Abstract
Background
Recent studies on the association between Glutathione S-transferase T1 (GSTT1) polymorphism and risk of prostate cancer showed inconclusive results. To clarify this possible association, we conducted a meta-analysis of published studies.
Methods
Data were collected from the following electronic databases: Pubmed, Embase, and Chinese Biomedical Database (CBM). The odds ratio (OR) and its 95% confidence interval (95%CI) was used to assess the strength of the association. We summarized the data on the association between GSTT1 null genotype and risk of prostate cancer in the overall population, and performed subgroup analyses by ethnicity, adjusted ORs, and types of controls.
Results
Ultimately, a total of 43 studies with a total of 26,393 subjects (9,934 cases and 16,459 controls) were eligible for meta-analysis. Overall, there was a significant association between GSTT1 null genotype and increased risk of prostate cancer (OR = 1.14, 95%CI 1.01–1.29, P = 0.034). Meta-analysis of adjusted ORs also showed a significant association between GSTT1 null genotype and increased risk of prostate cancer (OR = 1.34, 95%CI 1.09–1.64, P = 0.006). Similar results were found in the subgroup analyses by ethnicity and types of controls.
Conclusion
This meta-analysis demonstrates that GSTT1 null genotype is associated with prostate cancer susceptibility, and GSTT1 null genotype contributes to increased risk of prostate cancer.
Introduction
Prostate cancer is a common cause of cancer mortality and one of the most frequently diagnosed malignancies in men [1], [2]. Identifying risk factors for prostate cancer is critically important to develop potential interventions and to expand our understanding of the biology of this disease [2], [3]. Endogenous products and environmental factors could result in the production of reactive oxygen species (ROS) and nitrogen metabolites causing cell injury and genetic instability, and further result in the carcinogenesis in prostate [2]. Glutathione S-transferases (GSTs) play an active role in the detoxification of a variety of endogenous or exogenous carcinogens by glutathione (GSH) conjugation [4], [5]. These enzymes also play a crucial role in protection of DNA from oxidative damage by ROS [4], [5]. In humans, GST super family consists of many cytosolic, mitochondrial, and microsomal proteins, and the cytosolic family has eight distinct classes alpha, kappa, mu, omega, pi, sigma, theta, and zeta [6]. The theta class of GSTs is encoded by the Glutathione S-transferase T1 (GSTT1) gene located on the long arm of chromosome 22 (22q11.23), and the homozygous deletion (null genotype) of GSTT1 gene causes complete absence of GST enzymes activity [7], [8]. In 2009, a meta-analysis on the association between GSTT1 null genotype and prostate risk was reported. This meta-analysis including 22 studies (3,837 cases and 4,552 controls) concluded that there was no association between GSTT1 null genotype and prostate risk [9]. Nevertheless, this meta-analysis included relatively small sample size, and many new studies recently have examined the association between GSTT1 null genotype and prostate risk [10]–[20], but the results remain inconclusive and inconsistent. Hence, to clarify this possible association, we conducted an updated meta-analysis of published studies, which may provide an evidence for the association of GSTT1 null genotype and prostate risk.
Materials and Methods
Identification and Eligibility of Relevant Studies
Data were collected from the following electronic databases: Pubmed, Embase, and and Chinese Biomedical Database (CBM). Relevant publications were identified through a literature search using the following search strategy: (“Glutathione S-transferase T1” or “GSTT1” or “GSTT”) and (“prostate cancer” or “prostate carcinoma”). Additional literature was collected from cross-references within both original and review articles. No language restrictions were applied. A study was included in the current meta-analysis if: (1) it was published up to May 2012; (2) it was a case-control study; (3) the control subjects are prostate cancer-free regardless of whether they had benign prostate hyperplasia (BPH) or not. We excluded family-based studies of pedigrees with several affected cases per family because the analysis was based on linkage considerations. When a study reported the results on different ethnicities, we treated them as separate studies.
Data Extraction
Information was carefully extracted from all the eligible publications independently by two of the authors according to the inclusion criteria listed above. Disagreement was resolved by discussion among all authors. Data extracted from the selected studies included author, year of publication, country, ethnicity, definition of cases, characteristics of controls, total numbers of cases and controls, the genotype frequency of GSTT1 polymorphism, and adjusted odds ratio (OR) and its 95% confidence interval (95%CI). Different descents were categorized as Caucasians, East Asians, Africans, Indians, and Others. If original genotype frequency data were unavailable in relevant articles, a request was sent to the corresponding author for additional data. In deed, only two requests were sent, but no replies were obtained.
