Abstract
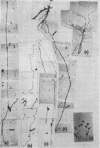
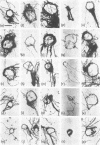
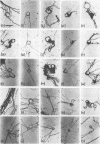
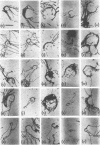
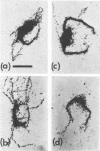
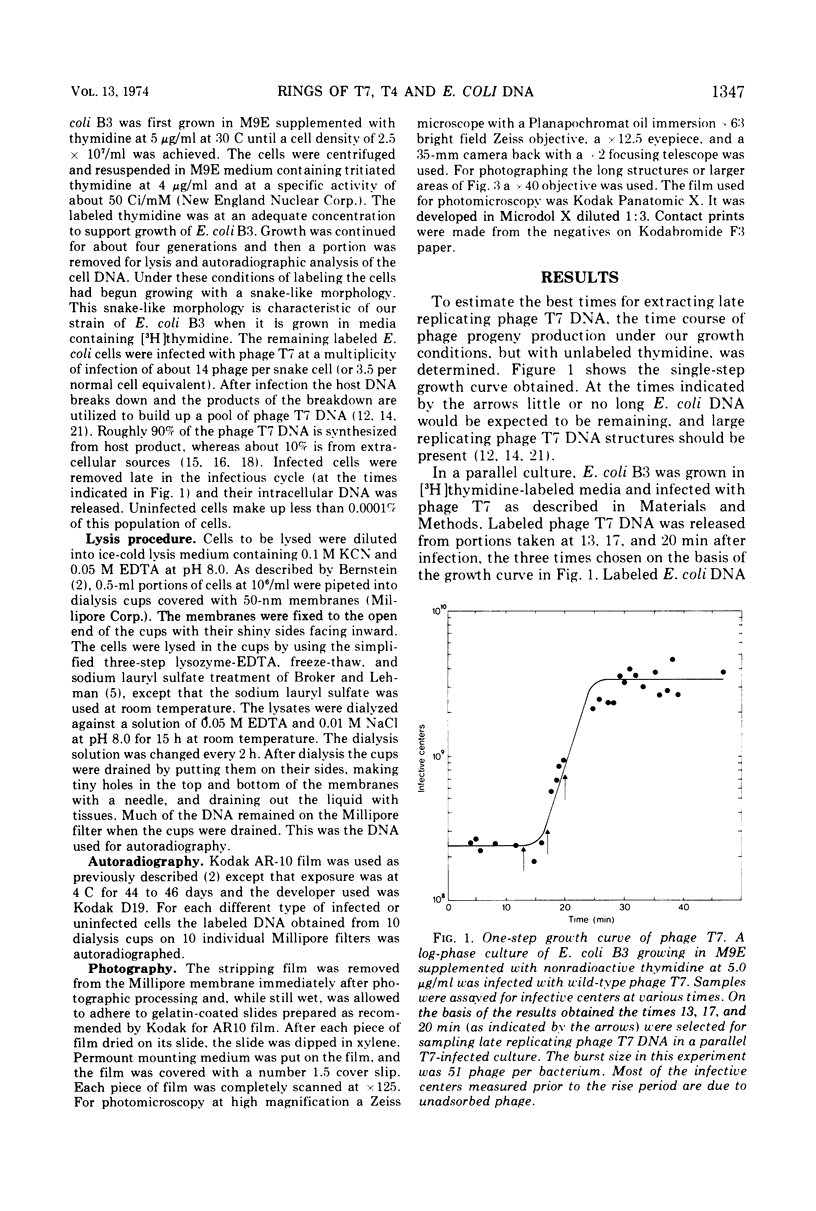
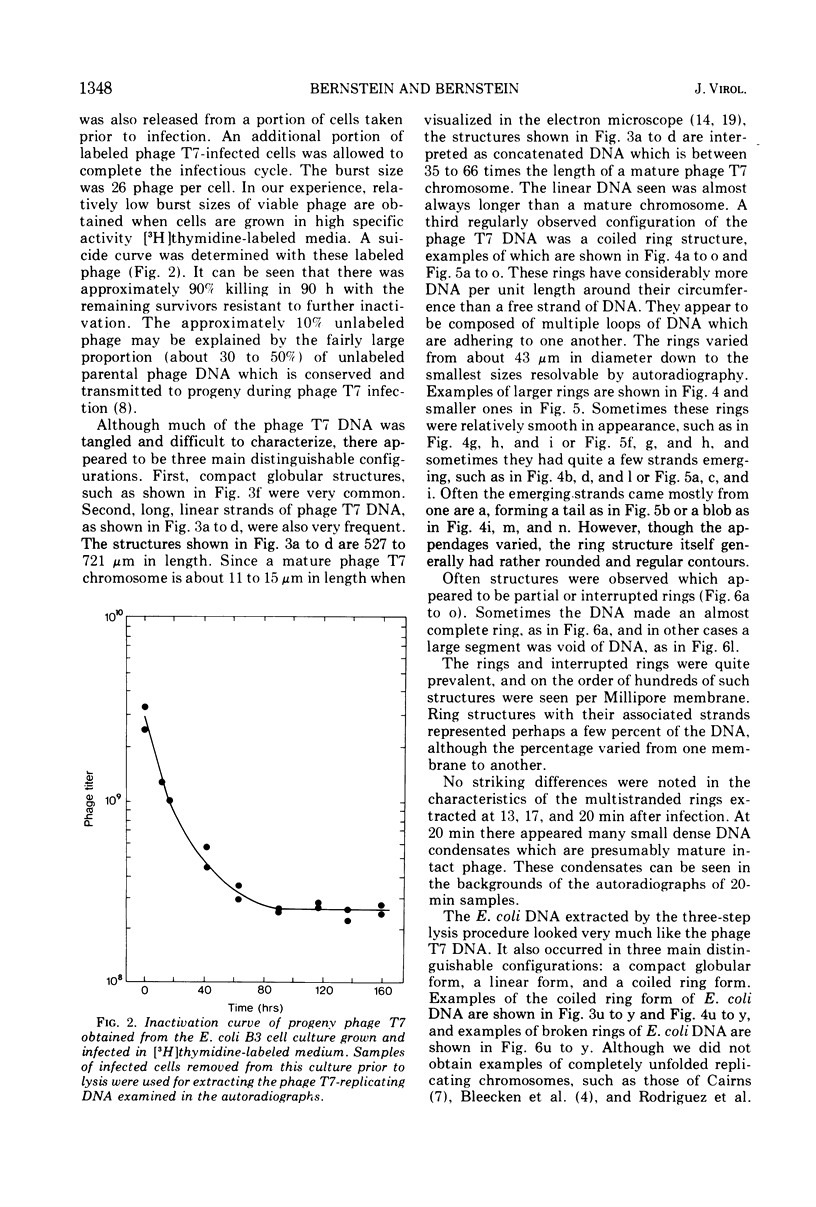
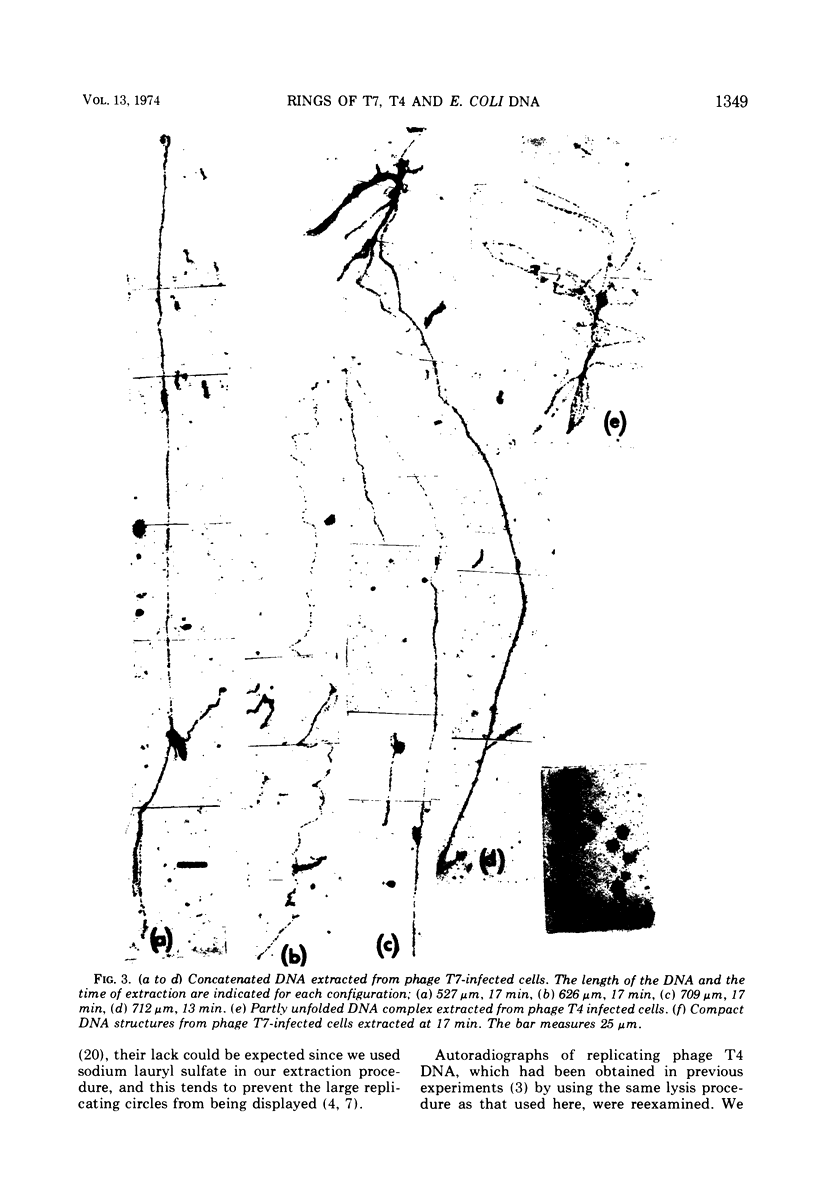
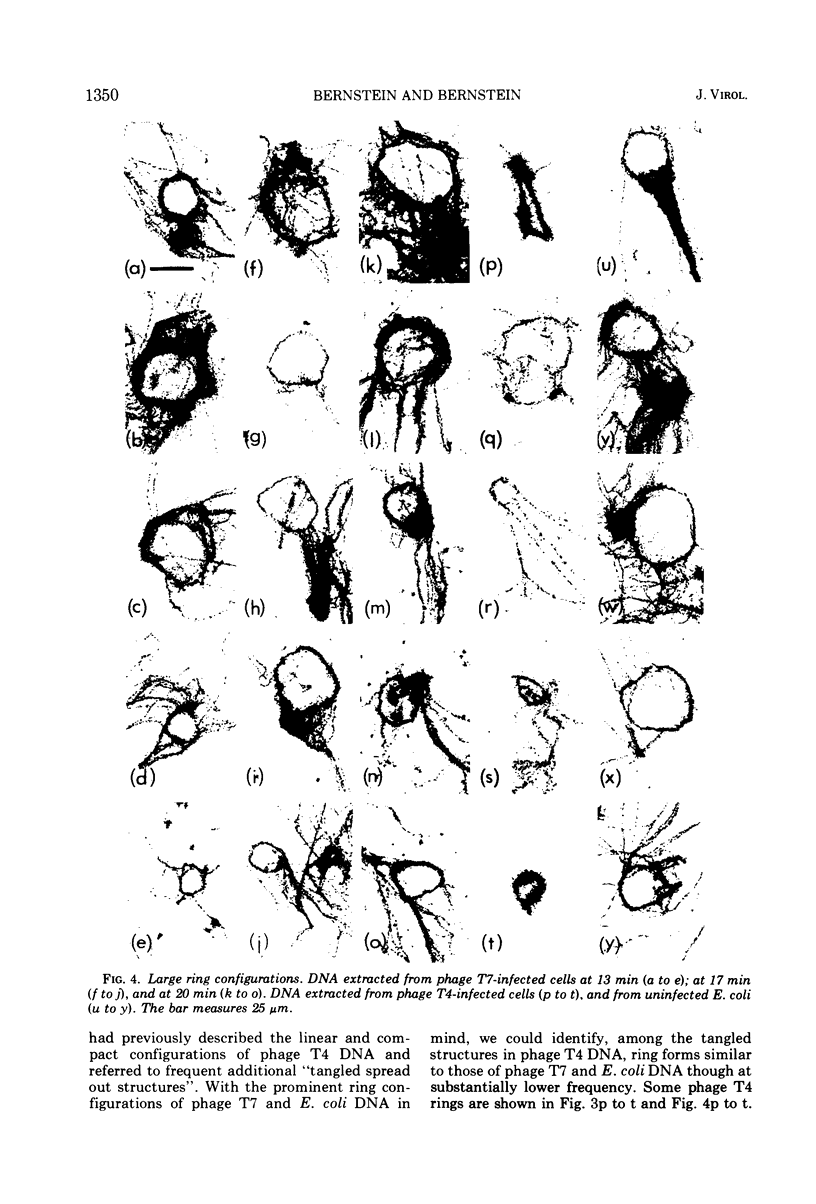
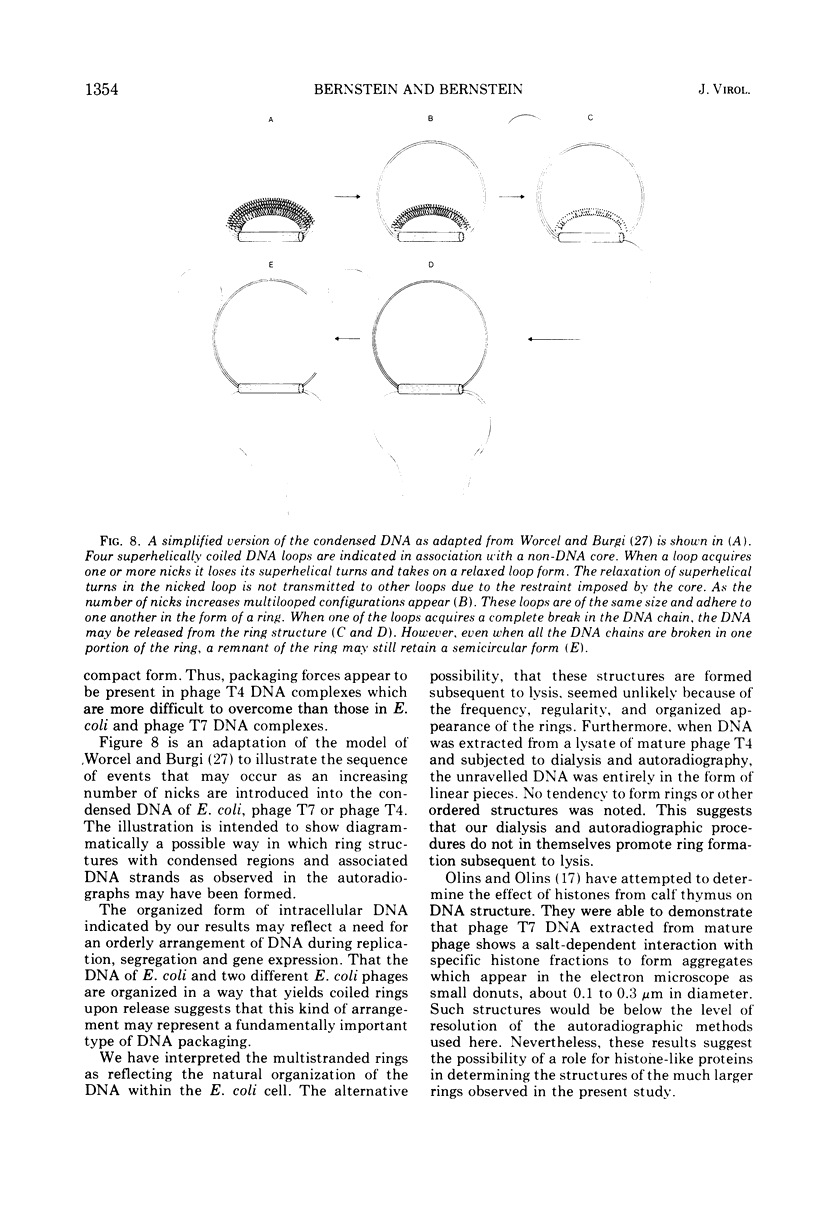
The replicating intracellular DNA of phage T7 was labeled at high specific activity with tritiated thymidine. The DNA of uninfected Escherichia coli was similarly labeled. Portions of cells which contained replicating phage T7 or E. coli DNA were lysed by a lysozyme, freeze-thaw, sodium lauryl sulfate procedure, and the DNA was spread on Millipore membranes for visualization by autoradiography. The DNA of phage T7 appeared to be highly concatenated reaching lengths of up to 721 μm. Much of the DNA of phage T7 and E. coli was retained in compact globular structures. In addition, orderly coiled rings of varying diameter up to about 43 μm were regularly observed. Similar coiled ring structures were also observed in autoradiographs of replicating phage T4 DNA which had been prepared in previous