Abstract
Chromosome counts of plants grown from open-pollinated seed from Japanese knotweed around the world have revealed the presence of extensive hybridisation with both native and other introduced taxa. These hybrids fit into three categories: inter- and intraspecific hybrids involving the taxa of Fallopia section Reynoutria (giant knotweeds), hybrids between Japanese knotweed and F. baldschuanica (Regel) Holub and hybrids between Japanese knotweed and the Australasian endemics of the genus Muehlenbeckia. In this minireview, the viability of the different classes of hybrid and the potential threats they pose are discussed in the context of recent examples of allopolyploid speciation, which generally involve hybridisation between a native and an alien species. Such wide hybridisations also challenge accepted taxonomic classifications. Japanese knotweed s.l. provides a fascinating example of the interplay between ploidy level, hybridisation and alien plant invasion. The octoploid (2n=88) Fallopia japonica var. japonica (Houtt.) Ronse Decraene is a single female clone throughout much of its adventive range, and provides an ideal system for investigating the potential for wide hybridisation.
Keywords: Fallopia, gynodioecy, polyploidy, invasive alien plant
Introduction
Although the threat to biodiversity posed by exotic invasive species has long been recognised, less attention has been paid to the role of hybridisation with exotic invasives. It is a well-known fact that, when related taxa that have long evolved in isolation are brought back into contact again (generally by human intervention), hybridisation followed by chromosome doubling can give rise to a new species, such as Senecio cambrensis (Rosser, 1955) or Spartina anglica (Marchant, 1963)—the latter a much more vigorous reclaimer of mud-flats than its parental species.
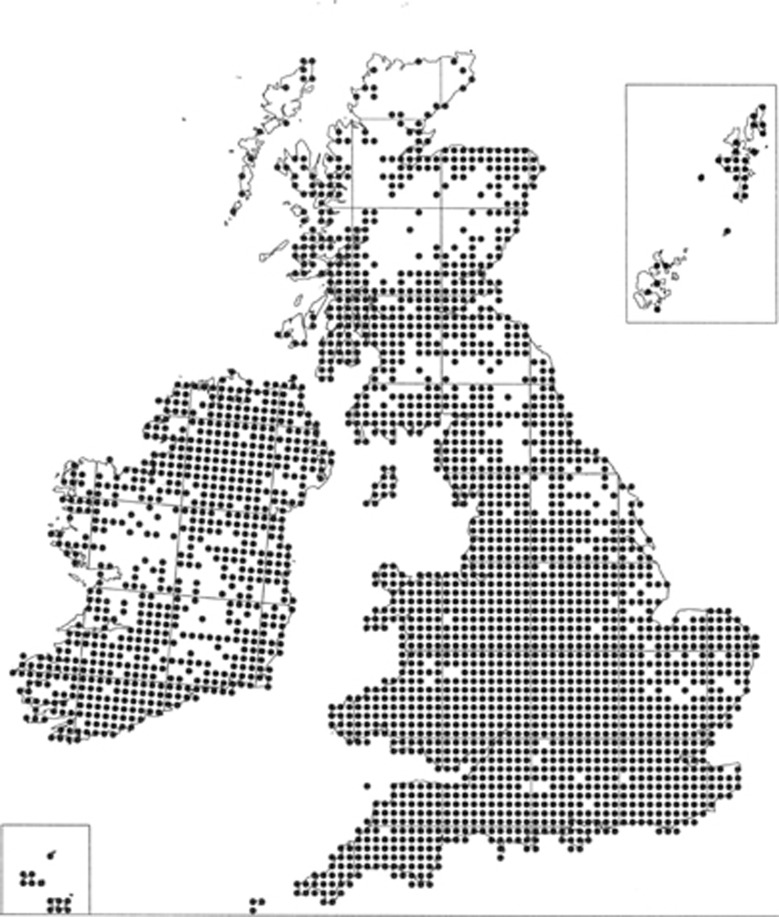
The Japanese knotweed invasion of Europe has been well documented by a series of historical, cytological, morphological and molecular approaches (Conolly, 1977; Bailey and Conolly, 2000; Hollingsworth and Bailey, 2000; Bímová et al., 2003; Pashley, 2006; Tiébré et al., 2007b). The position in North America is less well characterised from the historical, cytological and morphological perspectives (Forman and Kesseli, 2003), but has been subject to a number of molecular studies (Gammon et al., 2007, 2010; Grimsby et al., 2007). On both sides of the Atlantic, the plant is regarded as a serious threat to biodiversity and is legislated against in certain US States. In the UK, there is extremely stringent legislation regarding disposal of soil contaminated with rhizomes of Japanese knotweed (Wildlife and Countryside Act, 1981; Environmental Protection Act, 1990; Child and Wade, 2000), which has led to serious cost penalties in some high-profile redevelopments such as the Wembley and Olympic stadia. Less well known is its distribution in Tasmania (Bailey, unpublished) and the South Island of New Zealand (Webb et al., 1988).