Statistical methods
The strength of the association between GSTT1 null genotype and prostate cancer risk was assessed by calculating the pooled OR with its 95%CI. The pooled ORs were obtained using either the fixed-effects (Mantel-Haenszel's method) [21] or random-effects (DerSimonian and Laird method) models [22], and the significance of the pooled OR was determined by the Z-test. Heterogeneity assumption was checked by the Chi-square test based Q-statistic [23] and the I2 statistic [24]. A significant Q statistic (P<0.10) or I2 statistic (I2>50%) indicated obvious heterogeneity across studies, and the random effect model was selected to pool the ORs. Otherwise, the fixed effect model was selected to pool the ORs. Subgroup analyses were performed by ethnicity, adjusted ORs, and types of controls. Subgroup analyses were firstly performed by adjusted ORs including subgroup analysis of adjusted ORs and subgroup analysis of unadjusted ORs. Subgroup analyses were then performed ethnicity, and ethnicities were categorized as Caucasians, East Asians, Africans, Indians, and Others. Finally, Subgroup analyses were performed by the types of controls. Publication bias was investigated with the funnel plot. The funnel plot should be asymmetric when there is a publication bias, and the funnel plot asymmetry was further assessed by the method of Egger's linear regression test [25]. Analyses were performed using the software Stata version 11 (StataCorp LP, College Station, TX). A P value less than 0.05 was considered statistically significant, and all the P values were two sided.
Results
Characteristics of Eligible Studies
There were 97 papers relevant to the searching words, and 50 papers were excluded (39 overlapping records; 4 were not case-control studies; 3 did not explore GSTT1 polymorphism; 2 were meta-analysis; 2 were reviews), leaving 47 studies for full publication review [10]–[20], [26]–[61] (Figure S1). Of these, 6 studies were excluded (2 were reviews; 2 were case-only studies; 1 was family-based case-control study; 1 was overlapping study) [56]–[61], leaving 41 studies [10]–[20], [26]–[55] (Figure S1). One study reported the results on two different ethnicities [37] and one study reported the results on two groups [11], and we treated them as separate studies. Finally, a total of 43 independent studies including a total of 26, 393 subjects (9, 934 cases and 16, 459 controls) were used in the current meta-analysis [10]–[20], [26]–[55]. Characteristics of studies eligible for the current meta-analysis were presented in Table 1. 43 independent studies consisted of 21 Caucasians, 6 East Asians, 6 Indins, 2 Africans and 6 mixed populations. Adjusted ORs with corresponding 95%CIs were reported in 13 studies [11]–[13], [26], [28], . There were 7 studies used BPH patients as the controls [10], [12], [17], [18], [29], [35], [51], while only 4 studies used the controls excluding BPH patients [10], [12], [17], [28].
Table 1. Characteristics of 43 eligible studies in this meta-analysis.
| First author(Year) | Country | Ethnic group | Cases (GSTT1 Null frequency, %) | Controls (GSTT1 Null frequency, %) | Adjusted variables | Adjusted OR (95%CI) |
| Choubey VK 2012 (10) | India | Indians | 51 prostate cancer cases (9, 17.6%) | 134 controls without BPH (17, 12.7%); 244 BPH patients as controls (48, 19.7%) | None | -- |
| Catsburg C 2012 (Localized) (11) | USA | Mixed | 491 localized prostate cancer cases (80, 16.3%) | 736 controls (153, 20.8%) | Age and family history of prostate cancer | 1.68 (1.19–2.38) |
| Catsburg C 2012 (Advanced) (11) | USA | Mixed | 909 advanced prostate cancer cases (162, 17.8%) | 736 controls (153, 20.8%) | Age and family history of prostate cancer | 1.18 (0.92–1.52) |
