Abstract
In type 1 diabetes, loss of tolerance to β-cell antigens results in T-cell–dependent autoimmune destruction of β cells. The abrogation of autoreactive T-cell responses is a prerequisite to achieve long-lasting correction of the disease. The liver has unique immunomodulatory properties and hepatic gene transfer results in tolerance induction and suppression of autoimmune diseases, in part by regulatory T-cell (Treg) activation. Hence, the liver could be manipulated to treat or prevent diabetes onset through expression of key genes. IGF-I may be an immunomodulatory candidate because it prevents autoimmune diabetes when expressed in β cells or subcutaneously injected. Here, we demonstrate that transient, plasmid-derived IGF-I expression in mouse liver suppressed autoimmune diabetes progression. Suppression was associated with decreased islet inflammation and β-cell apoptosis, increased β-cell replication, and normalized β-cell mass. Permanent protection depended on exogenous IGF-I expression in liver nonparenchymal cells and was associated with increased percentage of intrapancreatic Tregs. Importantly, Treg depletion completely abolished IGF-I-mediated protection confirming the therapeutic potential of these cells in autoimmune diabetes. This study demonstrates that a nonviral gene therapy combining the immunological properties of the liver and IGF-I could be beneficial in the treatment of the disease.
In type 1 diabetes, the immune system attacks and destroys β cells. At the clinical onset of type 1 diabetes, 15–40% of β cells are still able to produce insulin, thus blocking further autoimmune destruction even at this stage, holding great promise for arresting disease progression (1). Yet earlier intervention in individuals with documented autoimmune disease but without clinically manifest diabetes is of course the ultimate goal in any future intervention strategy to prevent diabetes. Following this rationale, a growing number of clinical intervention studies, with the common goal of blocking autoimmune disease and reestablishing long-term tolerance to β cells based on immunomodulation, have been initiated in the past decade (2).
The liver has unique immunological properties that affect T-cell activation and immune regulation. Although the liver is an important site for T-cell activation, this takes place in the context of immunosuppressive cytokines and a distinctive local immune environment, so that exposure to antigens often results in tolerance rather than immunity (3). Tolerance promotion can be mediated, among other mechanisms, by the induction of regulatory T cells (Tregs) capable of inhibiting effector responses in the periphery (4). However, the key hepatic cell type responsible for initiating this phenomenon remains controversial. Whereas several reports point to hepatocytes, others indicate nonparenchymal cells (NPCs) as the cells that promote expansion of specific Treg populations able to limit autoreactive immunity (5). Engineering hepatic cells to express molecules able to induce Tregs therefore represents a potential therapeutic approach for the treatment of autoimmune disorders (5). A number of gene transfer studies have described the ectopic expression of autoantigens in the liver as a means to promote peripheral control of autoreactive lymphocytes by increasing either the number and/or the function of Tregs (6). Also, it has been shown repeatedly, in both mice and nonhuman primates, that upon in vivo liver-directed gene transfer of coagulation factor IX, transgene expression has the capacity to provide therapeutic circulating levels while inducing immune tolerance to the transgene product (7,8). Thus, in the context of an autoimmune disease such as type 1 diabetes, a liver-targeted gene therapy approach to modify peripheral control of autoreactive lymphocytes offers a unique possibility to prevent development and progression of the disease. To achieve this would require efficient and targeted expression to tolerogenic cells as well as utilization of an appropriate immunomodulatory transgene. Among the possible immunomodulatory candidate genes for type 1 diabetes is IGF-I. We have previously reported that expression of IGF-I in β cells of transgenic mice counteracts the cytotoxicity and insulitis induced by treatment with multiple low doses of streptozotocin (STZ) (9,10). Moreover, daily subcutaneous administration of human recombinant IGF-I to prediabetic NOD mice reduces the severity of insulitis and the incidence of type 1 diabetes (11–13). All these studies reveal IGF-I as a key factor able to induce protection from type 1 diabetes.
In this work, we report that after delivery of a plasmid expressing IGF-I to the liver by hydrodynamic tail vein (HTV) injection, the incidence of diabetes decreases in an autoimmune mouse model of the disease. We show that the expression of IGF-I in liver NPCs leads to an increase in intrapancreatic Tregs, resulting in decreased pancreatic infiltration, reduced β-cell apoptosis, and increased β-cell replication, suggesting a blockage of the autoimmune attack against the pancreas.
RESEARCH DESIGN AND METHODS
Animals.
Heterozygous male transgenic mice expressing human interferon (IFN)-β under the control of the rat insulin promoter-I were used (9). Diabetes was induced by intraperitoneal injection of very low doses of STZ (25 mg/kg body weight) dissolved in citrate buffer (pH 4.5) on 5 consecutive days. All mice were fed ad libitum with a standard chow diet (2018S Teklad Global; Harlan) and maintained under conditions of controlled temperature and light (12-h light/dark cycles). Animal care and experimental procedures were approved by the Ethics Committee in Animal and Human Experimentation of the Universitat Autònoma de Barcelona.
Plasmids and hydrodynamic injection.
The HTV procedure was performed as described (14). The CAG, hAAT, and CD68 promoters were cloned substituting the CMV promoter from the commercial pVAX plasmid (Invitrogen). Animals in all STZ control (STZ-Con) groups were injected with the CMV-containing version of pVAX. The hAAT promoter was kindly provided by Dr. Katherine High, University of Pennsylvania.
β-Cell mass, replication, and apoptosis.
Determination of β-cell mass, replication, and apoptosis was performed as described (9,15).
Hormone and metabolite assays.
Blood glucose levels were measured with a Glucometer Elite analyzer (Bayer, Leverkusen, Germany). Serum insulin concentrations were determined by radioimmunoassay (CIS Biointernational, Gif-Sur-Yvette, France). For the glucose tolerance test, awake mice that were fasted overnight (16 h) were administered an intraperitoneal injection of glucose (1 g/kg body weight). The circulating levels of IGF-I were determined by enzyme-linked immunosorbent assay in 15 μL serum samples using the OCTEIA Rat/Mouse IGF-I ELISA (Immunodiagnostic Systems, Boldon, U.K.) following the manufacturer’s instructions.
Isolation of peripheral blood mononuclear cells.
Peripheral blood obtained from mice was diluted 1:1 with PBS plus 0.1% BSA, layered onto an appropriate volume of Histopaque 1083 (Sigma Aldrich, St. Louis, MO) and centrifuged at 800g for 20 min. Peripheral blood mononuclear cells were isolated from the gradient interface and washed in PBS plus 0.1% BSA before staining with a specific antibody for flow cytometry analysis.
Isolation of hepatic NPCs.
Intrahepatic lymphocytes were isolated from liver as previously described (16).
Flow cytometry and cell sorting.
