences were compared using χ2 test or Fisher exact test when appropriate. Independent Student t test or one-way ANOVA were used to compare the means between the groups as appropriate. Data for GADA titer were transformed using log base 10 to normalize their distribution. Logistic regression models were used to adjust the potential confounding variables including geographic area, ethnicity, age, BMI, and sex for LADA. P < 0.05 was considered significant.
RESULTS
Frequency of GADA positivity and LADA in China.
Of 287 (5.9%) LADA cases, there was no sex difference (6.1% [178 of 2,906] male vs. 5.5% [109 of 1,974] female, P = 0.302). LADA frequency was not significantly age related when divided into four age subgroups (30–39, 40–49, 50–59, and ≥60 years old), even after adjustment for geography, ethnicity, sex, and BMI (P = 0.19). Among these 4,880 subjects, the different frequency of LADA in Han compared with other ethnic groups (5.9% [284 of 4,786] vs. 3.2% [3 of 94], respectively) was not significant (P = 0.322).
Geographic distribution of LADA.
To examine geographic variation in the prevalence of LADA, we divided Mainland China geographically along the Qin Mountain-Huai River into Southern China (with 16 cities, 27 hospitals, and 2,746 patients) and northern China (10 cities, 19 hospitals, and 2,117 patients). After adjustment for ethnicity, age, sex, and BMI, there was a significant difference between northern and southern China for LADA (6.5% [137 of 2,119] vs. 5.4% [150 of 2,761], P = 0.040). Frequency of LADA was highest in northeastern China (7.1% [58 of 815]), which has the highest latitude and coldest climate, and lowest in southwestern China (4.0% [20 of 502], P = 0.019) (Fig. 2).
FIG. 2.
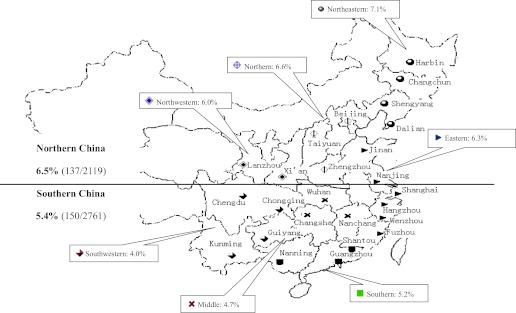
China map for LADA China Study.  , cities from the northeastern part of China;
, cities from the northeastern part of China;  , cities from the northern part of China;
, cities from the northern part of China;  , cities from northwestern China;
, cities from northwestern China;  , cities from eastern China;
, cities from eastern China;  , cities from central China;
, cities from central China;  , cities from southern China; and
, cities from southern China; and  , cities from southwestern China. LADA frequency was higher in northern than southern China after age, sex, and BMI adjustment (P < 0.040). (A high-quality color representation of this figure is available in the online issue.)
, cities from southwestern China. LADA frequency was higher in northern than southern China after age, sex, and BMI adjustment (P < 0.040). (A high-quality color representation of this figure is available in the online issue.)
Clinical characteristics of LADA.