Fallopia section Reynoutria includes Japanese knotweed (F. japonica), Giant knotweed (F. sachalinensis) and hybrids between these two species. These three taxa along with any backcrosses and F2s are conveniently referred to as Japanese knotweed s.l. These are all giant rhizomatous herbs originating from Asia, they are gynodioecious, with hermaphrodite and male-sterile (female) individuals. The hermaphrodites are self-incompatible and make poor female parents, so generally act as male plants (Bailey, 1989). New plants can originate vegetatively from very small fragments of rhizome, and in the introduced range this is the main means of spread. In the UK, Japanese knotweed occurs as two varieties, F. japonica var. japonica (2n=8 × =88) and F. japonica var. compacta (Houtt.) J. Bailey (2n=4 × =44). The single male-sterile clone of F. japonica var. japonica (Hollingsworth and Bailey, 2000), is thought to have originated from the commercial nursery garden of von Siebold in Leiden in the 1850s, when it was considered an attractive and valuable garden plant (Bailey and Conolly, 2000). Its vigorous vegetative reproduction has allowed clonal spread throughout the UK (Figure 1). Although both sexes (female and hermaphrodite) of F. japonica var. compacta occur in the UK, they share a single chloroplast haplotype (Hollingsworth et al., 1999). Similarly, the UK population of F. sachalinensis (F. Schmidt ex Maxim.) Ronse Decraene occurs as both sexes with just two different chloroplast haplotypes, a widespread one matching the Hokkaido populations and a second one restricted to a nursery garden in Colchester originating from Honshu (Pashley et al., 2007). This limited genetic diversity, combined with the different ploidy levels in Japanese knotweed s.l., allows the parentage of most UK hybrids to be assigned down to the female parent. This is in sharp contrast to the tremendous genetic and morphological variation found in Japan, where montane dwarf plants and tall lowland plants can share a chloroplast haplotype, the tall var. japonica plants occur at 4 × and 8 × ploidy levels and many regional varieties and subspecies exist (Bailey, 2003; Pashley, 2006).
Figure 1.
Distribution map of F. japonica var. japonica in the British Isles at a resolution of 10 km2. Courtesy of the Biological Records Centre.
Unique features of the Japanese knotweed invasion
To conduct a worldwide experiment on the limits of natural hybridisation in plants, there is hardly a better system than that presented by the Japanese knotweed invasion. There are a number of factors that combine to make Japanese knotweed so suitable for such work. Firstly, as a male-sterile clone of a gynodioecious species (Hollingsworth and Bailey, 2000), the natural limits of hybridisation can be explored in a system free from the complications of legitimate pollinations. It has very effective vegetative reproduction by woody rhizomes, is widespread and a single stand can occupy a large area., As a polyploid female parent, it is able to rescue crosses with diploids from the damaging deficiencies that can be associated with crosses between diploid species. Similarly, unbalanced aneuploid gametes from hybrids and backcrosses can also be rescued by the 8 × female background, as evidenced by the range of aneuploid progeny recovered from open-pollinated F. japonica growing next to F. x bohemica Table 2 (Bailey et al., 2008).
Both the probability of hybridisation and the ability to detect its occurrence even at at low frequencies, are enhanced by several features of the reproductive biology of the plants. The sheer numbers of flowers produced (up to 190 000 flowers per stem; Bailey, 1989) and stands made up from hundreds of stems make the plants highly attractive to pollinators and a long flowering season means overlap with a number of related taxa. Pollinated flowers develop a winged fruiting perianth whereas unfertilised ones drop off making it easy to detect the rate of pollen incorporation from other species. Finally, the fruit is a single-seeded achene; fruits with multiple ovules would be likely to abort if only the occasional ovule was fertilised, which would make it more difficult to detect the rate of pollination. Taken together, these factors create a highly sensitive and receptive system for studying the patterns of hybridisation.
Hybrids within Fallopia section Reynoutria
The first hybrids to be discovered in the UK in the 1980s (Bailey and Conolly, 1985) were between F. japonica and F. sachalinensis (=F. x bohemica 2n=66), a plant that appears more vigorous and troublesome in terms of invasiveness than either parent (Bímová et al., 2003). It was not actually recorded in its native Japan until long after its discovery in Europe. As a single male-sterile clone, any seed from F. japonica would inevitably be of hybrid origin; the subsequent introduction of hermaphrodite plants of F. sachalinenisis to Europe in the 1860s (Bailey and Conolly, 2000) allowed such hybrid seed to be formed.
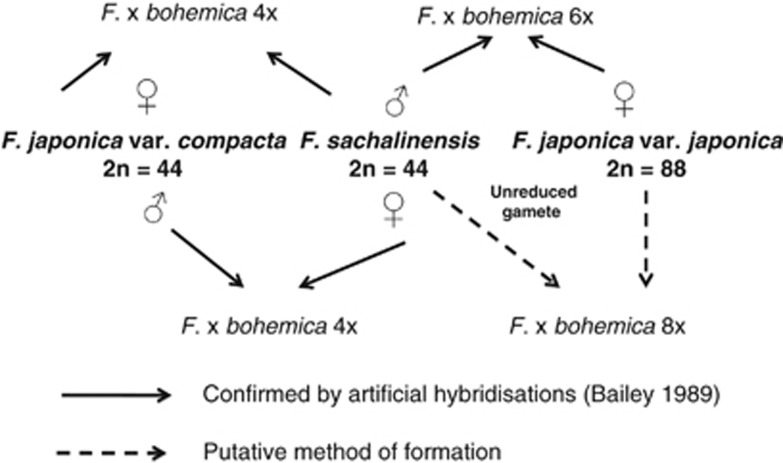
Figure 2 shows a hypothesis for how the 4 × and 6 × F. x bohemica hybrids were originally formed, and these crosses were resynthesised artificially at Leicester, in order to confirm the hybrid constitution of putative wild hybrids (Bailey, 1989). Since both sexes of var. compacta and sachalinensis occur, 4 × hybrids can occur with either taxon as the female parent, as confirmed by chloroplast haplotype studies (Hollingsworth et al., 1999). The origin of the 8 × F. x bohemica is more complex (Bailey and Wisskirchen, 2006), but for the UK plants, the most straightforward explanation is the pollination of F. japonica var. japonica by an unreduced F. sachalinensis gamete.
