The notion that type 2 diabetes and insulin resistance are associated with many macro- and microvascular defects (1,2) is unquestionable, but whether vascular defects precede and contribute to insulin resistance is less certain and has been a controversial topic. The most compelling evidence for a vascular involvement in insulin resistance has been in skeletal muscle (3), but recent research has also implicated its involvement in adipose tissue (4), which may then lead to whole body insulin resistance via inflammation (5).
The suggestion that the vasculature may be a potent contributor to insulin resistance in muscle came from early, indirect clinical studies in which insulin resistance was inversely associated with skeletal muscle capillary density in Pima Indians (6) and from studies of total blood flow during euglycemic-hyperinsulinemic clamps in normal and insulin-resistant subjects (7). Many subsequent studies by various research groups have reported corroborating data that vascular defects (especially in the microvasculature) can contribute to insulin resistance in muscle (rev. in 3 and reference list therein). The underlying consequence of the vascular defect is impaired delivery of insulin and/or glucose to the skeletal myocyte, which leads to insulin resistance. Because the muscle myocyte (and other tissues) also exhibit defects in insulin signaling and responsiveness in established states of obesity, hypertension, and diabetes (all of which are associated with insulin resistance), the significance of a vascular contribution is often questioned or undervalued.
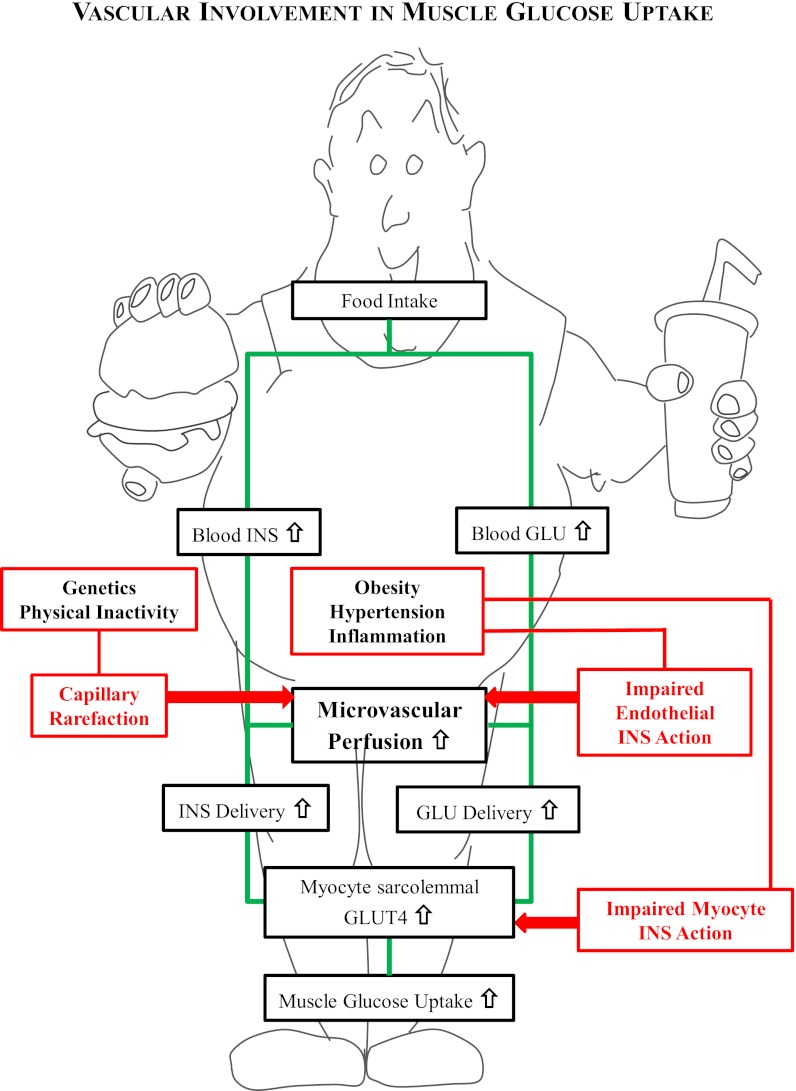
In the current issue of Diabetes, Bonner et al. (8) report data on the effects of muscle-specific vascular endothelial growth factor (VEGF) deletion on muscle insulin sensitivity. Their results provide further credence to the concept that insulin and glucose delivery to the skeletal myocyte is an important factor in muscle insulin resistance (Fig. 1). A VEGF knockout via cre-recombinase under the control of the muscle creatine kinase promoter allowed generation of mice with a 60% reduction of muscle capillary density. This is a valuable model because it allows an investigation of the chronic effects of impaired microvascular perfusion alone on insulin-mediated glucose metabolism without the complication of impaired insulin signaling in the tissues.
