Abstract
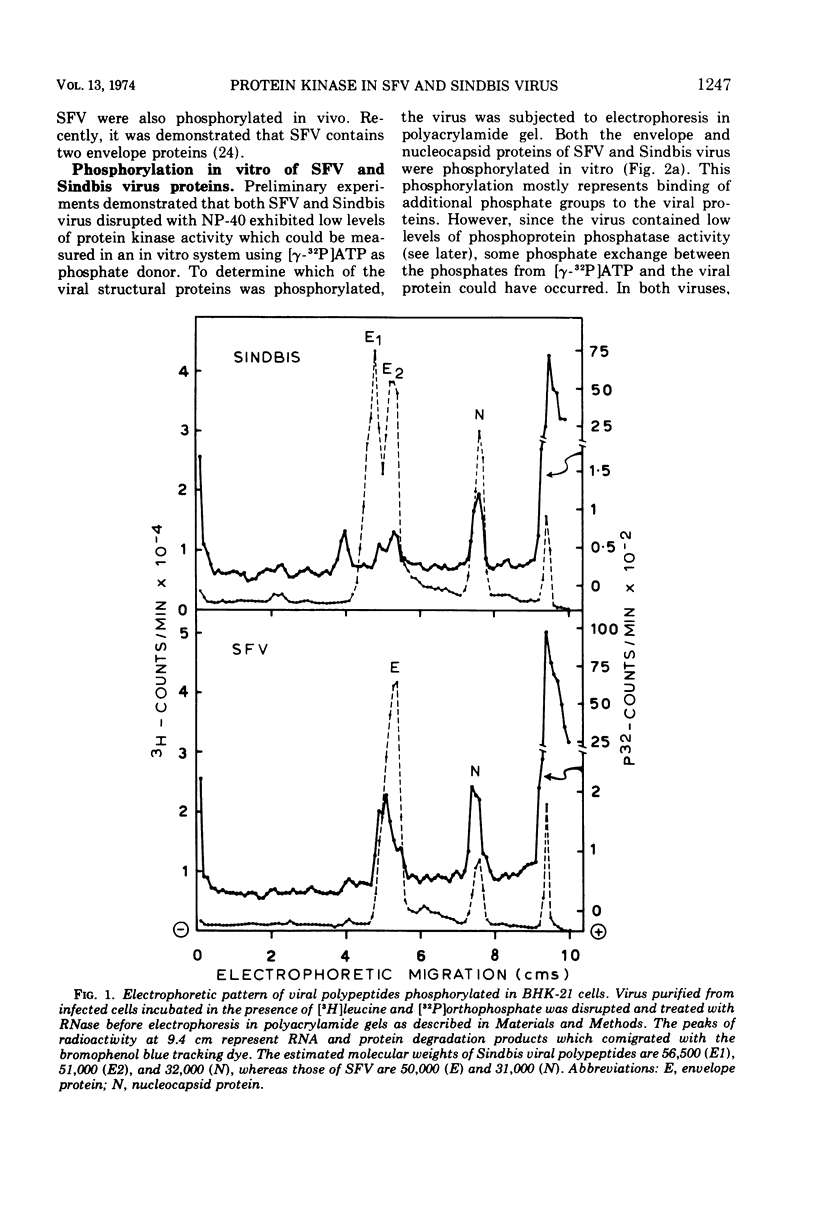
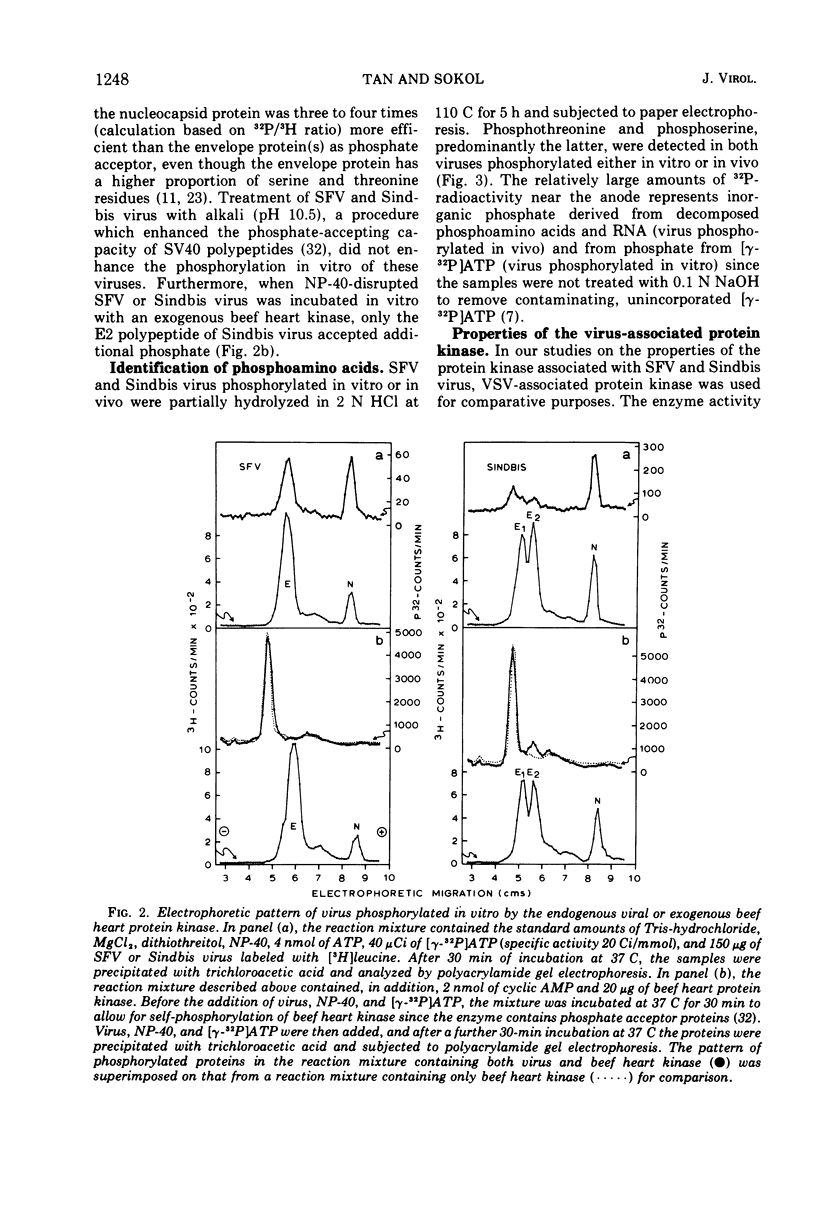
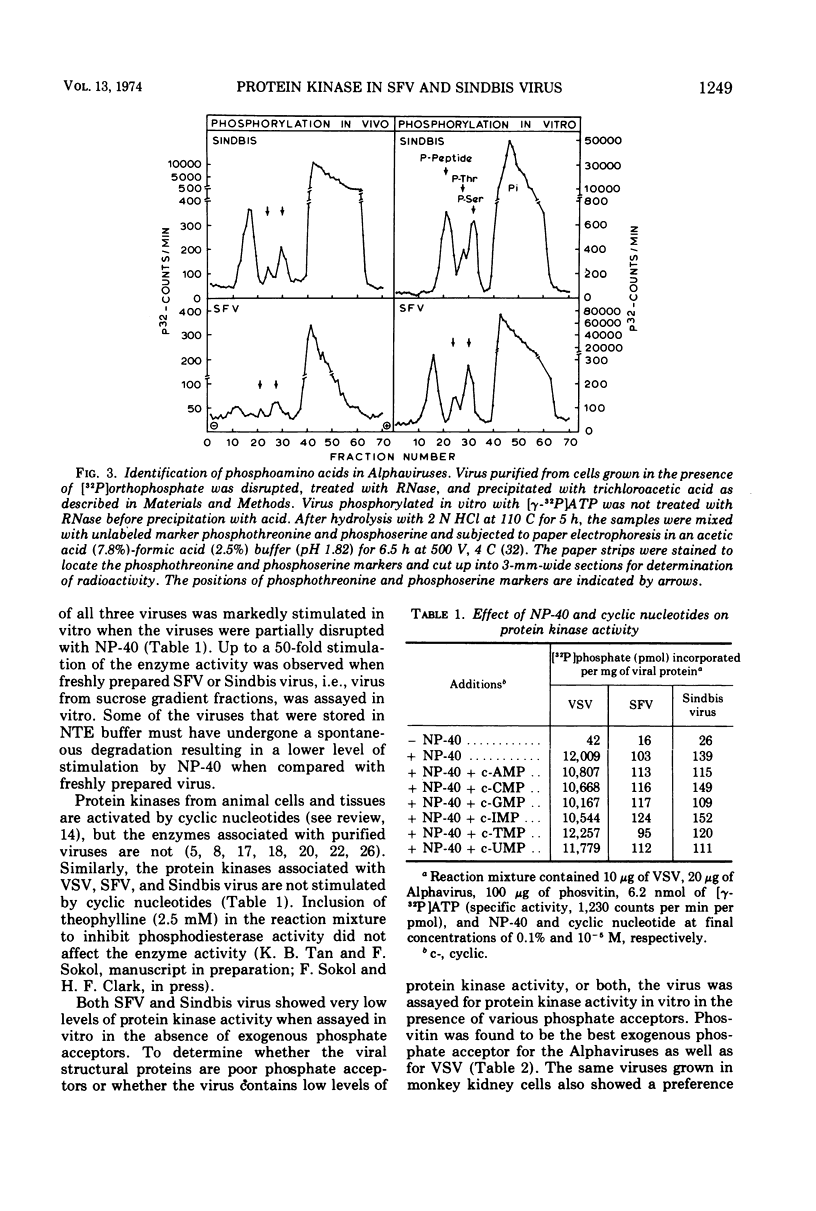
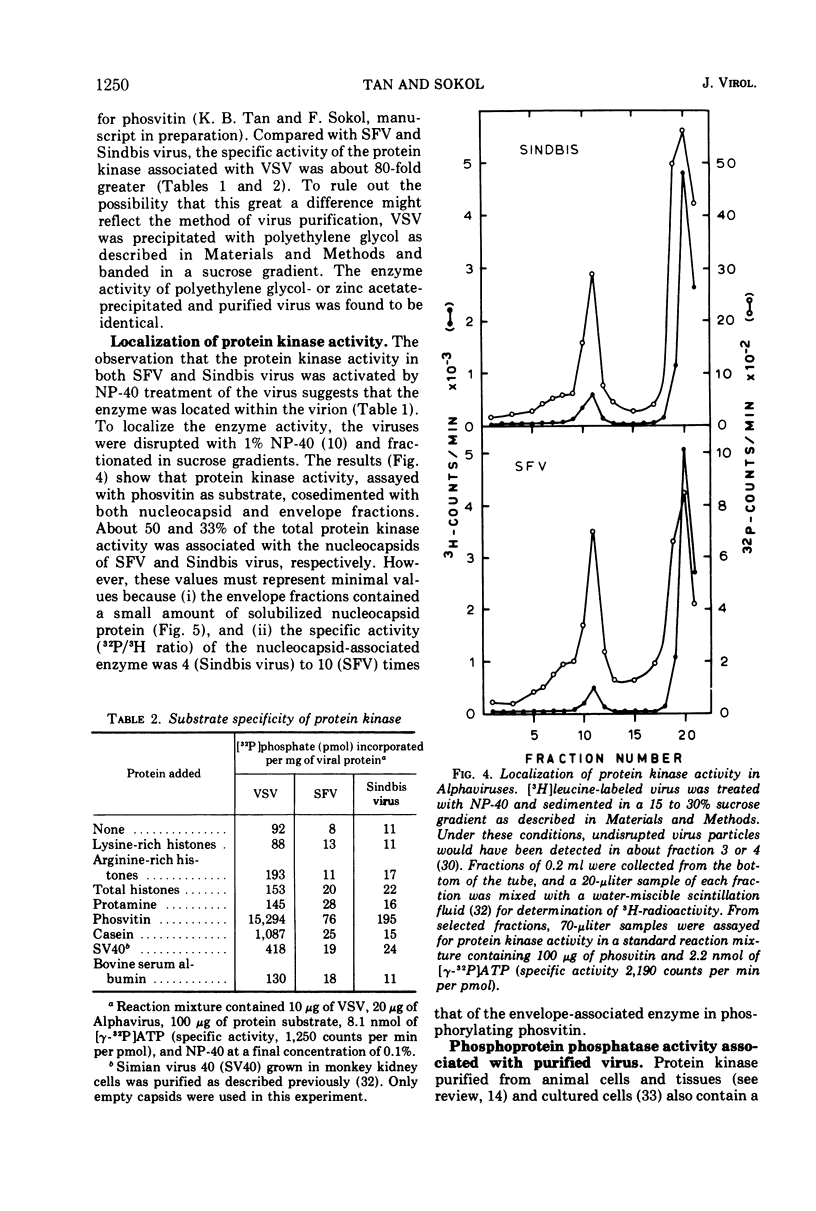
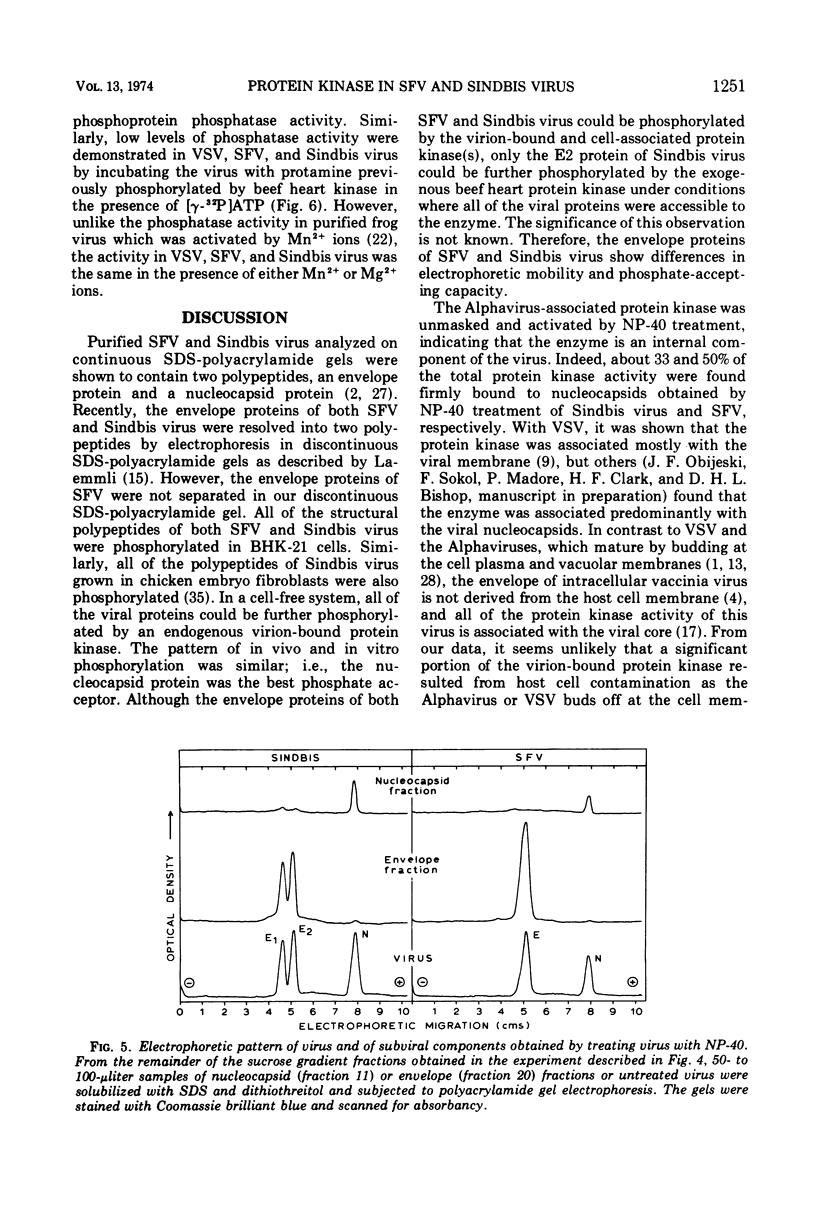
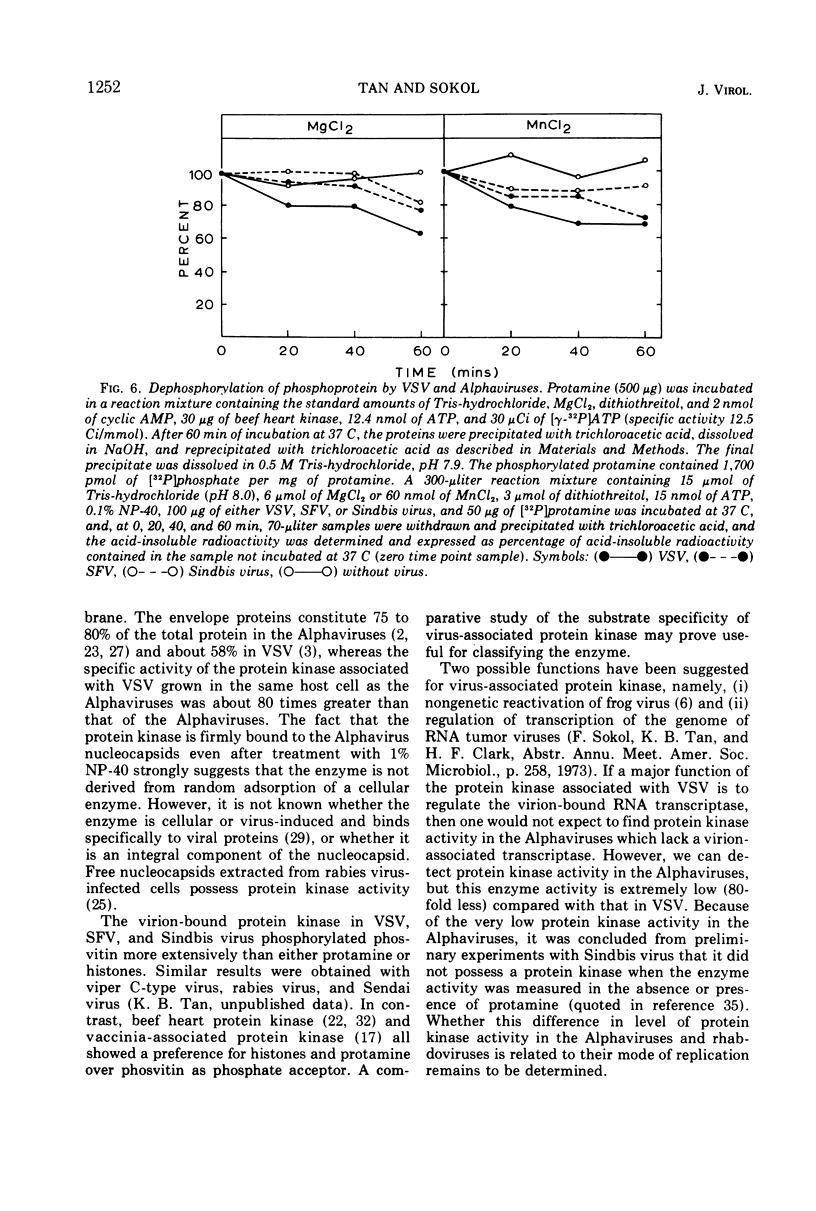
Semliki forest virus and Sindbis virus (Alphaviruses belonging to the togavirus group) grown in BHK-21 cells possessed very low levels of virion-associated protein kinase activity. For comparison, vesicular stomatitis virus, also grown in BHK-21 cells, contained a virion-bound protein kinase which had a specific activity 80 times greater than that of the Alphaviruses. The Alphavirus protein kinase was unmasked by the nonionic detergent Nonidet P-40 but was not activated by cyclic nucleotides. Phosvitin was the best exogenous phosphate acceptor for assaying the viral enzyme in vitro. Phosphoprotein phosphatase activity was also detected in the Alphaviruses. Both in vivo and in vitro, all of the viral structural polypeptides were phosphorylated, and the phosphorylated amino acids were