Cells from liver, spleen, peripheral blood, and pancreas were isolated as described above. CD11b, CD19, CD4, CD8, Gr-1, F4/80, CD25, FoxP3 (BioLegend, San Diego, CA), and CD11c (BD PharMingen, San José, CA) antibodies were used. Flow cytometric analysis was performed on a FC500 MPL (Beckman Coulter, Brea, CA) and data were analyzed using FCS 3 Express software (De Novo Software, Los Angeles, CA). CD11b+ cells were sorted using a FacsAria sorter (Becton Dickinson). RNA was isolated using Qiagen RNeasy Plus Micro kit following manufacturer’s instructions.
In vivo depletion of Tregs and natural killer cells.
For Treg depletion, anti-CD25 antibody (purified anti-mouse CD25 antibody, clone PC61; Biolegend) or normal rabbit serum were used. The antibody was injected intraperitoneally (30 µg in 40 µL of water) the same day as the first IGF-I injection, and a second administration was given 3 days later. For natural killer (NK) cells depletion, the antiasialo GM1 antibody (Anti-Mouse Asialo GM1; Diagnostica Longwood) was injected intraperitoneally (300 µg in 40 µL of water).
RT-PCR and quantitative PCR.
One µg RNA was retrotranscribed using the SuperScript VILO cDNA Synthesis Kit (Invitrogen) following manufacturer’s instructions. The following specific primers were used: GFP (F: 5′AAGTTCATCTGCACCACCG3′; R: 5′TCCTTGAAGAAGATGGTGCGC3′); Igf1 (F: 5′CTGGGCAACGTGCTGGTTATT3′; R: 5′GGGTTTGTGAAAGCATCTACGGAA3′); Il10 (F: 5′ ACTGCACCCACTTCCCAGT3′; R: 5′TGTCCAGCTGGTCCTTTGTT3′); Il7 (F: 5′ ATTATGGGTGGTGAGAGCCG3′; R: 5′GTTCCTGTCATTTTGTCCAATTCA3′); Tgfb1 (F: 5′GCAACATGTGGAACTCTACCAG3′; R: 5′ CAGCCACTCAGGCGTATCA3′); RBS (Rps26) (F: 5′ ATTCGCTGCACGAACTGCG 3′; R: 5′ CAGCAGGTCTGAATCGTGGT 3′).
Statistics.
Statistical analyses were performed using GraphPad Prism 4.0 software (GraphPad Software). All data are shown as mean ± SEM unless otherwise indicated. Unpaired t test was used to compare between two groups. Quantitative PCR data were analyzed using REST 2008 software (Corbett Research, Sydney, Australia).
RESULTS
Gene transfer to liver NPCs after HTV injection of plasmid DNA.
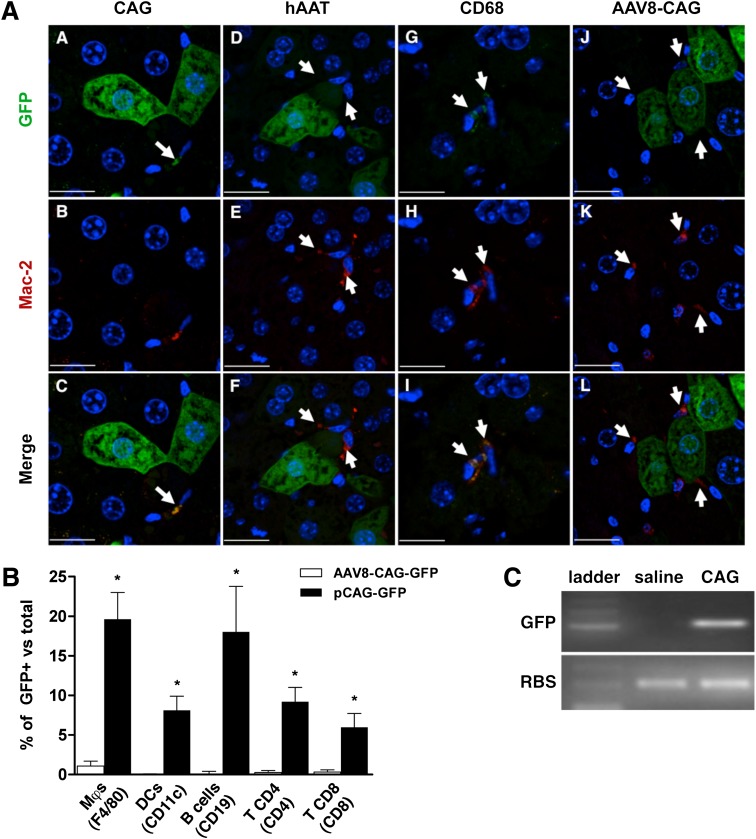
Hepatic cells can be efficiently transduced with both viral and nonviral strategies to express and secrete proteins. We hypothesized that for liver-mediated induction of tolerance to autoantigens, it would suffice with transient expression of the candidate gene. Such short-term expression can be achieved safely through HTV injection of plasmid DNA (14). However, because the full repertoire of cells transduced after HTV injection is unknown, and it is not clear which cells are required for tolerance induction, we analyzed GFP expression in several liver cell populations after injection of plasmid DNA with different promoter constructs. We found that in addition to hepatocytes (17), HTV injection of a plasmid-encoding GFP under control of the ubiquitously expressed CAG promoter (pCAG-GFP) efficiently transduced Kupffer cells (KCs) (Fig. 1A, panels A–C, and Supplementary Fig. 1A). In contrast, after HTV injection of a plasmid expressing GFP under the control of a hepatocyte-specific promoter (hAAT) (18), only GFP+ hepatocytes (but not KCs) were detected (Fig. 1A, panels D–F). However, when a myeloid-specific promoter (CD68) (19) was used to drive the expression of GFP, only GFP+ KCs could be readily detected (Fig. 1A, panels G–I, and Supplementary Fig. 1B). After HTV injection, hepatic expression of GFP plasmids was maximal at 24 h but progressively declined thereafter (Supplementary Fig. 2). As a control, injection of an adeno-associated virus serotype 8 expressing GFP led to long-term expression of GFP in hepatocytes, but KCs remained GFP-negative, consistent with the poor tropism of adeno-associated virus serotype 8 for KCs (20) (Fig. 1A, panels J–L). Flow cytometry analysis of liver NPCs showed that ∼20% of KCs and intrahepatic B lymphocytes, and 5–10% of dendritic cells (DCs) and T lymphocytes were GFP+ after HTV injection of pCAG-GFP (Fig. 1B). RT-PCR analysis of FACS-sorted KC confirmed GFP expression (Fig. 1C), demonstrating that liver NPCs can express foreign genes after HTV injection. Thus, this nonviral gene transfer approach may be used to transiently express genes of interest in liver parenchymal and/or NPCs. Our finding is in disagreement with previous data that showed plasmid-derived expression after HTV injection to be confined to hepatocytes (21).
FIG. 1.