FIG. 1.
In insulin-sensitive subjects, muscle glucose uptake after a meal is stimulated as outlined in the green pathway. The process involves an insulin-mediated stimulation of muscle microvascular perfusion that increases insulin (INS) and glucose (GLU) delivery to the myocyte, leading to GLUT4 translocation and thus increased muscle glucose uptake. Insulin resistance leading to decreased muscle glucose can occur by pathways outlined in red. If there is an impaired microvascular perfusion due to either capillary rarefaction (left side) or impairment of insulin signaling in the endothelium (right side), the decreased delivery of insulin can limit GLUT4 translocation in the myocyte and, along with decreased glucose delivery, reduce muscle glucose uptake.
The insulin sensitivity of these mice was assessed in the Mouse Metabolic Phenotyping Center at Vanderbilt University and shown to have markedly decreased insulin-stimulated glucose uptake in both skeletal muscle (40–45%) and cardiac muscle (63%), resulting in a 45% reduction of insulin-stimulated whole-body glucose disposal during a euglycemic insulin clamp in vivo. However, when insulin-mediated glucose uptake in muscle from these animals was examined in vitro by incubation (where insulin and glucose delivery are not dependent on the vascular system), there was no impairment in insulin action. Thus, the diminished insulin-stimulated glucose uptake observed in vivo is solely due to the impaired vascular delivery.
Results from the Vanderbilt group are remarkably similar to the recent study by Kubota et al. (9) that investigated mice lacking endothelial insulin receptor substrate-2 (IRS-2). The endothelial IRS-2 knockout mice demonstrated similar impairments to the VEGF knockout mice in insulin-stimulated muscle and whole-body glucose uptake in vivo. Muscle insulin action in vitro was also not impaired in the endothelial IRS-2 knockout animals. However, the endothelial IRS-2 knockout animals do not have decreased capillary density in their muscles; instead, they have decreased insulin and glucose delivery to their muscles due to lack of microvascular recruitment in muscle by insulin (Fig. 1). The degree of diminution of insulin-mediated muscle glucose uptake in the VEGF and IRS-2 knockout mice parallels the effects seen in vivo in both rats and humans when insulin-mediated microvascular recruitment has been acutely blocked (10).
A major difference, however, between the VEGF knockout animals and the endothelial IRS-2 knockouts is the impact on whole-body insulin sensitivity as assessed by the glucose infusion rate (GIR) during the insulin clamps. In the endothelial IRS-2 knockout, which lacked muscle microvascular recruitment by insulin, GIR was significantly reduced (45%), whereas VEGF knockout did not significantly change GIR compared with wild-type mice. The reason for these differences appears to lie in the responses in the liver. VEGF knockout animals have increased liver glycogen storage and glucose turnover that accounts for the lack of reduced GIR during the clamp studies. The reason a capillary rarefaction but not a microvascular recruitment impairment leads to altered hepatocyte function is a question that is ripe for further investigation.