found to be serine and threonine. The viral nucleocapsid protein was about four times more efficient as a phosphate acceptor than were the envelope proteins. From 33 to 50% of the total protein kinase was bound to the viral nucleocapsid, and the specific activity of this enzyme was 4 to 10 times greater than that associated with the viral envelope.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Acheson N. H., Tamm I. Structural proteins of Semliki Forest virus and its nucleocapsid. Virology. 1970 Jun;41(2):321–329. doi: 10.1016/0042-6822(70)90084-x. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F., Hull R. Model for vesicular stomatitis virus. J Virol. 1972 Aug;10(2):256–260. doi: 10.1128/jvi.10.2.256-260.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- Downer D. N., Rogers H. W., Randall C. C. Endogenous protein kinase and phosphate acceptor proteins in vaccinia virus. Virology. 1973 Mar;52(1):13–21. doi: 10.1016/0042-6822(73)90393-0. [DOI] [PubMed] [Google Scholar]
- Gravell M., Cromeans T. L. Viron-associated protein kinase and its involvement in nongenetic reactivation of frog polyhedral cytoplasmic deoxyribovirus. Virology. 1972 Jun;48(3):847–851. doi: 10.1016/0042-6822(72)90167-5. [DOI] [PubMed] [Google Scholar]
- Greenaway P. J. A possible error during assays for the enzymic phosphorylation of proteins and nucleic acids. Biochem Biophys Res Commun. 1972 May 12;47(3):639–644. doi: 10.1016/0006-291x(72)90926-6. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Twiddy E., Gilden R. V. Protein kinase associated with RNA tumor viruses and other budding RNA viruses. Virology. 1972 Feb;47(2):536–538. doi: 10.1016/0042-6822(72)90297-8. [DOI] [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Protein kinase and phosphoproteins of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):113–124. doi: 10.1128/jvi.13.1.113-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Burke D. C. Studies on the structural proteins of Semliki Forest virus. J Gen Virol. 1972 Jan;14(1):87–98. doi: 10.1099/0022-1317-14-1-87. