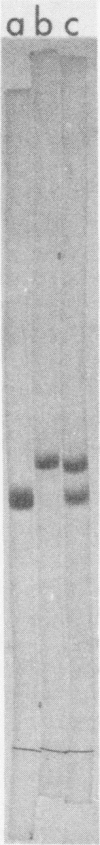
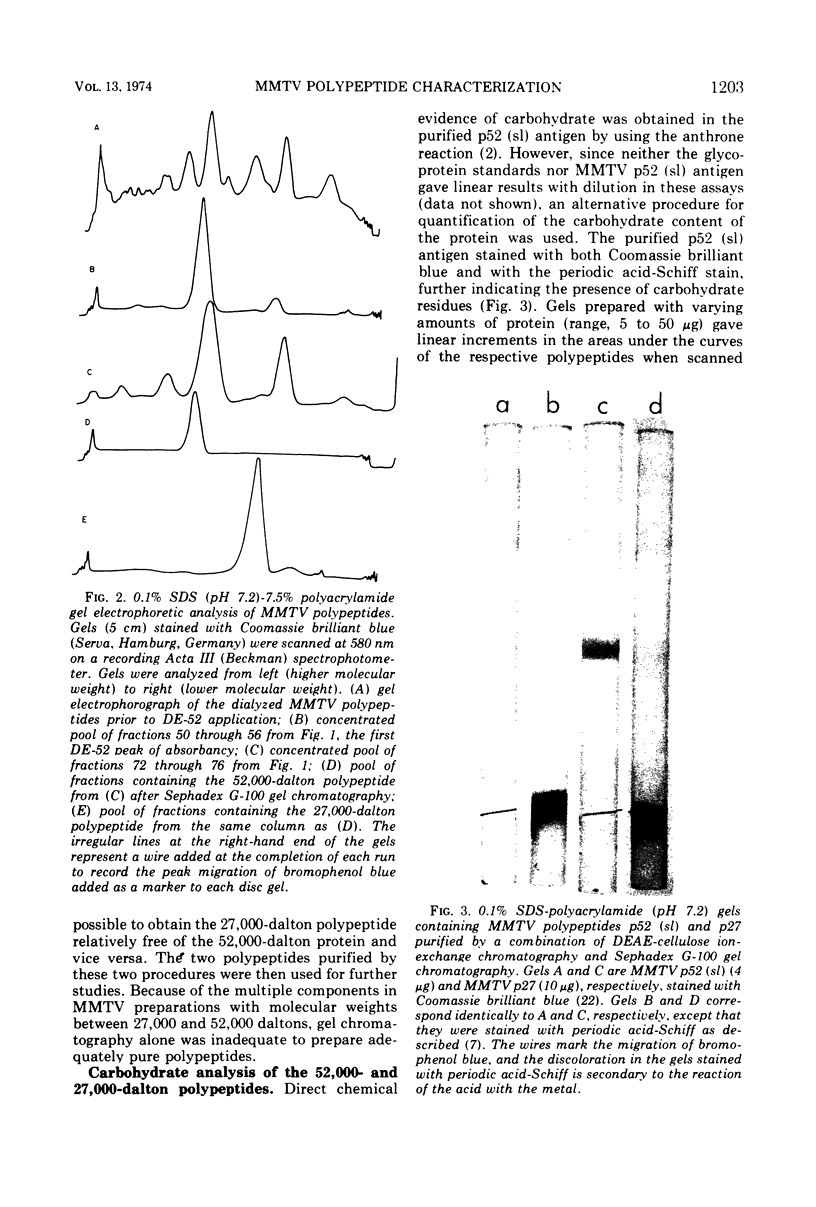
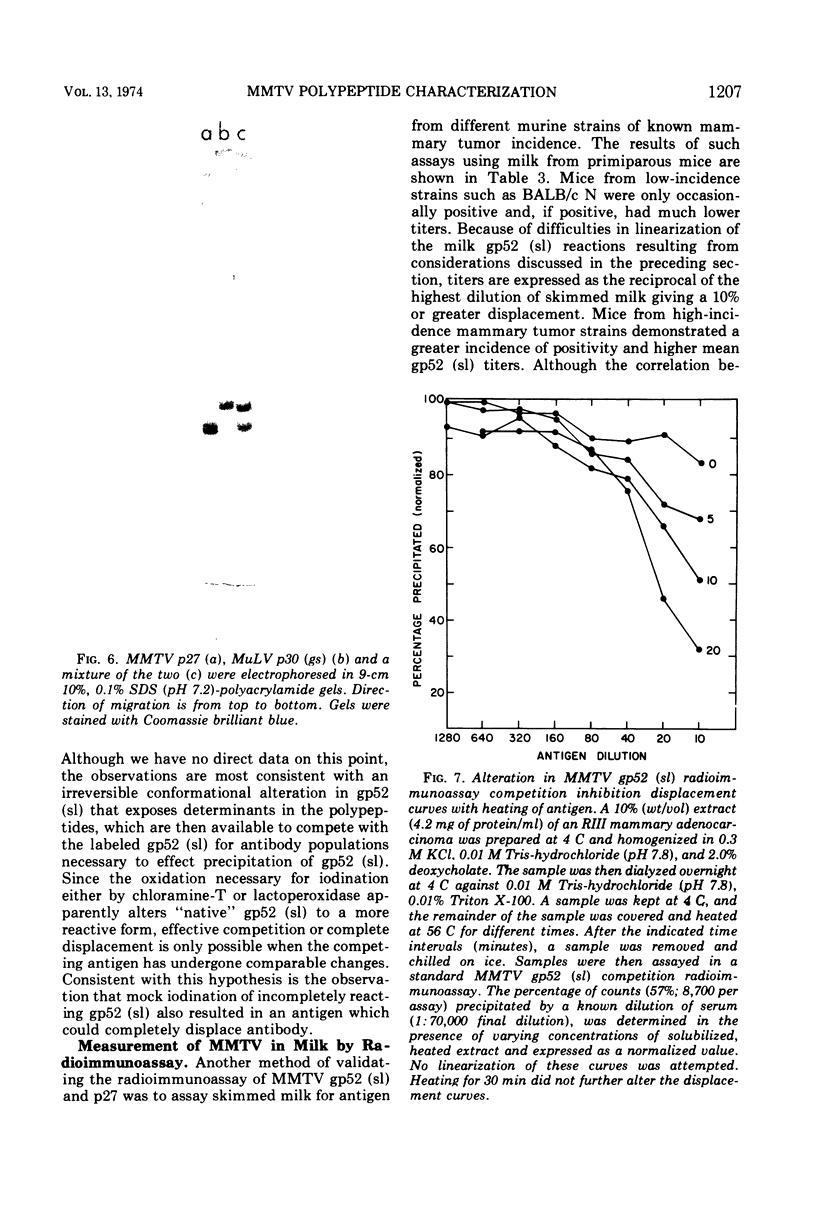
Abstract
A major murine mammary tumor viral (MMTV) antigen, sl, originally described by Nowinski et al. (1967, 1968, 1971), has been purified from RIII mouse milk MMTV by sequential ion-exchange and gel chromatography. The purified protein with sl antigenic reactivity contains carbohydrate, and has an apparent minimal molecular weight of 52,000. It can be designated as gp52 (sl). Another major MMTV viral protein with a molecular weight of 27,000 has also been isolated, and antisera have been prepared against it. Both MMTV gp52 (sl) and p27 viral polypeptides have been iodinated with 125I and used in immunoprecipitation and competition assays. The two MMTV proteins differ absolutely from each other and from major mouse type C viral polypeptides in molecular weight, immunological reactivity, and amino acid composition. Purified gp52 (sl) in radioimmunoprecipitation inhibition assays reacted in two distinct patterns. One pattern showed partial displacement of antibody which could be converted to the second, a complete displacement, by heating the antigen, presumably by exposing additional reactive determinants. Biologically, the patterns of major MMTV polypeptide expression in milk correlated with spontaneous mammary tumor incidence in different strains of mice, indicating that the sl antigen is group specific for MMTV or that several mouse strains contain the same virus type.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon R., Maron E. An immunological approach to the structural relationship between hen egg-white lysozyme and bovine -lactalbumin. J Mol Biol. 1971 Oct 14;61(1):225–235. doi: 10.1016/0022-2836(71)90219-1. [DOI] [PubMed] [Google Scholar]
- Blair P. B. Detection by immunodiffusion of mouse mammary tumor virus in milk samples and correlation with tumor development. Cancer Res. 1969 Apr;29(4):745–748. [PubMed] [Google Scholar]
- Blair P. B., Weiss D. W., Smith G. H. Studies on antigenic cross-reactivity of oncogenic RNA viruses. Cross-reactions between mouse mammary tumor viruses from different mouse strains. Isr J Med Sci. 1970 Sep-Oct;6(5):611–616. [PubMed] [Google Scholar]