Gene transfer to liver NPCs after HTV injection of plasmid DNA. A: Analysis of GFP and macrophage marker Mac-2 expression in hepatic cells. GFP and Mac-2 expression were examined 24 h after HTV injection of CAG-GFP (A–C), hAAT-GFP (D–F), and CD68-GFP (G–I) plasmids and 1 month after injection of an adeno-associated vector serotype 8 expressing GFP (AAV8-CAG-GFP) (J–L). White arrows indicate the localization of KCs. Scale bars: 18 µm (A–C); 22 µm (D–F); 16 µm (G–I); 24 µm (J–L). B: Flow cytometric analysis of GFP expression in liver NPCs 24 h after HTV injection of pCAG-GFP. Cell markers used to define populations are indicated in parentheses. Results are mean ± SEM of GFP+ cells within each population, n = 4 mice per group. *P < 0.05 vs. AAV8-CAG-GFP. C: Expression of GFP in NPCs. A single GFP-specific band was amplified in FACS-sorted CD11b+ cells from pCAG-GFP–treated livers. RBS, ribosomal protein S26. Data in B are representative of four independent experiments. The immunohistochemistry images are representative examples of three different mice per group. (A high-quality digital representation of this figure is available in the online issue.)
Plasmid-derived expression of IGF-I in liver prevents the development of type 1 diabetes.
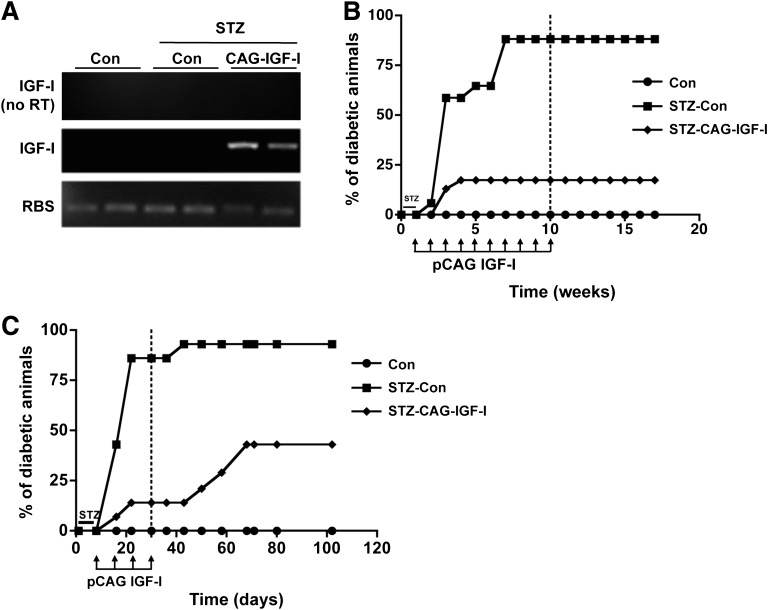
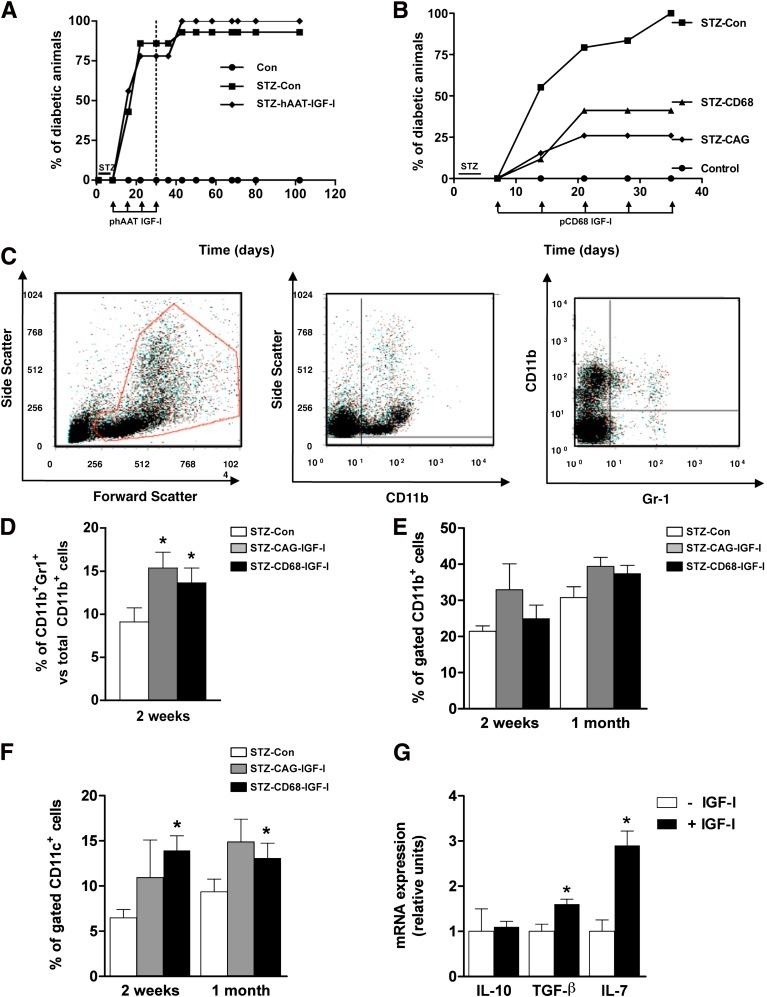
We then tested the ability of the liver to modulate autoreactive lymphocytes by combining the antidiabetic effects of IGF-I with the tolerogenic environment of the liver after HTV injection of IGF-I-expressing plasmids to a transgenic mouse model of type 1 diabetes. These mice express human interferon-β in β cells (TgIFN-β), which results in lymphocytic infiltration of islets and development of overt diabetes when mice are treated with very low doses of STZ, which do not induce diabetes in wild-type mice (9,22). By selecting different promoters, IGF-I was transiently expressed in hepatocytes, NPCs, or both. First, TgIFN-β mice were treated with STZ and 1 week later were administered weekly HTV injections of 5 μg of either pCAG-IGF-I (STZ-CAG-IGF-I) or a noncoding plasmid (STZ-Con). Non-STZ-treated TgIFN-β mice were used as controls. Hepatic transgene-specific IGF-I expression was confirmed by RT-PCR (Fig. 2A). Eight weeks post-STZ, 90% of STZ-Con mice were highly hyperglycemic, whereas ∼83% of STZ-CAG-IGF-I animals did not have development of diabetes (Fig. 2B). All mice showed similar levels of circulating IGF-I (Con, 671 ± 62; STZ-Con, 601 ± 183; STZ-CAG-IGF-I, 594 ± 164 ng/mL). After 10 IGF-I plasmid administrations, STZ-CAG-IGF-I normoglycemic mice reached permanent protection from type 1 diabetes and did not require further treatment (Fig. 2B). On the contrary, although a similar percentage of animals were initially protected from diabetes after receiving only four pCAG-IGF-I administrations, this protection was not sustained over time and 5 weeks after withdrawing the IGF-I treatment the percentage of diabetic animals increased to ∼43% (Fig. 2C), indicating that a longer IGF-I treatment was needed to fully protect from immune-mediated destruction of β cells.