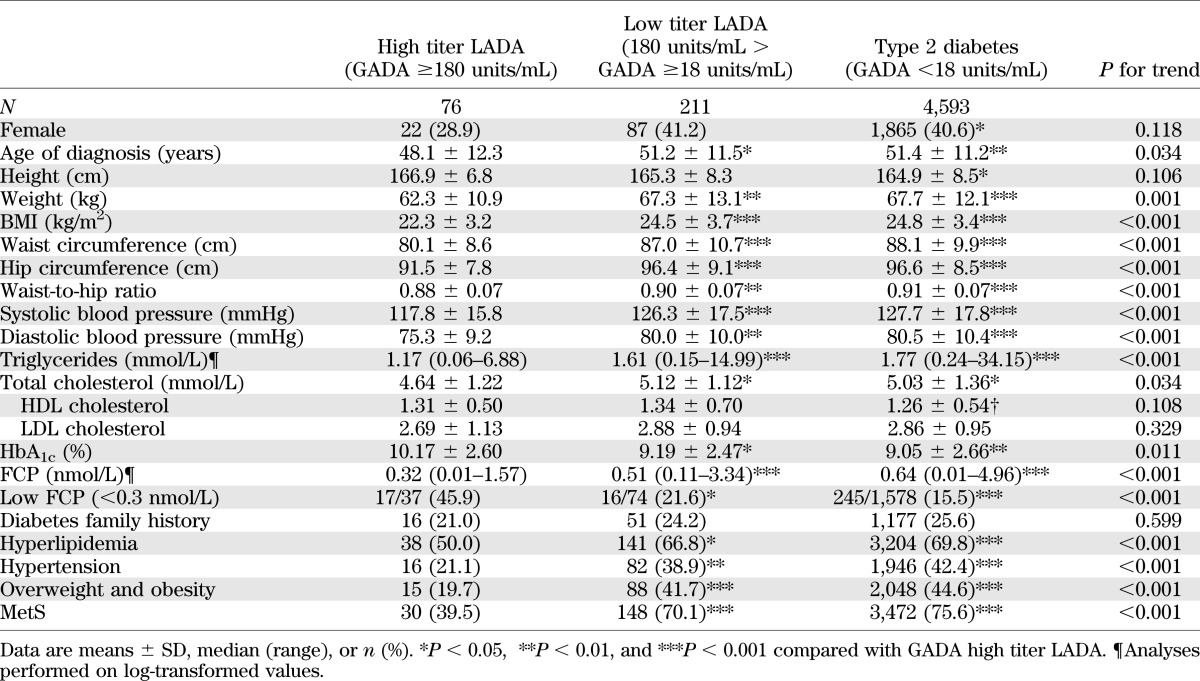
Compared with type 2 diabetic patients, LADA patients (Table 1) were leaner (BMI 23.9 ± 3.7 vs. 24.8 ± 3.4 kg/m2, P < 0.001; waist circumference 85.2 ± 10.6 vs. 88.1 ± 9.9 cm, P < 0.001) with lower β-cell function (FCP 0.45 ng/mL [interquartile range 0.01–3.34] vs. 0.64 ng/mL [0.01–4.96], P < 0.001) and less hypertriglyceridemia (62.4 vs. 69.8%, P = 0.008), hypertension (34.1 vs. 42.4%, P = 0.007), obesity (35.9 vs. 44.6%, P = 0.005), and metabolic syndrome (MetS) (62.0 vs. 75.6%, P < 0.001).
TABLE 1.
Clinical characteristics of type 2 diabetes and LADA
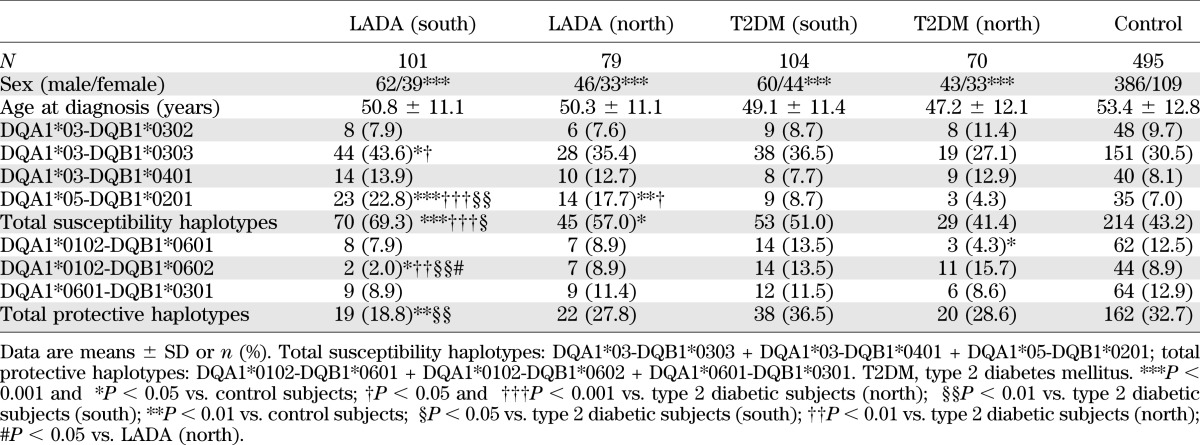
Compared with northern LADA patients, the southern LADA patients were leaner (BMI 23.1 ± 3.5 vs. 24.9 ± 3.8 kg/m2, P < 0.001; waist circumference 82.9 ± 10.2 vs. 87.7 ± 10.5 cm, P < 0.001), with less MetS (52.0% [78 of 150] vs. 73.0% [100 of 137], P < 0.001) and, importantly, had a higher GADA titer (0.1195 [0.06–1.50] vs. 0.089 [0.06–1.29], P = 0.037). Of note, β-cell function showed no significant differences between northern and southern subjects for either LADA or type 2 diabetes (data not shown).