Figure 2.
Diagram showing the putative origins by hybridisation between different ploidy levels and sexes of Japanese knotweed s.l., of the 4 × , 6 × and 8 × F. x bohemica plants in the UK.
The four ploidy levels of F. x bohemica differ in their worldwide distribution (Table 1), with the 6 × being the commonest worldwide. The 4 × and 8 × have only been recorded from the UK and the Czech Republic; although in the UK, the 4 × is more frequent than the 8 × , in the Czech Republic, the converse is true. The 10 × has only been recorded as a single established plant from the USA (Bailey, unpublished) and from Massachusetts Gammon et al. (2010). They also report 10 × F. x bohemica from artificial hybrids between F. japonica and 6 × F. x bohemica and also from open-pollinated seed from F. japonica. In the UK, 10 × individuals have been found only as seeds on F. japonica var. japonica growing next to hermaphroditic 6 × F. x bohemica (Table 2). The most logical origin being an unreduced 6 × gamete from the F. x bohemica male parent crossed with 8 × F. japonica.
Table 1. Ploidy levels of F. x bohemica plants established in the wild around the world.
| Country | Reference | 4x (%) | 6x (%) | 8x (%) | 10x (%) | Sample size |
|---|---|---|---|---|---|---|
| British Isles | Bailey and Wisskirchen, 2006 | 21 | 75 | 4 | 0 | 51 |
| Continental Europe (excluding Czech Republic) | Bailey and Wisskirchen, 2006 | 0 | 86.4 | 13.6 | 0 | 42 |
| Czech Republic | Mandák et al., 2003 | 2.1 | 92.5 | 5.3 | 0 | 94 |
| USA | Bailey, unpublished | 0 | 96.4 | 0 | 3.6 | 28 |
Table 2. Chromosome counts of backcrosses (natural & artificial) between F. japonica japonica and hermaphrodite F. x bohemica 6x.
| Site | Open or artificial pollination | 2n | Ploidy |
|---|---|---|---|
| Dolgellau (Wales) | Open | c68, 71, 76, 2 × 100, 101, 4 × 105, 107, 2 × 108, 2 × 110 | Aneuploid and decaploid |
| Albury Heath (England) | Open | 110 | Decaploid |
| Prior's Mesme (England) | Open | 96 | Aneuploid |
| Leicester (England) | Artificial | 110 | Decaploid |
Male meiosis is regular in the British 4 × and 8 × F. x bohemica, but highly irregular in the 6 × plants (Bailey, 1989; Bailey and Stace, 1992). This irregular meiosis does not necessarily result in infertility when backcrossing with F. japonica. Since large numbers of aneuploid gametes are produced, selection of more viable ones may be occurring at pollination. In addition, the female japonica gamete is already tetraploid, so it won't depend on receiving essential genes or chromosomes from the pollen parent to produce balanced gametes. These backcrosses, though perfectly viable, are generally aneuploid, unless the result of an unreduced gamete from the F. x bohemica (Table 2). Viable F2 F. x bohemica seed has been found in Dollgellau (Wales), but with a much lower seed set than found in Japanese knotweed plants pollinated by 6 × F. x bohemica. The fact that only a few viable seeds were found is presumably due to the lower chances of success when both parents produce irregular gametes (Bailey, unpublished). From these results, it is clear that there is considerable scope for changing the admixture of japonica and sachalinensis chromosomes in the F2s and backcrosses. This is of more than academic interest, since F. sachalinensis has a much more northerly distribution than F. japonica, and such rearrangements offer the potential to produce individuals better tailored to the climate that they find themselves in.
Hybrids between Fallopia sect. Reynoutria and Fallopia sect. Pleuropterus
Fallopia baldschuanica is in Fallopia section Pleuropterus, and is a vigorous woody climber frequently planted in gardens. Open-pollinated seed collected in the UK from F. japonica plants, which are out of pollination range of hermaphroditic section Reynoutria taxa, produces seedlings with 2n=54 (pentaploid) and a twining early growth form (Bailey, 1988, 2001). These seedlings are hybrids between F. japonica and F. baldschuanica, and germinate readily in cultivation; they were named Fallopia x conollyana (Bailey, 2001) in honour of Ann Conolly's major role in the study of this group. Tiébré et al. (2007a) and Engler et al. (2011) also report F. x conollyana from seed collected from open-pollinated F. japonica in Belgium and Germany, respectively.