experiments. Worcel and Burgi (27) have presented evidence that E. coli chromosomes, when gently extracted from cells, are in a multilooped and superhelically twisted configuration. The coiled rings which we have observed may correspond to the relaxed, multilooped configurations which they find when the superhelical twists have been relieved by one or more nicks in each loop.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein C. A comparison of the number of nucleotides per unit length in Escherichia coli and phage T4 chromosomes. Biophys J. 1970 Dec;10(12):1154–1172. doi: 10.1016/S0006-3495(70)86362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H., Bernstein C. Circular and branched circular concatenates as possible intermediates in bacteriophage T4 DNA replication. J Mol Biol. 1973 Jul 5;77(3):355–361. doi: 10.1016/0022-2836(73)90443-9. [DOI] [PubMed] [Google Scholar]
- Bleecken S., Strohbach G., Sarfert E. Autoradiography of bacterial chromosomes. Z Allg Mikrobiol. 1966;6(2):121–123. doi: 10.1002/jobm.3630060205. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Lehman I. R. Branched DNA molecules: intermediates in T4 recombination. J Mol Biol. 1971 Aug 28;60(1):131–149. doi: 10.1016/0022-2836(71)90453-0. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The application of autoradiography to the study of DNA viruses. Cold Spring Harb Symp Quant Biol. 1962;27:311–318. doi: 10.1101/sqb.1962.027.001.029. [DOI] [PubMed] [Google Scholar]
- Carlson K. Intracellular fate of deoxyribonucleic acid from T7 bacteriophages. J Virol. 1968 Oct;2(10):1230–1233. doi: 10.1128/jvi.2.10.1230-1233.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center M. S. Replicative intermediates of bacteriophage T7 deoxyribonucleic acid. J Virol. 1972 Jul;10(1):115–123. doi: 10.1128/jvi.10.1.115-123.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Worcel A. Letter: Electron microscopic visualization of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):107–109. doi: 10.1016/0022-2836(74)90577-4. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of replicating DNA in T4-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):127–143. doi: 10.1016/s0022-2836(66)80081-5. [DOI] [PubMed] [Google Scholar]
- Hausmann R., Gomez B. Amber mutants of bacteriophages T3 and T7 defective in phage-directed deoxyribonucleic acid synthesis. J Virol. 1967 Aug;1(4):779–792. doi: 10.1128/jvi.1.4.779-792.