Clinical characteristics according to GADA titer.
To analyze the distribution of GADA titer in LADA, we log transformed the data, which showed a possible bimodal distribution (Fig. 3). GADA positivity above or below 180 units/mL largely captured the two modes, with high GADA titer (≥180 units/mL) in 26.5% of patients (76 of 287) and a low GADA titer (<180 units/mL) in 73.5% (211 of 287). High GADA titer LADA subjects compared with low GADA titer LADA subjects were younger at diagnosis (48.1 ± 12.3 vs. 51.2 ± 11.5 years, P = 0.036) and leaner (BMI 22.3 ± 3.2 vs. 24.5 ± 3.7 kg/m2, P < 0.001, and waist circumference 80.1 ± 8.6 vs. 87.0 ± 10.7 cm, P < 0.001), with lower blood pressure (systolic 117.8 ± 15.8 vs. 126.3 ± 17.5 mmHg, P < 0.001; diastolic 75.3 ± 9.2 vs. 80.0 ± 10.0 mmHg, P = 0.001), lower triglyceride levels (1.17 mmol/L [0.06–6.88] vs. 1.61 mmol/L [0.15–14.99], P < 0.001), lower insulin secretion (FCP 0.32 ng/mL [0.01–1.57] vs. 0.51 [0.11–3.34], P < 0.001), and less MetS (39.5 vs. 70.1%, P < 0.001). However, when low GADA titer LADA were compared with type 2 diabetic patients (GADA-negative patients), with the exception of HDL cholesterol (1.26 ± 0.54 vs. 1.34 ± 0.70 mmol/L, P = 0.044), clinical features between them were not significantly different (Table 2).
FIG. 3.
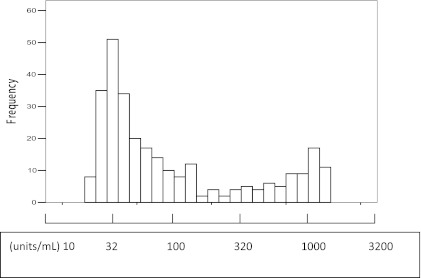
Bimodal GADA titer (log 10 transformed) in LADA patients. GADA positivity above or below 180 units/mL largely captured the two modes, with high GADA titer (≥180 units/mL) in 26.5% of patients (76 of 287) and a low GADA titer (<180 units/mL) in 73.5% (211 of 287).
TABLE 2.
Clinical characteristics in type 2 diabetic and LADA patients with high or low GADA titer
HLA-DQ genomic background differences.
In these Chinese patients, as for Europeans, the frequency of total diabetes-susceptibility haplotypes (i.e., DQA1*03-DQB1*0302, DQA1*03-DQB1*0303, DQA1*03-DQB1*0401, and DQA1*05-DQB1*0201) was significantly higher in LADA (63.9% [115 of 180]) than in both type 2 diabetic (47.1% [82 of 174]) and control (43.2% [214 of 495]) subjects, while the frequency of diabetes-protective haplotypes (i.e., DQA1*0102-DQB1*0601, DQA1*0102-DQB1*0602, and DQA1*0601-DQB1*0301) in LADA (22.8% [41 of 180]) was significantly lower than in both type 2 diabetic (33.3% [58 of 174]) and control (32.7% [162 of 495]) subjects (Table 3). The frequency of HLA-DQ diabetes-susceptibility DQA1*05-DQB1*0201 was greater in high GADA titer than in low GADA titer patients (38.8% [19 of 49] vs. 13.7% [18 of 131], respectively, P < 0.001]) (Table 3). Comparing subjects from the south and north of China, LADA subjects had a similar frequency of HLA-DQ diabetes-susceptibility (69.3% [70 of 101] vs. 57.0% [45 of 79], respectively) and diabetes-protective (18.8% [19 of 101] vs. 27.8% [22 of 79], respectively) haplotypes (Table 4).