FIG. 2.
Plasmid-derived expression of IGF-I in liver prevents the development of type 1 diabetes. A: Expression of IGF-I in the liver after HTV injection of pCAG-IGF-I. RNA was obtained from livers and analyzed by RT-PCR 24 h after the injection of 5 μg of pCAG-IGF-I. Upper lane: nonretrotranscribed (RT−) samples; middle lane: RT+ samples, detection using IGF-I oligonucleotides; bottom lane: RT+ samples, detection using Rps26 (housekeeping) oligonucleotides. B: Cumulative diabetes incidence after long-term CAG-IGF-I treatment (n = 20 animals per group). C: Cumulative diabetes incidence on short-term CAG-IGF-I treatment (n = 15 animals per group). Animals were considered diabetic when two consecutive measurements of blood glucose were >250 mg/dL. Dotted line indicates end of pIGF-I treatment.
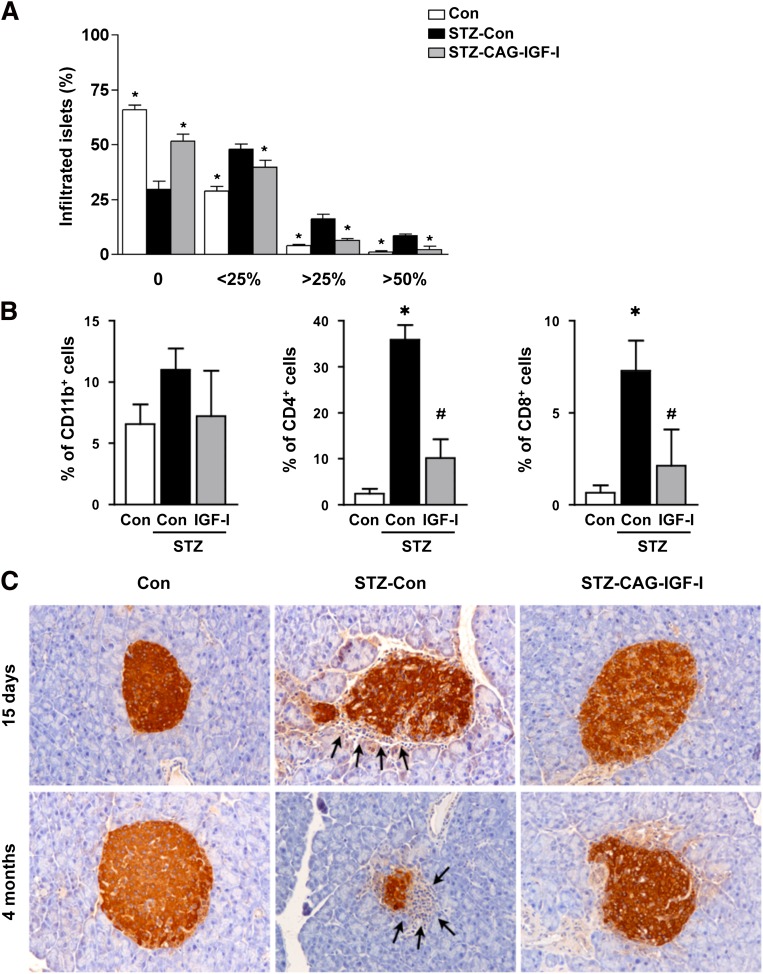
The early stages of diabetic pathogenesis are characterized by insulitis, an inflammation of the islets of Langerhans caused by lymphocytic infiltration. The insulitis score was determined 15 days and 4 months after STZ treatment. Approximately 30% of islets in non-STZ-treated control mice showed lymphocytic infiltration, mainly consisting of peri-insulitis (Fig. 3A), as previously reported (9). Fifteen days after STZ treatment, the percentage of infiltrated islets increased to 70% in STZ-Con mice, with more than 25% of islets highly infiltrated. In normoglycemic CAG-IGF-I--treated animals, insulitis was significantly lower, although not completely cleared, as determined by immunohistochemical and fluorescence-activated cell sorter analysis (Fig. 3B, C). This decreased infiltration suggests that pCAG-IGF-I treatment blocked the autoimmune attack against β cells. Four months after STZ treatment, only few insulin-positive cells could be detected in STZ-Con pancreas, and the remaining islets were small and highly infiltrated. In contrast, most of the islets in normoglycemic pCAG-IGF-I--treated mice were noninfiltrated (Fig. 3C).
FIG. 3.
Decreased pancreatic infiltration following IGF-I treatment. A: Insulitis score was determined in control (Con), STZ-Con, and STZ-CAG-IGF-I animals 15 days after STZ treatment. No infiltration (0), peri-insulitis (<25%), moderate insulitis (<50%), and severe insulitis (>50%). Results are mean ± SEM of four mice per group. *P < 0.05 vs. STZ-Con. B: Percentage of infiltrating macrophages (CD11b+), T CD4+ cells, and T CD8+ cells in islets. The presence of these cells in islets was quantified by flow cytometry from pancreas extracts 15 days after STZ treatment. Results are mean ± SEM of three animals per group. *P < 0.05 vs. Con. #P < 0.05 vs. STZ-Con. C: Immunohistochemical detection of insulin in pancreatic sections of Con, STZ-Con, and STZ-CAG-IGF-I–treated mice at 2 weeks and 4 months after STZ treatment. Arrows indicate lymphocytic infiltrate in islets. Data are representative of at least three independent experiments. (A high-quality digital representation of this figure is available in the online issue.)
Plasmid-derived expression of IGF-I in the liver normalizes pancreatic β-cell mass, insulinemia, and glucose tolerance.