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A. Visualization of replicating mammalian and T4 bacteriophage DNA. Cold Spring Harb Symp Quant Biol. 1968;33:509–524. doi: 10.1101/sqb.1968.033.01.059. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- LABAW L. W. The origin of phosphorus in Escherichia coli bacteriophages. J Bacteriol. 1951 Aug;62(2):169–173. doi: 10.1128/jb.62.2.169-173.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABAW L. W. The origin of phosphorus in the T1, T5, T6, and T7 bacteriophages of Escherichia coli. J Bacteriol. 1953 Oct;66(4):429–436. doi: 10.1128/jb.66.4.429-436.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins D. E., Olins A. L. Model nucleohistones: the interaction of F1 and F2al histones with native T7 DNA. J Mol Biol. 1971 May 14;57(3):437–455. doi: 10.1016/0022-2836(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Ritchie D. A., Thomas C. A., Jr, MacHattie L. A., Wensink P. C. Terminal repetition in non-permuted T3 and T7 bacteriophage DNA molecules. J Mol Biol. 1967 Feb 14;23(3):365–376. doi: 10.1016/s0022-2836(67)80111-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. L., Dalbey M. S., Davern C. I. Autoradiographic evidence for bidirectional DNA replication in Escherichia coli. J Mol Biol. 1973 Mar 15;74(4):599–604. doi: 10.1016/0022-2836(73)90050-8. [DOI] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski P. D., Kerr C. Degradation of Escherichia coli B deoxyribonucleic acid after infection with deoxyribonucleic acid-defective amber mutants of bacteriophage T7. J Virol. 1970 Aug;6(2):149–155. doi: 10.1128/jvi.6.2.149-155.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R. A., Thomas C. A., Jr Some special structural features of intracellular bacteriophage T7 concatemers. J Mol Biol. 1972 Jul 21;68(2):319–345. doi: 10.1016/0022-2836(72)90216-1. [DOI] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strätling W., Krause E., Knippers R. Fast sedimenting deoxyribonucleic acid in bacteriophage T7-infected cells. Virology. 1973 Jan;51(1):109–119. doi: 10.1016/0042-6822(73)90371-1. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Barnes J. E. Deoxyribonucleic acid synthesis in Escherichia coli infected with some deoxyribonucleic acid polymerase-less mutants of bacteriophage T4. Virology. 1966 Jan;28(1):100–107. doi: 10.1016/0042-6822(66)90310-2. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. doi: 10.1016/0022-2836(74)90576-2. [DOI] [PubMed] [Google Scholar]