TABLE 3.
Frequencies of HLA-DQ haplotypes with high or low GADA titer LADA and type 2 diabetic patients
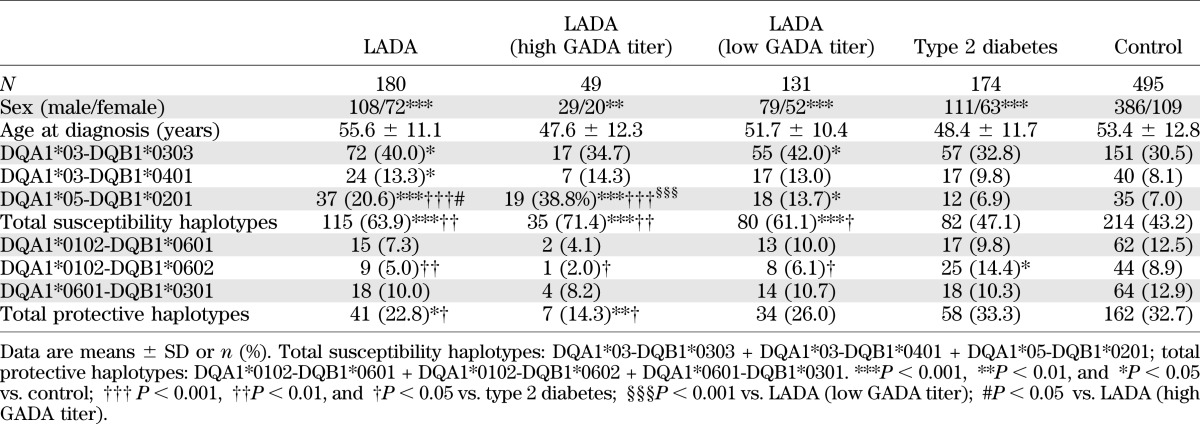
TABLE 4.
Frequencies of HLA-DQ haplotypes with LADA and type 2 diabetic patients in southern and northern China
DISCUSSION
This report is the first multicenter study in China to assess GADA in a large number of diabetic case subjects (4,880 patients) across a large group of centers (25 cities and 46 hospitals) throughout China. The study is also distinct in reporting the clinical, metabolic, and immunogenetic features of LADA in China. From these analyses, autoimmune diabetes and LADA emerge as a prevalent form of diabetes with features remarkably similar to those in adult-onset autoimmune diabetes and LADA in Europe and North America.
Differences in the prevalence of LADA worldwide can be ascribed to age at diagnosis, mode of ascertainment, number of autoantibodies tested, risk of false positivity for autoantibodies, disease duration at study, and the numbers of patients progressing to insulin treatment. When defined as LADA using the IDS criteria (12), the prevalence of LADA was 5.9% by GADA. Although several diabetes-associated autoantibodies can be used for LADA diagnosis, in Caucasians (23) GADA is the most prevalent autoantibody, as has been noted by us in a small Chinese study of a similar cohort (Z.Z., manuscript in preparation) (5,16). It is noteworthy that GADA positivity was defined as a titer above the 99.5th centile of the healthy background population so that only 1 of 200 subjects could have a false-positive result. Further, in this study, we centralized the assays and retested to confirm all positive results, further decreasing false positivity. There may be a bias toward transient early insulin therapy in the hospital-based clinics used in this study (vs. community-based clinics), so we ascertained cases with insulin independence at 6 months postdiagnosis. It is likely we excluded GADA-positive patients who started on insulin within 6 months, although a recent small population-based study of Tianjin in the northern part of China found LADA in 9.2% of adult-onset diabetic patients who were initially non–insulin requiring from a local community-based study (20). Age at diagnosis also has a major effect on GADA frequency, which was higher (11.7%) in younger patients (aged 15–30 years at diagnosis)—ascertained as part of this study but not reported here (data not shown)—than in those diagnosed later, in whom there was no age effect.