Fallopia x. conollyana represents a most unexpected hybrid between two non-sympatric taxa, an octoploid (2n=88) rhizomatous herb and a diploid (2n=20) twining woody climber. At the time of its discovery in the 1980s, it was regarded taxonomically as an intergeneric cross, and its existence provided additional support for Ronse Decraene and Akeroyd's (1988) decision to merge the genus Reynoutria with the older genus Fallopia. There are two major barriers to the spread of F. x conollyana: firstly, the general inability of seed from Japanese knotweed to overwinter, germinate and establish spontaneously in Europe, and secondly, the conflict between the parental overwintering strategies: woody stems versus rhizomes. In spite of this, there is a single established plant (covering several square metres) known in Haringey, London (Bailey, 1992). There is also the question of the disparity in ploidy levels of the parents. In cereals, there is some evidence that successful seed production in crosses between ploidy levels is greater when the higher ploidy is the female, rather than the reciprocal (Sharma, 1995). In the Brassicaceae, Scott et al. (1998) found that seed abortion occurred in both directions in crosses between 2 × and 6 × Arabidopsis plants, suggesting maternal or paternal excess in the endosperm as the cause. A 2:1 maternal:paternal balance in the endosperm is often suggested as necessary for successful seed formation. Clearly, this is not a limitation here as female 8 × F. japonica x 2 × F. baldschuanica gives an endosperm ratio of 8:1, and the 4 × /2 × crosses a female:male ratio of 4:1.
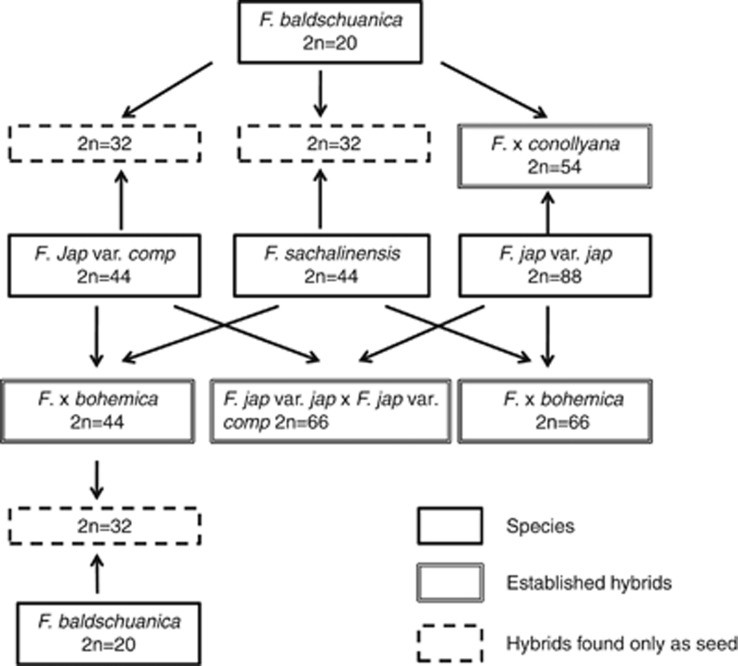
Hybrids between other members of Japanese knotweed s.l. and F. baldschuanica have also been recovered as open-pollinated seed from F. japonica var. compacta, F. sachalinensis and 4x F. x bohemica (Figure 3). The triploid F. sachalinensis x F. baldschuanica (2n=32) produces long, drooping indeterminate shoots that rarely flower (Bailey, 1989). The triple hybrid, 4 × F. x bohemica x F. baldschuanica (2n=32) is the only one of the baldschuanica hybrids to flower regularly (loc. cit.). The trigonous buds and flowers of the baldschuanica hybrids are more winged or keeled than those of F. japonica, which is an F. baldschuanica character. This is more obvious in the 3 × plants, which have a higher ratio of baldschuanica:japonica chromosomes (1:2) than the 5 × hybrids (1:4) (Figures 5(2) and (3)). Meiosis in the triploids is extremely irregular, with little sign of bivalent formation, but in the pentaploid F. x conollyana, there is regular pairing of the F. japonica complement, with the 10 F. baldschuanica chromosomes remaining univalent (Figures 4(1) and (2)).
Figure 3.
Diagram showing hybridisation between Japanese knotweed s.l. and F. baldschuanica, indicating which crosses are established in the wild and which have been found only as seed.
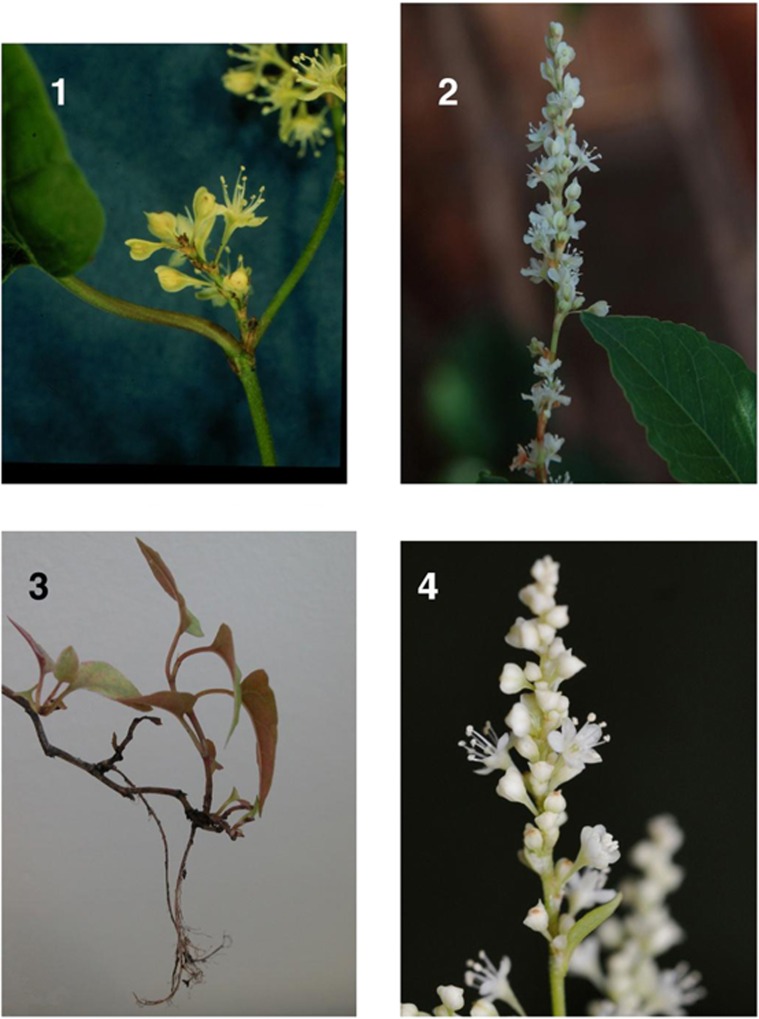
Figure 5.
