Abstract
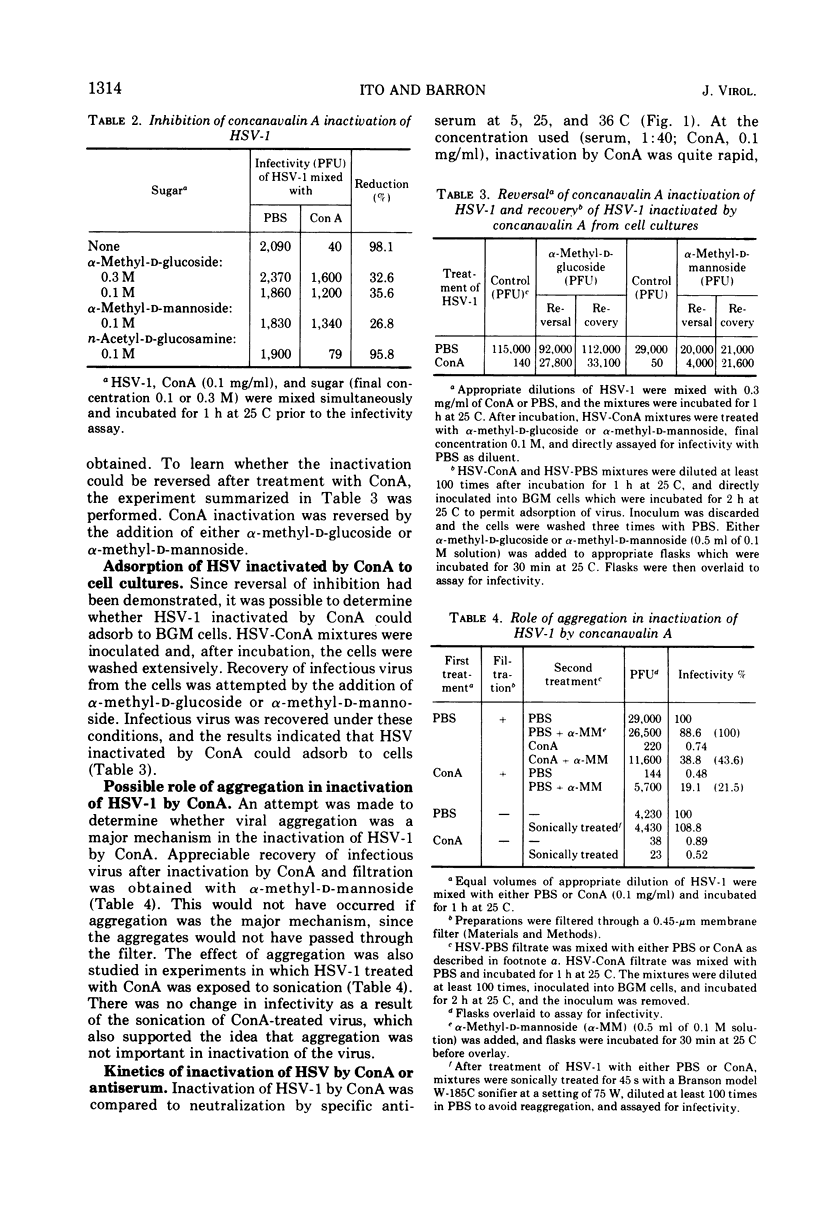
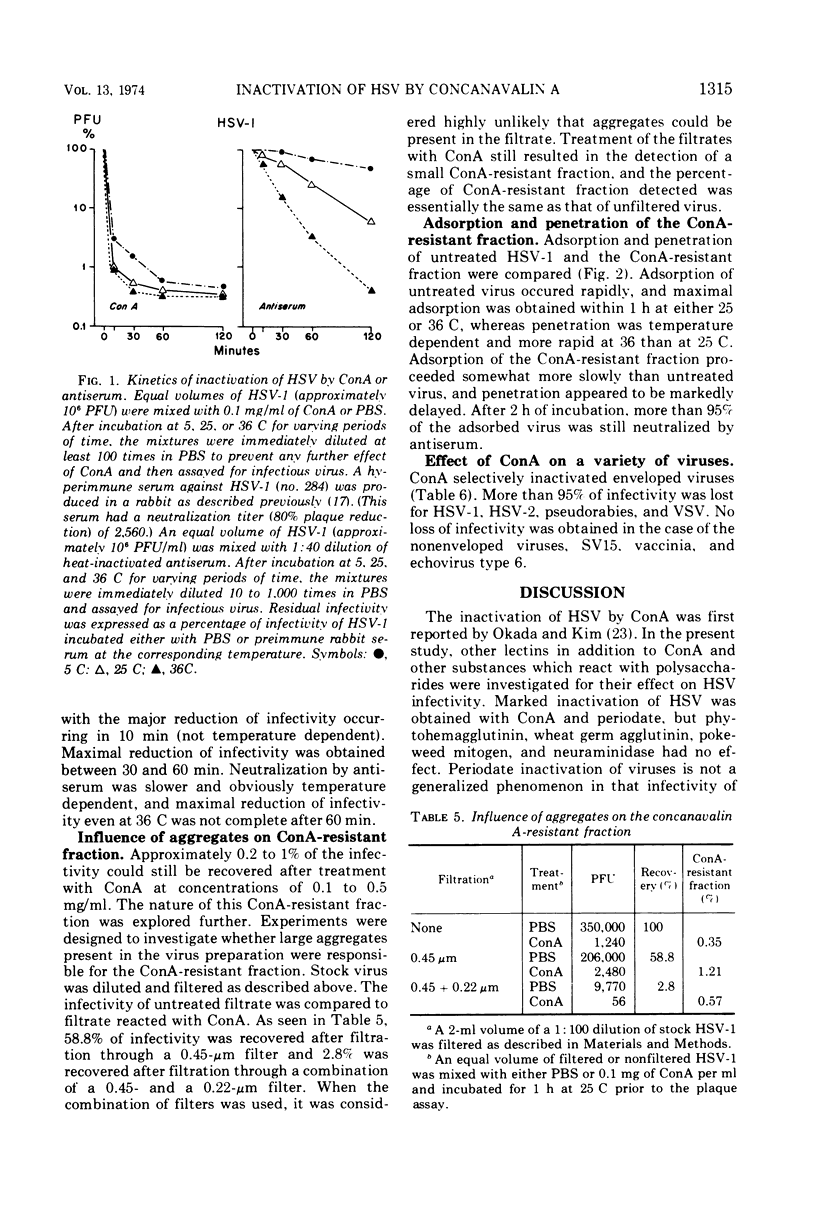
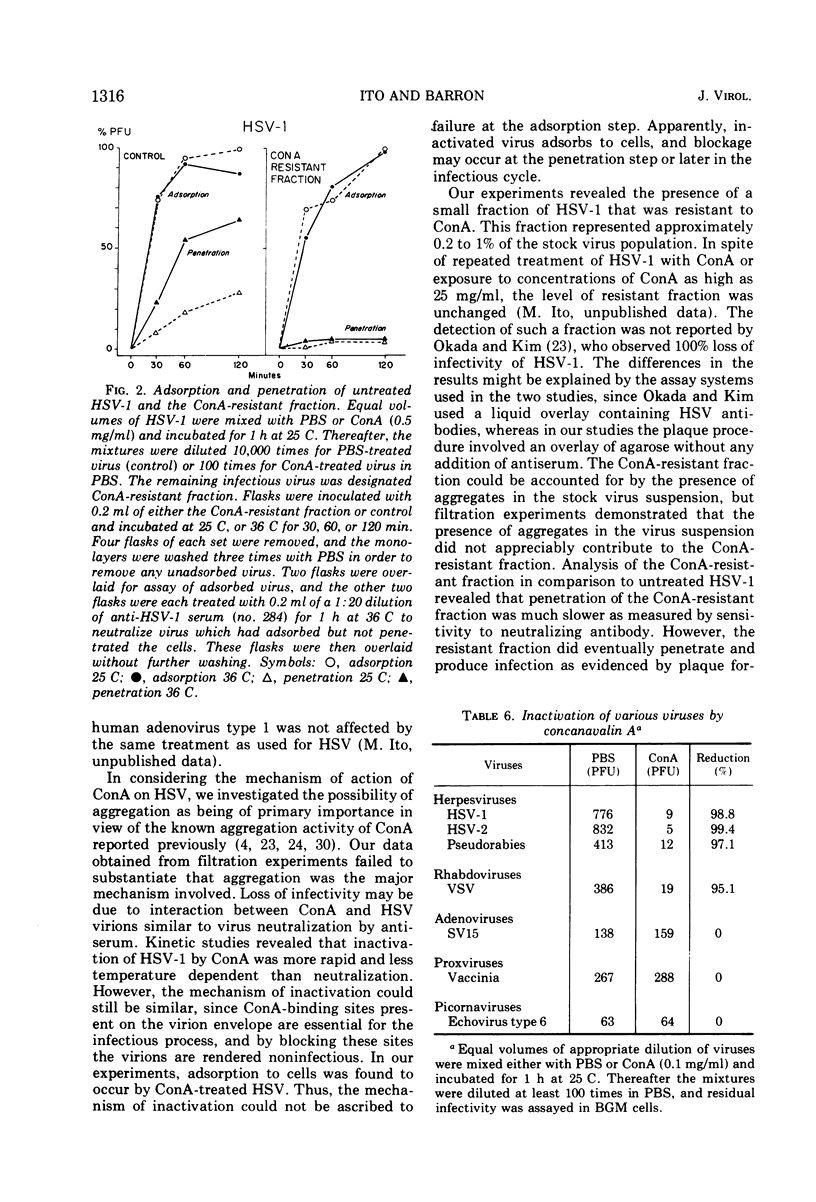
The infectivity of herpes simplex virus type 1 (HSV-1) was inactivated after treatment with either concanavalin A (ConA) or periodate. Phytohemagglutinin, wheat germ agglutinin, pokeweed mitogen, and neuraminidase failed to inactivate the virus. The effect of ConA could be specifically inhibited or reversed by the addition of α-methyl-d-glucoside or α-methyl-d-mannoside. Evidence was obtained that HSV-1 inactivated by ConA could adsorb to host cells. Viral aggregation was not a major mechanism in the inactivation of HSV-1 by ConA. Under the experimental conditions employed, inactivation of HSV-1 was faster by ConA than by antiserum and less temperature dependent. A ConA-resistant fraction was detected which appeared to adsorb less quickly than untreated virus, and penetration of ConA-resistant fraction was strikingly slow. The presence of aggregates in the virus preparation did not appear to account for the ConA-resistant fraction. Inactivation of viral infectivity by ConA was obtained only with enveloped viruses, since HSV-1, HSV-2, pseudorabies, and vesicular stomatitis virus were inactivated and vaccinia and echovirus type 6 were not.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Berg P. Quantitative binding of 125 I-concanavalin A to normal and transformed cells. J Virol. 1971 Nov;8(5):716–721. doi: 10.1128/jvi.8.5.716-721.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aub J. C., Sanford B. H., Cote M. N. Studies on reactivity of tumor and normal cells to a wheat germ agglutinin. Proc Natl Acad Sci U S A. 1965 Aug;54(2):396–399. doi: 10.1073/pnas.54.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron A. L., Olshevsky C., Cohen M. M. Characteristics of the BGM line of cells from African green monkey kidney. Brief report. Arch Gesamte Virusforsch. 1970;32(4):389–392. doi: 10.1007/BF01250067. [DOI] [PubMed] [Google Scholar]