A marked variation in the prevalence of classic childhood-onset type 1 diabetes in China has been described, with a north-south gradient (0.69 vs. 0.34/100,000/year, respectively) (8) similar to that reported in Europe (6,10). No comparable study has been performed in adult-onset autoimmune diabetes until now, when we also found a north-south frequency gradient for adult-onset autoimmune diabetes in China. The frequency of GADA positivity, as well as LADA, in the north of China, above a geographic line along the Qin Mountain-Huai River, significantly exceeded that in the south. This geographic line forms a sharp climatic and topographic divide, separating the monsoon-prone, subtropical forested south from the temperate, drier north, which has less average sunshine. Each area is associated with distinct lifestyles and diets, the staple being flour in the north and rice in the south. The difference is greatest (nearly twofold) when the northeast is compared with the southwest. In these two areas, the durations of winter are different: 4–6 months in the northeast but <1 month in the southwest. While the causality of this apparent polarity in LADA frequencies in China remains unclear, it cannot be explained by immune features (i.e., the higher GADA titers were in southern China) or HLA-DQ genetic differences (i.e., there were similar frequencies of diabetes-susceptibility and protective haplotypes between north and south), which suggests that nonimmunogenetic factors, including environment and lifestyle, may play a role.
As with studies of LADA in other countries, notably among Caucasians (11,18), the characteristics of LADA are distinct from those of GADA-negative type 2 diabetic patients. There was a remarkable phenotypic difference with LADA according to GADA titer: those with high titer being more distinct from type 2 diabetic patients than those with low titer. These differences relate to the same features that distinguished GADA-positive from GADA-negative patients, including age at diagnosis, BMI, and frequency of MetS. In fact, patients with low GADA titer were not dissimilar from type 2 diabetic patients, apart from having lower HDL cholesterol and higher HLA susceptibility haplotypes. Notably, FCP, a surrogate marker of insulin secretory capacity, was lower in GADA-positive patients, especially in those with high titer GADA. So although any GADA positivity reflects an autoimmune process, high GADA titer probably reflects a more severe process with greater loss of insulin secretory capacity. We (24) and others (18,25) have previously reported the relationship of bimodal GADA titer to clinical features in LADA. However, the current study’s large size provides the strongest confirmation to date of GADA titer bimodality after log transformation. Along with distinct clinical features, the presence and titer of autoantibodies may be important for risk stratification and accurate therapeutic choice for LADA patients (25). Since there were no categorical clinical features in such LADA patients, only by testing for diabetes-associated autoantibodies can they be identified. Moreover, patients with autoimmune diabetes need more rigorous follow-up, as they are prone to worse glucose control and the need for insulin therapy. A future expansion of this study to explore the relationship of this present observation to immune reactivity to different GAD peptides potentially associated with the development of high and low titers antibodies would be relevant. There have been some earlier reports about the different autoantibodies generated by different GAD epitopes (26).
In part, the similarity between Chinese and European LADA results from similarities in disease genetic risk. As with Caucasians, HLA diabetes-susceptibility and diabetes-protective haplotypes were more and less frequent, respectively, in both LADA and type 1 diabetic compared with type 2 diabetic and control subjects, in whom they were similar (18,27). However, the association with HLA diabetes susceptibility and diabetes-protective haplotypes was less striking in LADA than type 1 diabetic patients (17) and in low titer compared with high titer GADA-positive patients. Thus, as reported for Europeans, there is a spectrum of immunogenetic risk and clinical characteristics within Chinese adult-onset autoimmune diabetes, without evidence that LADA is a distinct form of diabetes (16,17,27).