Illustrations of the hybrid plants showing morphological features of flowers and growing points that are most indicative of hybridity. (1) Putative hybrid between F. japonica and Muehlenbeckia australis showing development of axillary shoot and roots at the apex of the stem. (2) Flowers of F. sachalinensis x F. baldschuanica (2n=32). Note the broad wings on the tepals of buds and flowers, which is a F. baldschuanica character. (3) Flowers of F. x conollyana (2n=32), again showing the broad wings characteristic of F. baldschuanica. (4) Male flowers of putative F. japonica x M. australis (2n=54), which are morphologically much more like those of F. japonica, with little winging of the tepals in buds and flowers.
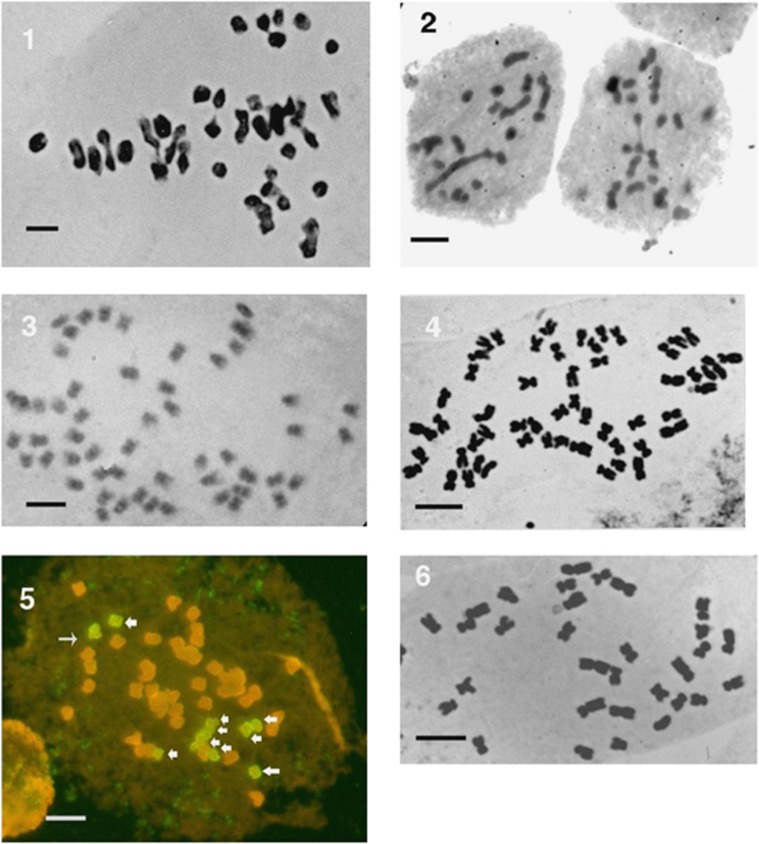
Figure 4.
Cytology; aceto-orcein-stained squashes examined with a Zeiss x 63 n.a.1.4 objective and transmitted light, apart from (5), which is a composite fluorescent image made with blue and green excitation. Mitotic preparations were made from root tip cells and meiotic squashes from pollen mother cells. Scale bar=5 μm. (1) Meiosis in F. x conollyana (2n=54) showing 22 bivalents (the F. japonica complement), the 10 larger F. baldschuanica chromosomes remain unpaired. (2) Meiosis in triploid F. x conollyana (F. japonica var. compacta x F. baldschuanica 2n=32) is much more irregular than in the 2n=54 plants; the 22 F. japonica chromosomes are unable to pair regularly and there is a mixture of multivalents, bivalents and univalents. (3) Mitosis in a putative F. japonica x M. australis hybrid (2n=54), all chromosomes are more or less the same size c.f. F. x conollyana (4). (4) F. conollyana (2n=54) mitosis showing the 10 conspicuously larger chromosomes from F. baldschuanica. (5) GISH image of a putative F. japonica x M. australis hybrid (2n=54), the 10 green chromosomes (arrowed) have labelled M. australis DNA hybridised to them. (6) Mitosis in a F. sachalinensis x F. baldschuanica hybrid (2n=32), the 10 F. baldschuanica chromosomes are conspicuously larger.