- Ben-Bassat H., Inbar M., Sachs L. Requirement for cell replication after SV40 infection for a structural change of the cell surface membrane. Virology. 1970 Apr;40(4):854–859. doi: 10.1016/0042-6822(70)90131-5. [DOI] [PubMed] [Google Scholar]
- Biquard J. M., Vigier P. Agglutination par al concanavaline A des fibroblastes d'embryon de poule transformés par le virus de Rous (SR-RSV) et un mutant thermosensible de ce virus. C R Acad Sci Hebd Seances Acad Sci D. 1972 Jan 3;274(1):144–147. [PubMed] [Google Scholar]
- Birdwell C. R., Strauss J. H. Agglutination of Sindbis virus and of cells infected with Sindbis virus by plant lectins. J Virol. 1973 Apr;11(4):502–507. doi: 10.1128/jvi.11.4.502-507.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Martin G. S. Agglutination of cells transformed by Rous sarcoma virus by wheat germ agglutinin and concanavalin A. Nat New Biol. 1972 May 3;237(70):9–12. doi: 10.1038/newbio237009a0. [DOI] [PubMed] [Google Scholar]
- Calafat J., Hageman P. C. Binding of Concanavalin A to the envelope of two murine RNA tumour viruses. J Gen Virol. 1972 Jan;14(1):103–106. doi: 10.1099/0022-1317-14-1-103. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Livingston D. C. Binding of 3 H-concanavalin A by normal and transformed cells. Nat New Biol. 1971 Aug 4;232(31):155–156. doi: 10.1038/newbio232155a0. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd Herpesvirus envelopment. J Virol. 