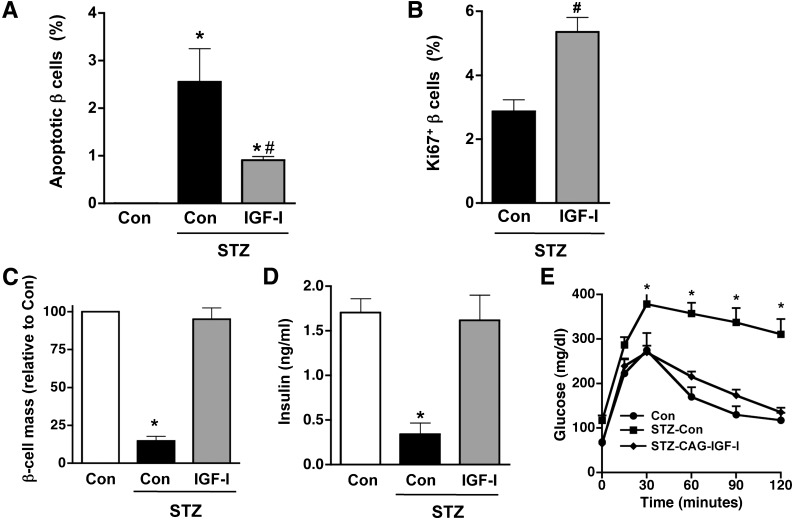
To study if exogenous IGF-I expression in the liver protected β cells from STZ-induced apoptosis, we identified apoptotic cells in paraffin sections with TUNEL staining and non-β cells with an antiglucagon, antisomatostatin, and antipancreatic polypeptide antibody cocktail. Two weeks after STZ treatment, a great increase in TUNEL-positive β cells was detected in islets of mock-treated mice. In contrast, islets of IGF-I-treated mice showed a 2.5-fold reduction in the number of apoptotic cells (Fig. 4A). Double immunostaining for insulin and the proliferation marker Ki67 was used to identify replicating β cells and determine whether replication of existing β cells occurred after IGF-I treatment. Two weeks after STZ treatment, a two-fold increase in Ki67-positive β cells was detected in IGF-I animals compared with STZ-treated controls (Fig. 4B). The higher replication rate and decreased apoptosis of β cells contributed to a striking increase (more than four-fold) in the number of β cells per pancreas area in STZ-IGF-I--treated mice compared with STZ-treated mice (Fig. 4C). As a result, circulating insulin levels measured 4 months after diabetes induction in CAG-IGF-I treated mice were normalized (Fig. 4D) and STZ-CAG-IGF-I mice showed normal tolerance to glucose (Fig. 4E). Thus, the abrogation of autoimmune β-cell attack was sufficient to preserve β-cell function in STZ-CAG-IGF-I--treated mice.
FIG. 4.
Plasmid-derived expression of IGF-I in liver normalizes β-cell mass, insulinemia, and glucose tolerance. TUNEL-positive (apoptotic) (A) and Ki67-positive (replicative) (B) β cells were counted 2 weeks after STZ injection as indicated in research design and methods. Results are mean ± SEM of four mice per group. *P < 0.05 vs. controls (Con). #P < 0.05 vs. STZ-Con. C: β-cell mass was measured 4 months after STZ administration. Serum insulin levels (D) and glucose tolerance (E) were measured 7 weeks after the first IGF-I administration. Results are mean ± SEM of four to five mice per group. For glucose tolerance test, blood glucose levels were measured after an intraperitoneal glucose injection of 1 g/kg body weight. *P < 0.05 vs. Con. Data are representative of three independent experiments.
Nonparenchymal cell-derived IGF-I protects islets from diabetes.
Because HTV injection mediates transduction of both hepatocytes and nonhepatocyte cells in the liver (Fig. 1), we used cell-type specific promoters to restrict IGF-I expression to target cells to discern the populations responsible for diabetes protection. A plasmid expressing IGF-I under the control of the hepatocyte-specific hAAT promoter (phAAT-IGF-I) was administered weekly for 4 weeks. Similar to STZ-Con mice, 100% of STZ-hAAT-IGF-I--treated mice had development of diabetes (Fig. 5A), indicating that hepatocyte-derived IGF-I is ineffective in protecting islets from lymphocytic infiltration. In contrast, when a plasmid expressing IGF-I under the control of the KC-specific CD68 promoter (pCD68-IGF-I) was administered to STZ-treated mice, ∼60% of mice were protected from diabetes, whereas 100% of STZ-Con mice had development of diabetes (Fig. 5B). In this experimental group, the expression in hepatic NPC of IGF-I derived from the plasmid was confirmed by RT-PCR (Supplementary Fig. 3). These results show that IGF-I expression in nonparenchymal liver cells is necessary and sufficient to suppress diabetes progression. The slightly lower degree of protection as compared with the CAG-IGF-I--treated mice could reflect differences in the expression levels of plasmid-derived IGF-I. Alternatively, it may suggest that other hepatic cell types also play a role in preventing diabetes onset. Several studies have shown that other NPC types such as liver sinusoidal endothelial cells (23), DCs (24), stellate cells (25), and lymphocytes (26) can mediate liver-derived immune tolerance in several animal models of autoimmune diseases.
FIG. 5.
KC-derived but not hepatocyte-derived IGF-I protects islets from diabetes and increases hepatic myeloid-derived suppressor cells and expression of IL-7 and TGF-β. A: Cumulative diabetes incidence after hAAT-IGF-I treatment. Dotted line indicates end of treatment (n = 15 animals per group). B: Cumulative diabetes incidence after CD68-IGF-I expression (n = 15 animals per group). Animals were considered diabetic when two consecutive measurements of blood glucose were >250 mg/dL. Data are representative of at least two independent experiments. Representative plots (C) and percentage of CD11b+Gr1+ MDSC cells (D). CD11b+ KCs (E) and CD11c+ DCs (F) as determined by flow cytometry at weeks 2 and 4 after pIGF-I treatment. Results are mean ± SEM of five animals per group. *P < 0.05 vs. STZ-Con. G: Expression of IL-10, TGF-β, and IL-7 from in vitro cultured DCs was measured by quantitative PCR as described in research design and methods. *P < 0.05 vs. mock. Data are representative of two independent experiments. (A high-quality color representation of this figure is available in the online issue.)
We measured by flow cytometry the frequency of several types of hepatic NPCs after pIGF-I treatment. The numbers of CD4+ T lymphocytes and NK cells were increased in livers of IGF-I-treated mice, whereas the percentage of hepatic CD8+ T cells was decreased in both CAG-IGF-I--treated and CD68-IGF-I--treated animals (Supplementary Fig. 4A, B). Importantly, no changes were detected in the frequency of peripheral macrophages, DCs or lymphocytes with either treatment (Supplementary Fig. 4C, D). The only exception was a decrease in circulating NK cells in the CAG-IGF-I group (Supplementary Fig. 4C). IGF-I treatment also led to a significant increase in liver CD11b+Gr1+ myeloid-derived suppressor cells (MDSCs), in both STZ-CAG-IGF-I and STZ-CD68-IGF-I mice 2 weeks post-STZ (Fig. 5C, D). Nevertheless, the percentage of CD11b+ cells remained unchanged (Fig. 5E). In addition to being potent suppressors of various T-cell functions, MDSCs can also induce Treg development (27). The percentage of hepatic DCs was also increased (Fig. 5F). To rule out the possibility that these differences were attributable to changes in total cell numbers (e.g., presence of an inflammatory infiltrate in response to higher numbers of CpG motifs in the IGF-I plasmid as compared with the noncoding one), the total amount of CD11b+ and CD11c+ cells was measured in a separate cohort of mice that received either CAG-IGF-I or noncoding plasmid and compared with an uninjected control group. Importantly, both plasmid-treated groups had equivalent CD11b+ and CD11c+ cell numbers in the liver (data not shown). To further determine the effects of IGF-I expression on DCs, the DCs from livers of healthy mice were isolated and cultured in vitro in the presence of exogenous IGF-I and the expression of key immunosuppressive cytokines was analyzed by quantitative PCR. IGF-I-treated DCs showed an increase in transforming growth factor (TGF)-β and interleukin (IL)-7 expression, whereas IL-10 remained unchanged (Fig. 5G). TGF-β is critically involved in peripheral conversion of conventional T cells to Tregs (28), whereas IL-7 is a survival factor for Tregs and is expressed by diabetes-suppressive immature DCs (29).