Despite the similarity of LADA patients between Chinese and Caucasian patients, some differences exist. First, the overall GADA frequency and LADA frequency in China is lower than reported in northern European multicenter studies such as the UK Prospective Diabetes Study (UKPDS) (11) or the Botnia Study (15) but slightly higher than reported in some southern European studies such as the Non–Insulin Requiring Autoimmune Diabetes (NIRAD) study (18), Asian studies for Japanese or Korean (28,29), or the North American/Northern European ADOPT (A Diabetes Outcome Progression Trial) (30). That the frequency of LADA in China is higher than in some European countries is surprising given the infrequency of childhood-onset autoimmune diabetes in China (6,8). Second, in the UKPDS study, GADA frequency decreased with age >30 years, while in China GADA frequency showed no such age affect. Third, the frequency of MetS and raised inflammation markers was higher in Chinese LADA than in type 1 diabetic subjects, as we previously reported (31), while in Caucasians they were similar (32). Fourth, the high titer GADA patients represented approximately one-quarter of the total number of Chinese LADA cases but approximately one-half of Italian LADA cases (18). Fifth, in contrast to the strong association of DQA1 03-DQB1*0302 with autoimmune diabetes in Caucasians, the most common susceptible genes of HLA-DQ in Chinese LADA were moderate-risk haplotypes including DQA1 03-DQB1*0303 and DQA1*05-DQB1*0201 both in this multicenter and our previous study (17). By implication, the immunogenotype of Chinese LADA is more moderate than in Caucasians. Thus, the autoimmune β-cell destruction may be less potent. However, to define global ethnic differences in LADA will require global collaboration using standardized ascertainment, assays, and definitions.
As with any large multicenter study, there are potential ascertainment biases and heterogeneity of clinical practice that might affect the cohort, so our results should be interpreted cautiously. Besides, GADAs are not uncommon autoantibodies that may appear without clinical evidence of established autoimmunity. So, despite the current use of GADA positivity to define autoimmune diabetes, debate continues as to whether GADAs reflect autoimmune disease. Nevertheless, it is clear that adult-onset autoimmune diabetes and LADA are now shown to be prevalent in China—in contrast to childhood-onset autoimmune diabetes. The worldwide congruence of characteristics of adult-onset diabetes associated with GADA, even extending to HLA risk and clinical features linked to GADA titer, strongly implies that universal genetic and environmental factors may predispose to the development of adult-onset autoimmune diabetes, as is the case for type 2 diabetes.
ACKNOWLEDGMENTS
This study was supported by the European Foundation for the Study of Diabetes grant awards for collaborative diabetes research between China and Europe, the National Department Public Benefit (Health) Research Foundation of China (201002002), Program for Changjiang Scholars and Innovative Research Team in University (IRT1195), and Hunan Provincial Natural Science Foundation of China (11JJ7005).
No potential conflicts of interest relevant to this article were reported.
Z.Z. designed the study, contributed to discussion, and wrote, reviewed, and edited the manuscript. Y.X. researched data, wrote the manuscript, and contributed to discussion. L.J., W.J., and G.N. researched data and contributed to discussion. G.H. and J.L. researched data. L.Y. designed the study and contributed to discussion. Z.L., W.A.H., and R.D.L. contributed to discussion and reviewed and edited the manuscript. Z.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented as an oral presentation at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The LADA China Study Group thanks all of the patients, investigators, doctors, nurses, and technicians involved at the 46 participating centers of the LADA China Study for collecting blood samples and data. The authors thank L. Ho-Le, M. Hawa, and S. Paschou (all from Blizard Institute, London, U.K.) for editing the manuscript.
Appendix