1968 Jan;2(1):48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geder L., Skinner G. R. Differentiation between type 1 and type 2 strains of herpes simplex virus by an indirect immunofluorescent technique. J Gen Virol. 1971 Aug;12(2):179–182. doi: 10.1099/0022-1317-12-2-179. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Barron A. L. Studies on a strain of vaccinia virus defective in surface antigen production. Proc Soc Exp Biol Med. 1972 May;140(1):374–377. doi: 10.3181/00379727-140-36461. [DOI] [PubMed] [Google Scholar]
- Ito M., Barron A. L. Surface antigen produced by herpes simplex virus (HSV). J Immunol. 1972 Mar;108(3):711–718. [PubMed] [Google Scholar]
- Ito M., Barron A. L. Surface antigens produced by Herpesviruses Varicella-Zoster virus. Infect Immun. 1973 Jul;8(1):48–521. doi: 10.1128/iai.8.1.48-52.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeller M., Doljanski F. Agglutination of normal and rous sarcoma virus-transformed chick embryo cells by concanavalin A and wheat germ agglutinin. Nat New Biol. 1972 Feb 9;235(58):184–185. doi: 10.1038/newbio235184a0. [DOI] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Kim J. Interaction of concanavalin A with enveloped viruses and host cells. Virology. 1972 Nov;50(2):507–515. doi: 10.1016/0042-6822(72)90401-1. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Ellwood D. C., Appleyard G., Stanley J. L. Agglutination of an arbovirus by concanavalin A. Nat New Biol. 1971 Sep 8;233(36):50–51. doi: 10.1038/newbio233050a0. [DOI] [PubMed] [Google Scholar]
- Peterknecht W., Bitter-Suermann D., Falke D. Immundahärenz zum Nachweis virusspezifischer Antikörper und Antigene. II. Immunadhärenz-Hämadsorption zum Nachweis zell- und virusspeczifischer Antigene auf Kulturzellen. Z Med Mikrobiol Immunol. 1968;154(3):234–244. [PubMed] [Google Scholar]