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphorylation of nuclear protein early in the course of gene activation in lymphocytes. Science. 1966 Nov 11;154(3750):780–781. doi: 10.1126/science.154.3750.780. [DOI] [PubMed] [Google Scholar]
- Knudson D. L. Rhabdoviruses. J Gen Virol. 1973 Jun;20(Suppl):105–130. doi: 10.1099/0022-1317-20-Supplement-105. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- Käriäinen L., Söderlund H. Properties of Semliki Forest virus nucleocapsid. 1. Sensitivity to pancreatic ribonuclease. Virology. 1971 Jan;43(1):291–299. doi: 10.1016/0042-6822(71)90246-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Protein kinase and specific phosphate acceptor proteins associated with vaccinia virus cores. J Virol. 1972 Sep;10(3):417–424. doi: 10.1128/jvi.10.3.417-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall C. C., Rogers H. W., Downer D. N., Gentry G. A. Protein kinase activity in equine herpesvirus. J Virol. 1972 Feb;9(2):216–222. doi: 10.1128/jvi.9.2.216-222.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemond H., Moss B. Phosphoprotein component of vaccinia virions. J Virol. 1973 Jun;11(6):961–970. doi: 10.1128/jvi.11.6.961-970.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. S., Gravell M., Darlington R. Protein kinase in enveloped herpes simplex virions. Virology. 1972 Oct;50(1):287–290. doi: 10.1016/0042-6822(72)90374-1. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Silberstein H., August J. T. Phosphorylation of animal virus proteins by a virion protein kinase. J Virol. 1973 Sep;12(3):511–522. doi: 10.1128/jvi.12.3.511-522.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Keränen S., Käriänen L. Identification of a precursor for one of the Semliki forest virus membrane proteins. FEBS Lett. 1973 Jan 15;29(2):87–91. doi: 10.1016/0014-5793(73)80532-0. [DOI] [PubMed] [Google Scholar]
- Simons K., Käriäinen L. Characterization of the Semliki Forest virus core and envelope protein. Biochem Biophys Res Commun. 1970 Mar 12;38(5):981–988. doi: 10.1016/0006-291x(70)90818-1. [DOI] [PubMed] [Google Scholar]