Roles of Treg and NK cells in diabetes modulation.
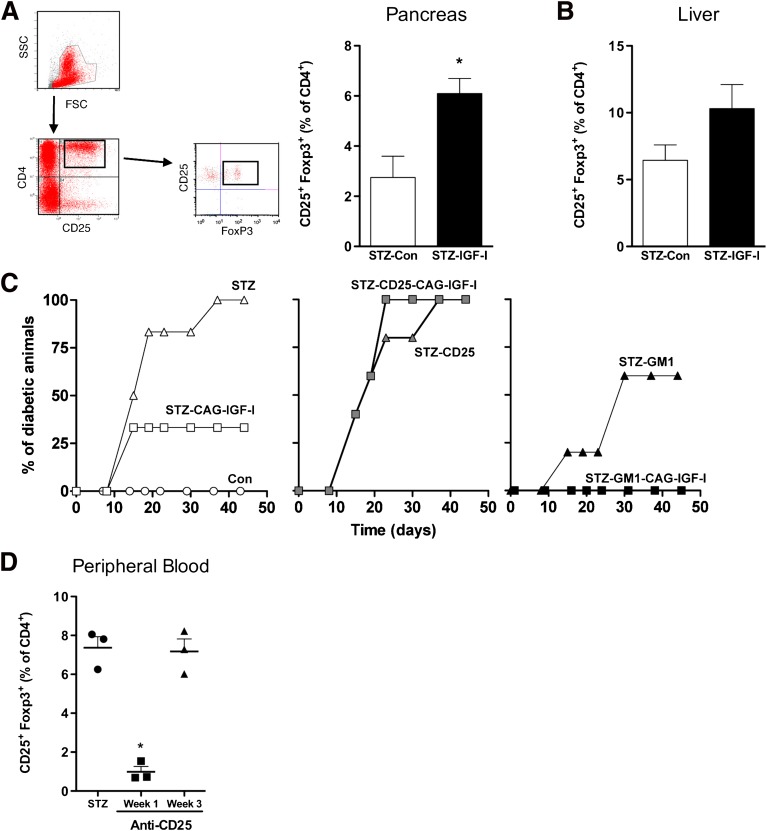
Tregs play a major role in the control of type 1 diabetes (30). To define if changes in Tregs populations underlie the ability of hepatic IGF-I expression to protect from diabetes, we analyzed the percentage of intrahepatic and intrapancreatic Tregs after pCAG-IGF-I treatment. A significant (approximately two-fold) increase of CD4+CD25+Foxp3+ Tregs in the pancreas of IGF-I-treated animals was found (Fig. 6A). An increase of approximately two-fold in intrahepatic Tregs, albeit nonsignificant, also was observed when compared with STZ-Con mice (Fig. 6B). The contribution of Tregs in the prevention of diabetes was further studied by depleting this cell population with an anti-CD25 antibody as previously described (31). Treg depletion in the absence of STZ failed to trigger diabetes onset (Supplementary Fig. 5). As expected from previous experiments, IGF-I treatment prevented the development of diabetes in ∼70% of STZ-treated mice, whereas the depletion of CD25+ lymphocytes completely abolished the IGF-I-mediated protective effect (Fig. 6C, left and middle panels). Flow cytometric analysis of peripheral blood lymphocytes showed that the levels of Treg cells declined most significantly at week 1 and gradually returned to normal within 3 weeks (Fig. 6D). However, animals remained diabetic.
FIG. 6.
IGF-I treatment increases the number and function of Treg cells and decreases NK cell number. A: Representative plot (left) and percentage of CD4+CD25+FoxP3+ Tregs in pancreas (right) and (B) liver was measured by flow cytometry 1 month after the induction of diabetes. Values are mean ± SEM of three animals per group. *P < 0.05 vs. STZ-Con. C: Cumulative diabetes incidence after Treg depletion using an anti-CD25 monoclonal antibody resulted in the loss of IGF-I protective effects (middle panel, n = 6 mice per group). Depletion of NK cells using an antiasialo GM1 polyclonal antibody (right panel) resulted in all IGF-I-treated animals becoming diabetes-free (n = 6 mice per group). Data are representative of two independent experiments. D: Depletion of peripheral Treg cells was evaluated 1 and 3 weeks after treatment with anti-CD25 antibody. *P < 0.05 vs. STZ-Con. (A high-quality color representation of this figure is available in the online issue.)
Our observation of a decrease in circulating and an increase in intrahepatic NK cells after pIGF-I treatment, as well as recent work from others (32), suggest a prominent role of this lymphocyte population in diabetes development. Also, it has been described for humans and mice that Tregs can suppress NK cell functions, including cytotoxicity (33,34). To assess the relevance of NK cells in the development of diabetes, we administered an antiasialo GM1 antibody to deplete this lymphocyte population (32,35). Depletion of NK cells reduced the incidence of diabetes to 60% in STZ-Con animals, whereas it completely abolished the incidence of the disease in IGF-I-treated animals (Fig. 6C, right panel). This and the reduction of NK population in peripheral blood mononuclear cells in IGF-I-treated animals indicate that prevention from diabetes after IGF-I treatment may be mediated, at least in part, by a decrease in the number of NK cells. Collectively, these results highlight the central role of Tregs and NK cells, in our animal model, in preventing or accelerating the disease, respectively.
DISCUSSION
In the current study, we showed that plasmid-derived IGF-I expression in the liver of prediabetic TgIFN-β mice protects from type 1 diabetes. Whereas concurrent expression of IGF-I in hepatocytes and NPCs led to disease protection, NPC-specific IGF-I expression was sufficient to prevent the onset of the disease. Our results suggest a key role of NPCs in promoting expansion of Treg populations able to limit autoreactive immunity because hepatic IGF-I expression caused enhanced CD4+CD25+Foxp3+ Treg number and tolerogenic function. We previously proposed that IGF-I is an interesting candidate to prevent type 1 diabetes (9,10). However, to our knowledge, this is the first study to report the use of a gene therapy approach using IGF-I as a protective agent against autoimmune diabetes.
First, we characterized the liver cell populations that were transduced after hydrodynamic injection of plasmid DNA. It was unexpectedly observed that in addition to hepatocytes, other hepatic cells were also transduced. Previous work by others had established that plasmid DNA injected under HTV conditions could be found within endothelial cells and KCs (17,21). However, actual expression in NPCs was not detected. The discrepancy with our work may be attributable to the lack of use of specific antibodies against NPCs in the previous studies to unequivocally identify transduced NPCs. Thus, it is possible that transduced NPCs were misidentified as being hepatocytes. Through immunohistochemical analysis, PCR and flow cytometry data to show GFP-positive NPCs after HTV injection, we provide strong evidence that NPCs are able to express plasmid-derived DNA after HTV. Because a significant percentage of nonparenchymal immune liver cells, such as KCs and DCs, are transduced after HTV injection, our data may have implications for the interpretation of gene therapy experiments targeting the liver using this nonviral technique.