To appreciate how significant the vascular effects of insulin can be, it is interesting to compare GIR and muscle glucose uptake effects in the knockout animals with the effects in high-fat diet–fed mice, in which there is a 66% reduction of GIR and a 52% reduction in insulin-mediated muscle glucose uptake (data from 9). High-fat feeding leads to both an impairment of insulin-mediated microvascular recruitment and myocyte insulin resistance, which can be observed in vitro. This additional effect of myocyte insulin resistance has only marginally added to the impact of the vascular defect alone on GIR (cf. 45% reduction endothelial IRS-2) and insulin-mediated muscle glucose uptake (cf. 45% reduction VEGF and endothelial IRS knockout). Interestingly, increasing muscle capillarization by overexpression of angiopoietin-1 (11) or treatment with beraprost (9) in mice overcomes the effects of high-fat feeding.
One factor that is still to be fully resolved is how capillary rarefaction or impaired microvascular recruitment decreases muscle glucose uptake. Although impaired perfusion will reduce delivery of both insulin and glucose, it is not clear which is the major limiting component. The Vanderbilt group has data that support glucose delivery as being limiting (12), but the current study has observed decreased p85/p-IRS-1 in response to insulin in muscle. The latter observation supports instead that insulin delivery is crucial. However, no impairment of Akt phosphorylation was observed in the muscle of VEGF knockout animals, and others have demonstrated that decreased insulin signaling upstream of Akt does not necessarily impact insulin action (13). Only measures of surface GLUT4 content in the muscle of these VEGF and endothelial IRS-2–knockout animals would fully resolve these questions. However, it is not an easy task to obtain accurate and meaningful data when the protocols require in vivo experiments to observe the vascular contribution.
Overall, the study by Bonner et al. (8) highlights that it is not only the myocyte insulin resistance that needs to be targeted for the treatment of insulin and diabetes but that defects in the muscle microvasculature must also be considered. Development of therapeutic agents that overcome the vascular defects associated with insulin resistance could significantly enhance the efficacy of currently used antidiabetic drugs that act on the myocyte. Potentially such drugs may also provide greater cardiovascular protection for diabetic patients.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 572.
REFERENCES
- 1.DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol 2011;108(Suppl.):3B–24B [DOI] [PubMed] [Google Scholar]
- 2.De Boer MP, Meijer RI, Wijnstok NJ, et al. Microvascular dysfunction: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Microcirculation 2012;19:5–18 [DOI] [PubMed] [Google Scholar]
- 3.Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab 2008;295:E732–E750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karpe F, Fielding BA, Ilic V, Macdonald IA, Summers LK, Frayn KN. Impaired postprandial adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes 2002;51:2467–2473 [DOI] [PubMed] [Google Scholar]
- 5.Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009;58:718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lillioja S, Young AA, Culter CL, et al. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 1987;80:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron AD, Steinberg H, Brechtel G, Johnson A. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol 1994;266:E248–E253 [DOI] [PubMed] [Google Scholar]
- 8.Bonner JS, Lantier L, Hasenour CM, James FD, Bracy DP, Wasserman DH. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes 2013;62:572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubota T, Kubota N, Kumagai H, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 2011;13:294–307 [DOI] [PubMed] [Google Scholar]
- 10.Rattigan S, Bussey CT, Ross RM, Richards SM. Obesity, insulin resistance, and capillary recruitment. Microcirculation 2007;14:299–309 [DOI] [PubMed] [Google Scholar]
- 11.Sung HK, Kim YW, Choi SJ, et al. COMP-angiopoietin-1 enhances skeletal muscle blood flow and insulin sensitivity in mice. Am J Physiol Endocrinol Metab 2009;297:E402–E409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fueger PT, Shearer J, Bracy DP, et al. Control of muscle glucose uptake: test of the rate-limiting step paradigm in conscious, unrestrained mice. J Physiol 2005;562:925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleasby ME, Reinten TA, Cooney GJ, James DE, Kraegen EW. Functional studies of Akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced IRS-1 expression. Mol Endocrinol 2007;21:215–228 [DOI] [PubMed] [Google Scholar]