Hybrids between Fallopia sect. Reynoutria and Muehlenbeckia
Since their discovery in the 1980s, the F. baldschuanica hybrids have been regarded as a botanical curiosity. This view changed in 2005, when seeds were obtained from open pollinated Japanese knotweed from New Zealand. This produced plants superficially similar to F. x conollyana with 2n=54. However, in spite of a chromosome number in common with F. x conollyana, they lacked the 10 conspicuously larger chromosomes so characteristic of F. x conollyana (Figures 4(4)–(6)), the stems were more ridged to touch, and axillary roots and shoots were formed when the arching tips of the stems touched the soil (Figure 5(1)). The most likely putative parent, Muehlenbeckia australis (2n=20) (Polygonaceae), is a scrambling liana that roots when growing points touch the soil and is morphologically similar to F. baldschuanica. Support for Muehlenbeckia australis as the male parent was provided by a GISH analysis using labelled M. australis as the probe (Figure 4(5), Bailey unpublished, using techniques from Schwarzacher and Heslop-Harrison, 2000). These hybrids flower more readily than the F. baldschuanica hybrids (Figure 5(4)).
The Fallopia x Muehlenbeckia hybrid is in some respects even more unlikely than F. x conollyana; there are the same discontinuities of chromosome number (20 versus 88), growth form (shrubby scrambler versus rhizomatous herb) and the parents are in different genera—but in this case, to a genus restricted to Australasia and South America. More remarkably, the parental growth strategies appear to be complementary rather than conflicting, since the hybrid has acquired the ability to root from the shoot tips (Figure 5(1)). If this is the case, we face a formidable new taxon, as some species of Muehlenbeckia already form impenetrable thickets by this means. The extent of this hybridisation is currently under investigation, and although viable seed is being produced, it is not yet known if any of the hybrids are established in New Zealand.
Discussion
The Polygonaceae is rather a large family and indepth taxonomic analysis has in the past been restricted to sectional treatments such as Polygonum s.l. The taxa comprising Fallopia have generally been considered under Polygonum s.l. and the Muehlenbeckia taxa under the Coccolobeae; this, combined with their disjunct distributions has meant that the similarities between these two genera have not generally been highlighted. Haraldson (1978) considers Bilderdykia and Reynoutria (our Fallopia) to be in the Coccolobeae close to Muehlenbeckia, though Muehlenbeckia was not one of the genera she studied. With the onset of molecular approaches, it becomes much more practical to study larger groupings of species and accordingly after the initial work by Lamb-Frye and Kron (2003), a series of papers (Galasso et al., 2009; Sanchez et al., 2009; Sanchez et al., 2011; Schuster et al., 2011a) have produced well-supported clades featuring Muehlenbeckia and Fallopia (sensu Ronse de Craene). Schuster et al. (2011b) using matK, ndhF, 39rps16-59trnK, trnL-trnF, 39trnV-ndhC, the 2nd intron of LEAFY and ITS, show separate Reynoutria, Muehlenbeckia and Fallopia clades that together make a larger Reynoutria/Muehlenbeckia/Fallopia clade. Rather than making this Reynoutria/Muehlenbeckia/Fallopia clade into a single monophyletic genus Fallopia, they choose to resurrect Reynoutria and Fallopia as two separate genera. The choice is either a broad delimitation of Fallopia to include Muehlenbeckia or three separate genera in which viable intergeneric hybrids between Reynoutria and Fallopia and Reynoutria and Muehlenbeckia are readily formed.
The eukaryote cell is clearly highly accommodating, judging by the production of viable human/tobacco somatic cell fusions (Jones et al., 1976). Two different genomes share the same cytoplasm in F1 hybrids and allopolyploids, though there can be a tendency for one genome to be lost; for example, in some Hordeum hybrids (Bennett et al., 1976). Very wide hybridisations between cereals and other grasses are possible, but only by ovule pollination and early stage embryo rescue (Sharma, 1995). In the Polygonaceae, F. japonica can cross naturally with both F. baldschuanica and Muehlenbeckia taxa to give viable and germinable progeny. Is this some special propensity of the Polygonaceae, or is it a much more widespread plant phenomenon that has gone undetected, due to lack of research or lack of a suitable system? For whatever reason, the apparent absence of breeding barriers between long isolated taxa, in a relatively poorly studied group such as the Polygonaceae, is a phenomenon of some interest. The Japanese knotweed invasion of Europe is unusual in that it is mainly by the clonal spread of one female individual. There are clear parallels with the case of Canadian pondweed Elodea canadensis, (2n=24) introduced to the UK in 1836, where a vigorous female clone spread vegetatively, causing navigation problems in certain UK waterways. It is no longer such a problem and is being replaced by a related alien E. nuttallii (2n=48) (Simpson, 1984; Stace, 1997). F. japonica arrived as a polyploid clone, and although there is not sufficient evidence to suggest that polyploidy is responsible for its invasive success, the enforced hybridisation due to the absence of male plants has done much to increase the genetic diversity of Japanese knotweed s.l. and also created some most unexpected hybrids between highly divergent parents. In terms of threats posed, F. x bohemica and its backcrosses are of major importance, particularly in North America (Bram and McNair, 2004), where recruitment from seed is much more common than in Europe. F. x conollyana, inspite of its frequent production as seed, rarely germinates in the wild and even when it does, is not a very strong competitor. Not enough is known about the hybrids between F. japonica and Muehlenbeckia, but they may be in a different league from the other hybrids mentioned. Should they be able to combine the invasive potential of both parents with some heterosis, there is the potential for a most formidable addition to the alien taxa that have caused so much damage to New Zealand's biodiversity. Work is currently underway to evaluate the threats posed by this plant in New Zealand.