We took advantage of the transient expression of transgenes in specific liver cell types mediated by plasmid HTV to examine whether exogenous IGF-I expression in the liver could prevent autoimmune diabetes in a transgenic mouse model of the disease. TgIFN-β mice treated with very low doses of STZ comprised the model used in the study presented herein. We (9,22) and others (35,36) have established the TgIFN-β mice as a model of type 1 diabetes devoid of some of the limitations present in the most widely used NOD mouse model; namely, there is a major gender bias in the incidence of diabetes: ∼80% of disease incidence in NOD females versus ∼0–40% in males. NOD mice harbor a plethora of genetically fixed immune defects, such as dysfunctional NK cells and lack of hemolytic complement, that distinguish these mice from human patients. Also, the incidence of disease is highest when NOD mice are maintained in a relatively germ-free environment but dramatically decreases when in conventional housing facilities. It is now acknowledged that type 1 diabetes is such a complex and heterogeneous disease that the use, or overuse as already pointed out by some authors (37), of the NOD model as the single model of the disease may result in a limited landscape of both basic and translational research in the field of autoimmune diabetes. On the contrary, TgIFN-β mice show normal reproduction and life span, and both male and female have development of diabetes after treatment with very low doses of STZ. Thus, the use of these transgenic mice offers advantages because studies in diabetic male or female mice can be easily programmed, depending on the experimental needs. Furthermore, when TgIFN-β mice are backcrossed with NOD mice, development of diabetes is clearly accelerated (38). These features indicate that TgIFN-β mice may be an important tool for dissecting tolerance mechanisms and assaying new treatments for this disease.
A large body of evidence indicates that Tregs play a central role in retarding and/or suppressing diabetes onset (39). Recently, Grinberg-Bleyer et al. (40) have showed that increasing the number of Treg cells locally in the pancreas can reverse established disease in NOD mice. In our work, an increase in intrapancreatic Treg cells was detected in IGF-I-treated mice compared with those treated with a noncoding plasmid. The induction of TGF-β production after the exposure of isolated liver-derived mononuclear cells to IGF-I in vitro suggests that the increase in the number of Tregs may be the result of augmented conversion of conventional T cells into Tregs (41). However, we also detected an increase in IL-7 transcription in cultured DCs exposed to IGF-I. Giannoukakis et al. (29) have shown that DCs derived from NOD mice engineered ex vivo to become immunoregulatory increased their prevalence of Tregs via the action of IL-7. Their work described IL-7 as a survival factor for Tregs. Thus, the observed increase in Tregs also may be the result of an improvement in Treg survival.
Despite the importance of Tregs in diabetes prevention in the experiments presented herein, the participation of other key players cannot be ruled out. A role for NK cells in the development of diabetes was also envisaged in this study. We observed a decrease in the levels of circulating NK cells in IGF-I-treated animals along with an increase in intrahepatic NK cells. This observation bears similarities to the studies of John and Crispe (42) in which they described an inverse correlation between the frequency of activated CD8+ T cells trapped in the liver and their frequency in the circulating pool. We also detected an increase in CD4+ cells in the liver after treatment with IGF-I plasmids. Studies reporting accumulation of activated CD8+ cells (43) and NK cells (44) during tolerance induction suggest an important role for the liver in this process. Whereas CD8+ cell accumulation seems to lead to destruction of activated CD8+ cells, in the presence of the antigen NK cells seem to play an anti-inflammatory role in the liver. In addition, the recent finding that MDSCs affect NK activity via TGF-β and that downregulation of splenic and liver NK cell function is inversely correlated with the marked increase of MDSCs in liver and spleen (45) might explain the relevance of NK cells in our studies. It also has been described that Tregs could suppress NK cell functions, including cytotoxicity, NKG2D expression, and IFN production in humans and mice (33,34).
Collectively, our data suggest that complex interactions between different cellular types (Tregs, NK cells, and MDSCs) may be responsible for the observed diabetes prevention after IGF-I treatment. Although our study using HTV injection of plasmid DNA to prevent autoimmune diabetes represents a proof of concept in mice, recent progress toward a clinically applicable hydrodynamic gene delivery procedure (46) may favor the translation of such in vivo approaches for the treatment of autoimmune diseases in human subjects.
ACKNOWLEDGMENTS
This work was supported by grants from Ministerio de Ciencia e Innovación (SAF2005-01262 and SAF2008-00962) and from the European Community (FP6 CLINIGENE, LSHB-CT-2006-018933). X.M.A., J.A., and A.R. were recipients of a predoctoral fellowship from Ministerio de Educación, Cultura y Deporte, and D.C. received a predoctoral fellowship from Instituto de Salud Carlos III, Spain. C.J.M. was the recipient of a postdoctoral fellowship from Ministerio de Educación, Cultura y Deporte, Spain.
No potential conflicts of interest relevant to this article were reported.
X.M.A., S.T., and F.B. designed experiments and wrote the manuscript. X.M.A., S.T., C.R., D.C., J.A., M.O., A.Ri., A.Ru., C.J.M., and A.C. performed experiments. F.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1776/-/DC1.
†Deceased.