- Ponce de Leon M., Hessle H., Cohen G. H. Separation of Herpes simplex virus-induced antigens by Concanavalin A affinity chromatography. J Virol. 1973 Oct;12(4):766–774. doi: 10.1128/jvi.12.4.766-774.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg S., Raskas H. J. Surface changes of human cells productively infected with human adenoviruses. Virology. 1972 Jun;48(3):631–637. doi: 10.1016/0042-6822(72)90147-x. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Adam E., Melnick J. L., Rawls W. E. Use of the 51 Cr release test to demonstrate patterns of antibody response in humans to herpesvirus types 1 and 2. J Immunol. 1972 Sep;109(3):554–564. [PubMed] [Google Scholar]
- Stewart M. L., Summers D. F., Soeiro R., Fields B. N., Maizel J. V., Jr Purification of oncornaviruses by agglutination with concanacalin A (murine leukemia virus-phytohemagglutinin-friend virus). Proc Natl Acad Sci U S A. 1973 May;70(5):1308–1312. doi: 10.1073/pnas.70.5.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia S. S., Lowry S., Rawls W. E., Melnick J. L., McMillan V. Detection of early cell surface changes in herpes simplex virus infected cells by agglutination with concanavalin A. J Gen Virol. 1972 Apr;15(1):93–97. doi: 10.1099/0022-1317-15-1-93. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Virus aggregation as the cause of the non-neutralizable persistent fraction. J Virol. 1967 Jun;1(3):478–488. doi: 10.1128/jvi.1.3.478-488.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Tevethia S. S. Expression of concanavalin A binding sites in rabbit kidney cells infected with vaccinia virus. Virology. 1971 Jul;45(1):313–316. doi: 10.1016/0042-6822(71)90140-1. [DOI] [PubMed] [Google Scholar]