Data archiving
There were no data to deposit.
Acknowledgments
I thank the late Ann Conolly for starting me on this work and for her unstinting and generous help; my PhD students Michelle Hollingsworth and Catherine Pashley; Tim Senior for collecting and sending plant material from New Zealand; and the generations of undergraduate project students such as Stuart Desjardins, who have kept the project active. I am also grateful to the two anonymous referees and Barbara Mable for their helpful suggestions.
The author declares no conflict of interest.
References
- Bailey JP, Conolly AP. Chromosome numbers of some alien Reynoutria species in the British Isles. Watsonia. 1985;15:270–271. [Google Scholar]
- Bailey JP. Putative Reynoutria japonica Houtt. x Fallopia baldschuanica (Regel) Holub hybrids discovered in Britain. Watsonia. 1988;17:163–164. [Google Scholar]
- Bailey JP.1989. Cytology and breeding behaviour of giant alien Polygonum species in britain. Phd thesis University Of Leicester http://hdl.handle.net/2381/9471 .
- Bailey JP. The Haringey Knotweed. Urban Nature Magazine. 1992;1:50–51. [Google Scholar]
- Bailey JP. Fallopia x conollyana, the railway-yard knotweed. Watsonia. 2001;23:539–541. [Google Scholar]
- Bailey JP.2003Japanese Knotweed s.l. at home and abroadIn: Child L, Brock JH, Prach K, Pysek P, Wade PM, Williamson M (eds).Plant Invasions—Ecological Threats and Management Solutions Backhuys Publishers: Leiden; 183–196. [Google Scholar]
- Bailey JP, Bímová K, Mandák B. Asexual spread versus sexual reproduction and evolution in Japanese Knotweed s.l.—the ‘Battle of the Clones?'. Biol Invasions. 2008;11:1189–1203. [Google Scholar]
- Bailey JP, Conolly AP. Prize-Winners to Pariahs - a History of Japanese Knotweed s.l. (Polygonaceae) in the British Isles. Watsonia. 2000;23:93–110. [Google Scholar]
- Bailey JP, Stace CA. Chromosome number, morphology, pairing, and DNA values of species and hybrids in the genus Fallopia (Polygonaceae) Pl Syst Evol. 1992;180:29–52. [Google Scholar]
- Bailey JP, Wisskirchen R. The distribution and origins of Fallopia x bohemica (Polygonaceae) in Europe. Nord J Bot. 2006;24:173–200. [Google Scholar]
- Bennett MD, Finch RA, Barclay IR. The time rate and mechanism of chromosome elimination in Hordeum hybrids. Chromosoma. 1976;54:175–200. [Google Scholar]
- Bímová K, Mandák B, Pyšek P. Experimental study of vegetative regeneration in four invasive Reynoutria taxa (Polygonaceae) Plant Ecol. 2003;166:1–11. [Google Scholar]
- Bram MR, McNair JN. Seed germinability and its seasonal onset of Japanese Knotweed (Polygonum cuspidatum) Weed Sci. 2004;52:759–767. [Google Scholar]
- Child L, Wade M. The Japanese Knotweed Manual. Packard Publishing: Chichester, UK; 2000. [Google Scholar]
- Conolly AP. The distribution and history in the British Isles of some alien species of Polygonum and Reynoutria. Watsonia. 1977;11:291–311. [Google Scholar]
- Engler J, Abt K, Buhk C. Seed characteristics and germination limitations in the highly invasive Fallopia japonica s.l. (Polygonaceae) Ecol Res. 2011;26:555–562. [Google Scholar]
- Environmental Protection Act . HMSO: London; 1990. [Google Scholar]
- Forman J, Kesseli R. Sexual reproduction in the invasive species Fallopia japonica (Polygonaceae) Am J Bot. 2003;90:586–592. doi: 10.3732/ajb.90.4.586. [DOI] [PubMed] [Google Scholar]
- Galasso G, Banfi E, De Mattia F, Grassi F, Sgorbati S, Labra M. Molecular phylogeny of Polygonum L.s.l. (Polygonoideae, Polygonaceae), focusing on European taxa: preliminary results and systematic considerations based on rbcL plastidal sequence data. Atti Soc It Sci nat Museo civ nat Milano. 2009;150:113–148. [Google Scholar]
- Gammon MA, Grimsby JL, Tsirelson D, Kesseli R. Molecular and morphological evidence reveals introgressions in swarms of the invasive taxa Fallopia japonica, F. sachalinensis, and F. xbohemica (Polygonaceae) in the United States. Am J Bot. 2007;94:948–956. doi: 10.3732/ajb.94.6.948. [DOI] [PubMed] [Google Scholar]
- Gammon MA, Baack E, Forman Orth J, Kesseli R. Viability, growth, and fertility of knotweed cytotypes in North America. Invasive Plant Sci Manag. 2010;3:208–218. [Google Scholar]