LADA China Study Group (investigators and hospitals): Linong Ji, Peking University People’s Hospital; Xiaohui Guo, Peking University First Hospital; Tianpei Hong, Peking University Third Hospital; Jumin Lu, The General Hospital of the People's Liberation Army; Zhangrong Xu, The 306th Hospital of the People's Liberation Army; Yingsheng Zhou, Beijing Hospital; Weiping Jia, Shanghai Jiao Tong University Affiliated 6th People's Hospital; Ning Guang, Shanghai Jiao Tong University Affiliated Rui-Jin Hospital; Renming Hu, Hua Shan Hospital, Fudan University; Xin Gao, Zhongshan Hospital, Fudan University; Yanbing Li, The First Affiliated Hospital, Sun Yat-sen University; Huazhang Yang, Guangdong General Hospital; Shaoda Lin, the First Affiliated Hospital, Shantou University; Shenren Chen, the Second Affiliated Hospital, Shantou University; Lulu Chen, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology; Yancheng Xu, Zhongnan Hospital of Wuhan University; Hong Li, First Affiliated Hospital of Medical School of Zhejiang University; Wei Gu, Second Affiliated Hospital of Medical School of Zhejiang University; Dawang Wang, The First Affiliated Hospital of Wenzhou Medical School; Qifu Li, The First Affiliated Hospital, Chongqing Medical University; Gangyi Yang, The Second Affiliated Hospital, Chongqing Medical University; Haoming Tian, West China Hospital, Sichuan University; Dalong Zhu, Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School; Chao Liu, Jiangsu Province Hospital, the First Affiliated Hospital with Nanjing Medical University; Qiuhe Ji, Xijing Hospital, Fourth Military Medical University; Zhongyan Shan, The First Hospital of China Medical University; Jianling Du, First Affiliated Hospital of Dalian Medical University; Benli Su, Second Affiliated Hospital of Dalian Medical University; Yan Liu, The Norman Bethune 1st hospital of Jilin University; Huanqi Ge, The Norman Bethune 2nd hospital of Jilin University; Yadong Sun, People’s Hospital of Jilin Province; Qiang Li, The 2nd Affiliated Hospital of Harbin Medical University; Jiajun Zhao, Shandong Provincial Hospital; Lingli Ouyang, The First Affiliated Hospital of Guangxi Medical University; Yuexin Bai, The First Affiliated Hospital of Zhengzhou University; Yuanming Xue, The First People’s Hospital of Yunnan Province; Xulei Tang, The First Affiliated Hospital of Lanzhou University; Lixin Shi, The Affiliated Hospital of Guiyang Medical College; Xiaoyang Lai, The Second Affiliated Hospital of Nanchang University; Jie Liu, Shanxi Provincial People’s Hospital; Liyong Yang, The First Affiliated Hospital of Fujian Medical University; Huiju Zhong, Xiangya Hospital of Central South University; Zhiguang Zhou, The Second Xiangya Hospital of Central South University; Jianying Liu, The First Affiliated Hospital of Nanchang University; Jing Yang, The First Hospital of Shanxi Medical University; and Yongde Peng, Shanghai First People’s Hospital.
Footnotes
A complete list of the investigators of the LADA China Study Group can be found in the APPENDIX.
See accompanying commentary, p. 339.
REFERENCES
- 1.Yang W, Lu J, Weng J, et al. China National Diabetes and Metabolic Disorders Study Group Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–1101 [DOI] [PubMed] [Google Scholar]
- 2.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 3.Danaei G, Finucane MM, Lu Y, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011;378:31–40 [DOI] [PubMed] [Google Scholar]
- 4.Mbanya JC, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-Saharan Africa. Lancet 2010;375:2254–2266 [DOI] [PubMed] [Google Scholar]
- 5.Rolandsson O, Palmer JP. Latent autoimmune diabetes in adults (LADA) is dead: long live autoimmune diabetes! Diabetologia 2010;53:1250–1253 [DOI] [PubMed] [Google Scholar]
- 6.DIAMOND Project Group Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med 2006;23:857–866 [DOI] [PubMed] [Google Scholar]
- 7.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008;371:1777–1782 [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Wang K, Li T, et al. Childhood diabetes in China. Enormous variation by place and ethnic group. Diabetes Care 1998;21:525–529 [DOI] [PubMed] [Google Scholar]
- 9.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 2007;8:286–298 [DOI] [PubMed] [Google Scholar]
- 10.EURODIAB ACE Study Group Variation and trends in incidence of childhood diabetes in Europe. Lancet 2000;355:873–876 [PubMed] [Google Scholar]
- 11.Turner R, Stratton I, Horton V, et al. UK Prospective Diabetes Study Group UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. Lancet 1997;350:1288–1293 [DOI] [PubMed] [Google Scholar]