- Sokol F., Clark H. F. Phosphoproteins, structural components of rhabdoviruses. Virology. 1973 Mar;52(1):246–263. doi: 10.1016/0042-6822(73)90413-3. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Protein kinase and phosphate acceptor proteins in Rauscher murine leukaemia virus. Nat New Biol. 1971 Sep 29;233(39):137–140. doi: 10.1038/newbio233137a0. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B. Electron microscopy of cells infected with Semliki forest virus temperature-sensitive mutants: correlation of ultrastructural and physiological observations. J Virol. 1970 May;5(5):632–638. doi: 10.1128/jvi.5.5.632-638.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., McAuslan B. R. Binding of deoxyribonucleic acid-dependent deoxyribonucleic acid polymerase to poxvirus. J Virol. 1972 Jan;9(1):70–74. doi: 10.1128/jvi.9.1.70-74.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., Sambrook J. F., Bellett A. J. Semliki forest virus temperature-sensitive mutants: isolation and characterization. Virology. 1969 Jul;38(3):427–439. doi: 10.1016/0042-6822(69)90155-x. [DOI] [PubMed] [Google Scholar]
- Tan K. B., Sokol F. Phosphorylation of simian virus 40 proteins in a cell-free system. J Virol. 1973 Oct;12(4):696–703. doi: 10.1128/jvi.12.4.696-703.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., Sokol F. Protein kinase stimulated by cyclic GMP in uninfected and simian virus 40-infected monkey kidney cells. J Virol. 1974 Jan;13(1):234–236. doi: 10.1128/jvi.13.1.234-236.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., Sokol F. Structural proteins of simian virus 40: phosphoproteins. J Virol. 1972 Nov;10(5):985–994. doi: 10.1128/jvi.10.5.985-994.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao M., Doerfler W. Phosphorylation of adenovirus polypeptides. Eur J Biochem. 1972 Jun 9;27(3):448–452. doi: 10.1111/j.1432-1033.1972.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Waite M. R., Lubin M., Jones K. J., Bose H. R. Phosphorylated proteins of Sindbis virus. J Virol. 1974 Jan;13(1):244–246. doi: 10.1128/jvi.13.1.244-246.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]