REFERENCES
- 1.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature; 2010;464:1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluestone JA, Auchincloss H, Nepom GT, Rotrosen D, St Clair EW, Turka LA. The Immune Tolerance Network at 10 years: tolerance research at the bedside. Nat Rev Immunol 2010;10:797–803 [DOI] [PubMed] [Google Scholar]
- 3.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 2009;27:147–163 [DOI] [PubMed] [Google Scholar]
- 4.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol 2003;3:51–62 [DOI] [PubMed] [Google Scholar]
- 5.Tiegs G, Lohse A. Immune tolerance: What is unique about the liver. J Autoimmun 2010;34:1–6 [DOI] [PubMed] [Google Scholar]
- 6.Hoffman BE, Herzog RW. Coaxing the liver into preventing autoimmune disease in the brain. J Clin Invest 2008;118:3271–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobrzynski E, Fitzgerald JC, Cao O, Mingozzi F, Wang L, Herzog RW. Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc Nat Acad Sci USA 2006;103:4592–4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver . Blood; 2007;110:2334–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casellas A, Salavert A, Agudo J, et al. Expression of IGF-I in pancreatic islets prevents lymphocytic infiltration and protects mice from type 1 diabetes. Diabetes; 2006;55:3246–3255 [DOI] [PubMed] [Google Scholar]
- 10.George M, Ayuso E, Casellas A, Costa C, Devedjian JC, Bosch F. Beta cell expression of IGF-I leads to recovery from type 1 diabetes. J Clin Invest 2002;109:1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergerot I, Fabien N, Maguer V, Thivolet C. Insulin-like growth factor-1 (IGF-1) protects NOD mice from insulitis and diabetes. Clin Exp Immunol 1995;102:335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Salojin KV, Mi Q-S, et al. Insulin-like growth factor (IGF)-I/IGF-binding protein-3 complex: therapeutic efficacy and mechanism of protection against type 1 diabetes. Endocrinology 2004;145:627–638 [DOI] [PubMed] [Google Scholar]
- 13.Kaino Y, Hirai H, Ito T, Kida K. Insulin-like growth factor I (IGF-I) delays the onset of diabetes in non-obese diabetic (NOD) mice. Diabetes Res Clin Pract 1996;34:7–11 [DOI] [PubMed] [Google Scholar]
- 14.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 1999;6:1258–1266 [DOI] [PubMed] [Google Scholar]
- 15.Agudo J, Ayuso E, Jimenez V, et al. IGF-I mediates regeneration of endocrine pancreas by increasing beta cell replication through cell cycle protein modulation in mice. Diabetologia 2008;51:1862–1872 [DOI] [PubMed] [Google Scholar]
- 16.Crispe IN. Isolation of mouse intrahepatic lymphocytes. In Current Protocols in Immunology. Coligan JE, Ed. New York, Wiley, 2001, p. 812–831 [DOI] [PubMed] [Google Scholar]
- 17.Budker VG, Subbotin VM, Budker T, Sebestyén MG, Zhang G, Wolff JA. Mechanism of plasmid delivery by hydrodynamic tail vein injection. II. Morphological studies. J Gene Med 2006;8:874–888 [DOI] [PubMed] [Google Scholar]
- 18.Mingozzi F, Liu YL, Dobrzynski E, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest 2003;111:1347–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottfried E, Kunz-Schughart LA, Weber A, et al. Expression of CD68 in non-myeloid cell types. Scand J Immunol 2008;67:453–463 [DOI] [PubMed] [Google Scholar]
- 20.Nakai H, Wu X, Fuess S, et al. Large-scale molecular characterization of adeno-associated virus vector integration in mouse liver. J Virol 2005;79:3606–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebestyén MG, Budker VG, Budker T, et al. Mechanism of plasmid delivery by hydrodynamic tail vein injection. I. Hepatocyte uptake of various molecules. J Gene Med 2006;8:852–873 [DOI] [PubMed] [Google Scholar]
- 22.Pelegrin M, Devedjian JC, Costa C, et al. Evidence from transgenic mice that interferon-beta may be involved in the onset of diabetes mellitus. J Biol Chem 1998;273:12332–12340 [DOI] [PubMed] [Google Scholar]
- 23.Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology; 2008;47:296–305 [DOI] [PubMed] [Google Scholar]
- 24.Bamboat ZM, Stableford JA, Plitas G, et al. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol 2009;182:1901–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CH, Kuo LM, Chang Y, et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology; 2006;44:1171–1181 [DOI] [PubMed] [Google Scholar]
- 26.Klugewitz K, Blumenthal-Barby F, Schrage A, Knolle PA, Hamann A, Crispe IN. Immunomodulatory effects of the liver: deletion of activated CD4+ effector cells and suppression of IFN-gamma-producing cells after intravenous protein immunization. J Immunol 2002;169:2407–2413 [DOI] [PubMed] [Google Scholar]
- 27.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shevach EM, Davidson TS, Huter EN, Dipaolo RA, Andersson J. Role of TGF-Beta in the induction of Foxp3 expression and T regulatory cell function. J Clin Immunol 2008;28:640–646 [DOI] [PubMed] [Google Scholar]
- 29.Harnaha J, Machen J, Wright M, et al. Interleukin-7 is a survival factor for CD4+ CD25+ T-cells and is expressed by diabetes-suppressive dendritic cells. Diabetes 2006;55:158–170 [PubMed] [Google Scholar]
- 30.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 2006;212:8–27 [DOI] [PubMed] [Google Scholar]
- 31.Cao O, Dobrzynski E, Wang L, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer . Blood; 2007;110:1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gur C, Porgador A, Elboim M, et al. The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat Immunol 2010;11:121–128 [DOI] [PubMed] [Google Scholar]
- 33.Ghiringhelli F, Ménard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 2005;202:1075–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ralainirina N, Poli A, Michel T, et al. Control of NK cell functions by CD4+CD25+ regulatory T cells. J Leukoc Biol 2007;81:144–153 [DOI] [PubMed] [Google Scholar]
- 35.Alba A, Planas R, Clemente X, et al. Natural killer cells are required for accelerated type 1 diabetes driven by interferon-beta. Clin Exp Immunol 2008;151:467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alba A, Puertas MC, Carrillo J, et al. IFN beta accelerates autoimmune type 1 diabetes in nonobese diabetic mice and breaks the tolerance to beta cells in nondiabetes-prone mice. J Immunol 2004;173:6667–6675 [DOI] [PubMed] [Google Scholar]
- 37.von Herrath MG, Nepom GT. Animal models of human type 1 diabetes. Nat Immunol 2009;10:129–132 [DOI] [PubMed] [Google Scholar]
- 38.Alba A, Puertas MC, Carrillo J, et al. IFN beta accelerates autoimmune type 1 diabetes in nonobese diabetic mice and breaks the tolerance to beta cells in nondiabetes-prone mice. J Immunol 2004;173:6667–6675 [DOI] [PubMed] [Google Scholar]
- 39.You S, Thieblemont N, Alyanakian MA, Bach JF, Chatenoud L. Transforming growth factor-beta and T-cell-mediated immunoregulation in the control of autoimmune diabetes. Immunol Rev 2006;212:185–02 [DOI] [PubMed] [Google Scholar]
- 40.Grinberg-Bleyer Y, Baeyens A, You S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 2010;207:1871–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chatenoud L. CD3-specific antibody-induced active tolerance: from bench to bedside. Nat Rev Immunol 2003;3:123–132 [DOI] [PubMed] [Google Scholar]
- 42.John B, Crispe IN. Passive and active mechanisms trap activated CD8+ T cells in the liver. J Immunol 2004;172:5222–5229 [DOI] [PubMed] [Google Scholar]
- 43.Shibolet O, Alper R, Zolotarov L, et al. The role of intrahepatic CD8+ T cell trapping and NK1.1+ cells in liver-mediated immune regulation. Clin Immunol 2004;111:82–92 [DOI] [PubMed] [Google Scholar]
- 44.Samsonov D, Trop S, Alper R, Diment J, Ilan Y. Enhancement of immune tolerance via induction of NK1.1 positive liver-associated-lymphocytes under immunosuppressive conditions. J Hepatol 2000;32:812–820 [DOI] [PubMed] [Google Scholar]
- 45.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-{beta}1. J Immunol 2009;182:240 [DOI] [PubMed] [Google Scholar]
- 46.Suda T, Suda K, Liu D. Computer-assisted hydrodynamic gene delivery. Mol Ther 2008;16:1098–1104 [DOI] [PubMed] [Google Scholar]