- 12.Fourlanos S, Dotta F, Greenbaum CJ, et al. Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia 2005;48:2206–2212 [DOI] [PubMed] [Google Scholar]
- 13.Gale EA. Latent autoimmune diabetes in adults: a guide for the perplexed. Diabetologia 2005;48:2195–2199 [DOI] [PubMed] [Google Scholar]
- 14.Leslie RD, Kolb H, Schloot NC, et al. Diabetes classification: grey zones, sound and smoke: Action LADA 1. Diabetes Metab Res Rev 2008;24:511–519 [DOI] [PubMed] [Google Scholar]
- 15.Tuomi T, Carlsson A, Li H, et al. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes 1999;48:150–157 [DOI] [PubMed] [Google Scholar]
- 16.Leslie RD, Williams R, Pozzilli P. Clinical review: Type 1 diabetes and latent autoimmune diabetes in adults: one end of the rainbow. J Clin Endocrinol Metab 2006;91:1654–1659 [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Zhou ZG, Wang JP, Zhang C, Huang G. From Type 1, through LADA, to type 2 diabetes: a continuous spectrum? Ann N Y Acad Sci 2008;1150:99–102 [DOI] [PubMed] [Google Scholar]
- 18.Buzzetti R, Di Pietro S, Giaccari A, et al. Non Insulin Requiring Autoimmune Diabetes Study Group High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes. Diabetes Care 2007;30:932–938 [DOI] [PubMed] [Google Scholar]
- 19.Li X, Zhou Z, Huang G, et al. Study on the positive frequency and distribution of glutamic acid decarboxylase antibody in phenotypic type 2 diabetic patients. Chin J Epidemiol 2005;26:800–803 [PubMed] [Google Scholar]
- 20.Qi X, Sun J, Wang J, et al. Prevalence and correlates of latent autoimmune diabetes in adults in Tianjin, China: a population-based cross-sectional study. Diabetes Care 2011;34:66–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grubin CE, Daniels T, Toivola B, et al. A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia 1994;37:344–350 [DOI] [PubMed] [Google Scholar]
- 22.Petersen JS, Hejnaes KR, Moody A, et al. Detection of GAD65 antibodies in diabetes and other autoimmune diseases using a simple radioligand assay. Diabetes 1994;43:459–467 [DOI] [PubMed] [Google Scholar]
- 23.Palmer JP, Hampe CS, Chiu H, Goel A, Brooks-Worrell BM. Is latent autoimmune diabetes in adults distinct from type 1 diabetes or just type 1 diabetes at an older age? Diabetes 2005;54(Suppl. 2):S62–S67 [DOI] [PubMed] [Google Scholar]
- 24.Li X, Yang L, Zhou Z, Huang G, Yan X. Glutamic acid decarboxylase 65 autoantibody levels discriminate two subtypes of latent autoimmune diabetes in adults. Chin Med J (Engl) 2003;116:1728–1732 [PubMed] [Google Scholar]
- 25.van Deutekom AW, Heine RJ, Simsek S. The islet autoantibody titres: their clinical relevance in latent autoimmune diabetes in adults (LADA) and the classification of diabetes mellitus. Diabet Med 2008;25:117–125 [DOI] [PubMed] [Google Scholar]
- 26.Rharbaoui F, Granier C, Kellou M, et al. Peptide specificity of high-titer anti-glutamic acid decarboxylase (GAD)65 autoantibodies. Immunol Lett 1998;62:123–130 [DOI] [PubMed] [Google Scholar]
- 27.Cervin C, Lyssenko V, Bakhtadze E, et al. Genetic similarities between latent autoimmune diabetes in adults, type 1 diabetes, and type 2 diabetes. Diabetes 2008;57:1433–1437 [DOI] [PubMed] [Google Scholar]
- 28.Takeda H, Kawasaki E, Shimizu I, et al. Ehime Study Clinical, autoimmune, and genetic characteristics of adult-onset diabetic patients with GAD autoantibodies in Japan (Ehime Study). Diabetes Care 2002;25:995–1001 [DOI] [PubMed] [Google Scholar]
- 29.Park Y, Hong S, Park L, et al. KNDP collaboratory Group LADA prevalence estimation and insulin dependency during follow-up. Diabetes Metab Res Rev 2011;27:975–979 [DOI] [PubMed] [Google Scholar]
- 30.Zinman B, Kahn SE, Haffner SM, O’Neill MC, Heise MA, Freed MI, ADOPT Study Group Phenotypic characteristics of GAD antibody-positive recently diagnosed patients with type 2 diabetes in North America and Europe. Diabetes 2004;53:3193–3200 [DOI] [PubMed] [Google Scholar]
- 31.Xiang Y, Zhou P, Li X, et al. Heterogeneity of altered cytokine levels across the clinical spectrum of diabetes in China. Diabetes Care 2011;34:1639–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawa MI, Thivolet C, Mauricio D, et al. Action LADA Group Metabolic syndrome and autoimmune diabetes: action LADA 3. Diabetes Care 2009;32:160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]