- Grimsby JL, Tsirelson D, Gammon MA, Kesseli R. Genetic diversity and clonal reproduction in Fallopia sp. (Polygonaceae) Am J Bot. 2007;94:957–964. doi: 10.3732/ajb.94.6.957. [DOI] [PubMed] [Google Scholar]
- Haraldson K. Anatomy and taxonomy in Polygonaceae subfam. Polygonoideae Meis. Emend. Jaretzky. Symbolae botanicae Upsalienses. 1978;22:2. [Google Scholar]
- Hollingsworth ML, Bailey JP, Hollingsworth PM, Ferris C. Chloroplast DNA variation and hybridisation between invasive populations of Japanese Knotweed and Giant Knotweed (Fallopia, Polygonaceae) Bot J Lin Soc. 1999;129:139–154. [Google Scholar]
- Hollingsworth ML, Bailey JP. Evidence for massive clonal growth in the invasive weed Fallopia japonica (Japanese Knotweed) Bot J Linn Soc. 2000;133:463–472. [Google Scholar]
- Jones CW, Mastrangelo IA, Smith HH, Liu HZ, Meck RA. Interkingdom fusion between human (HeLa) cells and tobacco hybrid (GGLL) protoplasts. Science. 1976;193:401–403. doi: 10.1126/science.935875. [DOI] [PubMed] [Google Scholar]
- Lamb-Frye AS, Kron KA. Phylogeny and character evolution in Polygonaceae. Syst Bot. 2003;28:326–332. [Google Scholar]
- Mandák B, Pyšek P, Lysák M, Suda J, Krahulcová A, Bímová K. Variation in DNA-ploidy levels of Reynoutria taxa in the Czech Republic. Ann Bot. 2003;92:265–272. doi: 10.1093/aob/mcg141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant CJ. Corrected chromosome numbers for Spartina x townsendii and its parent species. Nature Lond. 1963;199:929. [Google Scholar]
- Pashley CH.2006. The use of molecular markers in the study of the origin and evolution of Japanese Knotweed sensu lato. PhD thesis University of Leicester http://hdl.handle.net/2381/9700 .
- Pashley CH, Bailey JP, Ferris C. Clonal diversity in British populations of the alien invasive Giant Knotweed, Fallopia sachalinensis (F. Schmidt) Ronse Decraene, in the context of European and Japanese plants. Watsonia. 2007;26:359–371. [Google Scholar]
- Ronse Decraene L-P, Akeroyd JR. Generic limits in Polygonum and related genera (Polygonaceae) on the basis of floral characters. Bot J Lin Soc. 1988;98:321–337. [Google Scholar]
- Rosser EM. A new British species of Senecio. Watsonia. 1955;3:228. [Google Scholar]
- Sanchez A, Schuster TM, Kron KA. A Large-scale phylogeny of Polygonaceae based on molecular data. Int J Plant Sci. 2009;179:1044–1055. [Google Scholar]
- Sanchez A, Schuster TM, Burke JM, Kron KA. Taxonomy of Polygonoideae (Polygonaceae): A new tribal classification. Taxon. 2011;60:151–160. [Google Scholar]
- Schwarzacher T, Heslop-Harrison JS. Practical In Situ Hybridization. Bios Scientific Publishers Limited: Oxford; 2000. [Google Scholar]
- Schuster TM, Reveal JL, Kron KA. Phylogeny of Polygoneae (Polygonaceae: Polygonoideae) Taxon. 2011a;60:1653–1666. [Google Scholar]
- Schuster TM, Wilson KL, Kron KA. Phylogenetic relationships of Muehlenbeckia, Fallopia and Reynoutria (Polygonaceae) investigated with chloroplast and nuclear sequence data. Int J Plant Sci. 2011b;172:1053–1066. [Google Scholar]
- Scott RJ, Spielman M, Bailey J, Dickinson HG. Parent-of origin effects on seed development in Arabidopsis thaliana. Development. 1998;125:3329–3341. doi: 10.1242/dev.125.17.3329. [DOI] [PubMed] [Google Scholar]
- Sharma HC. How wide can a wide cross be. Euphytica. 1995;82:43–64. [Google Scholar]
- Simpson DA. A short history of the introduction and spread of Elodea Michx in the British Isles. Watsonia. 1984;15:1–9. [Google Scholar]
- Stace CA. New Flora of the British Isles. C.U.P: Cambridge; 1997. [Google Scholar]
- Tiébré M-S, Vanderhoeven S, Saad L, Mahy G. Hybridization and sexual reproduction in the invasive alien Fallopia (Polygonaceae) complex in Belgium. Ann Bot. 2007a;99:193–203. doi: 10.1093/aob/mcl242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiébré M-S, Bizoux J-P, Hardy OJ, Bailey JP, Mahy G. Hybridisation and morphogenetic variation in the invasive alien Fallopia (Polygonaceae) Complex in Belgium (Western Europe) Am J of Bot. 2007b;94:1900–1910. doi: 10.3732/ajb.94.11.1900. [DOI] [PubMed] [Google Scholar]
- Webb CJ, Sykes WR, Garnock-Jones PJ. Flora of New Zealand. Vol IV. Botany Division DSIR: Christchurch, New Zealand; 1988. [Google Scholar]
- Wildlife & Countryside Act . HMSO: London; 1981. [Google Scholar]