- Daams J. H., Calafat J., Lasfargues E. Y., Kramarsky B., Bentvelzen P. Mammary tumor virus associated antigens on the membrane of infected mouse spleen cells. Virology. 1970 May;41(1):184–186. doi: 10.1016/0042-6822(70)90068-1. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hilgers J. H., Theuns G. J., van Nie R. Mammary tumor virus (MTV) antigens in normal and mammary tumor-bearing mice. Int J Cancer. 1973 Nov 15;12(3):568–576. doi: 10.1002/ijc.2910120305. [DOI] [PubMed] [Google Scholar]
- Hilgers J., Williams W. C., Myers B., Dmochowski L. Detection of antigens of the mouse mammary tumor (MTV) and murine leukemia virus (MuLV) in cells of cultures derived from mammary tumors of mice of several strains. Virology. 1971 Aug;45(2):470–483. doi: 10.1016/0042-6822(71)90347-3. [DOI] [PubMed] [Google Scholar]
- Howk R. S., Rye L. A., Killeen L. A., Scolnick E. M., Parks W. P. Characterization and separation of viral DNA polymerase in mouse milk. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2117–2121. doi: 10.1073/pnas.70.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Boyse E. A., de Harven E., Geering G. Group-specific viral antigens in the milk and tissues of mice naturally infected with mammary tumor virus or Gross leukemia virus. Virology. 1968 Apr;34(4):617–629. doi: 10.1016/0042-6822(68)90083-4. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Moore D. H., Geering G., Boyse E. A. A soluble antigen of the mammary tumor virus. Virology. 1967 Jan;31(1):1–14. doi: 10.1016/0042-6822(67)90002-5. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Copeland T., Summers M., Gilden R. V. Amino terminal sequences of mammalian type C RNA tumor virus group-specific antigens. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1549–1555. doi: 10.1016/0006-291x(72)90890-x. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Foreman C., Kelloff G., Gilden R. V. The group-specific antigen and other structural proteins of hamster and mouse C-type viruses. Virology. 1971 Mar;43(3):665–674. doi: 10.1016/0042-6822(71)90290-x. [DOI] [PubMed] [Google Scholar]
- Teramoto Y. A., Puentes M. J., Young L. J., Cardiff R. D. Structure of the mouse mammary tumor virus: polypeptides and glycoproteins. J Virol. 1974 Feb;13(2):411–418. doi: 10.1128/jvi.13.2.411-418.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]