| Hemelrijck MV 2012 (26) | Germany | Caucasians | 203 prostate cancer cases (35, 17.2%) | 360 controls (64, 17.8%) | Time of recruitment and family history of prostate cancer | 1.08 (0.93–1.25) |
| Kumar V 2011 (17) | India | Indians | 57 prostate cancer cases (29, 50.9%) | 46 controls without BPH (22, 47.8%); 53 BPH patients as controls (32, 60.4%) | None | -- |
| Kwon DD 2011 (16) | Korea | East Asians | 166 prostate cancer cases (85, 51.2%) | 327 controls (163, 49.8%) | None | -- |
| Safarinejad MR 2011 (13) | Iran | Caucasians | 168 prostate cancer cases (58, 34.5%) | 336 controls (70, 20.8%) | Age, body mass index, occupational status, educational level and smoking status | 3.21 (2.52–6.21) |
| Ashtiani ZO 2011 (18) | Iran | Caucasians | 110 prostate cancer cases (38, 34.5%) | 100 BPH patients as controls (47, 47.0%) | None | -- |
| Rodrigues IS 2011 (14) | Brasil | Caucasians | 154 prostate cancer cases (42, 27.3%) | 154 controls (40, 26.0%) | None | -- |
| Thakur H 2011 (12) | India | Indians | 150 prostate cancer cases (39, 26.0%) | 172 controls without BPH (22, 12.8%); 150 BPH patients as controls (18, 12.0%) | Age, smoking, drinking and non vegetarian diet | 2.39 (1.36–4.2) |
| Norskov MS 2011 (15) | Denmark | Caucasians | 128 prostate cancer cases (26, 20.3%) | 4409 controls (656, 14.9%) | None | -- |
| Souiden Y 2010 (20) | Tunisia | Caucasians | 110 prostate cancer cases (30, 27.3%) | 122 controls (18, 14.8%) | None | -- |
| Steinbrecher A 2010 (19) | Germany | Caucasians | 248 prostate cancer cases (44, 17.7%) | 492 controls (77, 15.7%) | None | -- |
| Lavender NA 2009 (28) | USA | Africans | 189 prostate cancer cases (36, 19.0%) | 584 controls without BPH (102, 17.5%) | Age, prostate specific antigen, and west African ancestry | 1.15 (0.66–2.02) |
| Sivonova M 2009 (27) | Slovakia | Caucasians | 129 prostate cancer cases (24, 18.6%) | 228 controls (45, 19.7%) | None | -- |
| Lima MM Jr 2008 (29) | Brasil | Caucasians | 125 prostate cancer cases (42, 33.6%) | 100 BPH patients as controls (22, 22.0%) | None | -- |
| Davydova NA 2008 (30) | Russia | Caucasians | 61 prostate cancer cases (37, 60.7%) | 100 controls (43, 43.0%) | None | -- |
| Mallick S 2007 (31) | Guadeloupe | Mixed | 134 prostate cancer cases (30, 22.4%) | 134 controls (49, 36.6%) | None | -- |
| Cunningham JM 2007 (32) | USA | Mixed | 499 prostate cancer cases (185, 37.1%) | 493 controls (212, 43.0%) | None | -- |
| Mittal RD 2006 (35) | India | Indians | 54 prostate cancer cases (24, 44.4%) | 105 BPH patients as controls (30, 28.6%) | None | -- |
| Lindstrom S 2006 (36) | Sweden | Caucasians | 1299 prostate cancer cases (165, 12.7%) | 728 controls (107, 14.7%) | None | -- |
| Yang J 2006 (33) | China | East Asians | 163 prostate cancer cases (89, 54.6%) | 202 controls (95, 47.0%) | None | -- |
| Silig Y 2006 (34) | Turkey | Caucasians | 152 prostate cancer cases (34, 22.3%) | 169 controls (31, 18.3%) | Age, smoking, and family history of cancer. | 1.28 (0.74–2.27) |
| Agalliu I 2006 (Caucasians) (37) | USA | Caucasians | 558 prostate cancer cases (92, 16.5%) | 522 controls (88, 16.9%) | Age, family history of prostate cancer, and PSA testing history. | 1.04 (0.73–1.47) |
| Agalliu I 2006 (Africans) (37) | USA | Africans | 31 prostate cancer cases (7, 20.6%) | 15 controls (7, 46.7%) | Age, family history of prostate cancer, and PSA testing history. | 0.65 (0.13–3.33) |
| Nam RK 2005 (40) | Canada | Mixed | 996 prostate cancer cases (189, 19.0%) | 1092 controls (248, 22.7%) | Age, ethnic background, family history of prostate cancer, and PSA. | 0.81 (0.60–1.0) |
| Caceres DD 2005 (42) | Chile | Mixed | 100 prostate cancer cases (6, 6.0%) | 129 controls (14, 10.9%) | None | -- |
| Srivastava DS 2005 (39) | India | Indians | 127 prostate cancer cases (41, 32.3%) | 144 controls (29, 20.1%) | None | -- |
| Wang YL 2005 (38) | China | East Asians | 81 prostate cancer cases (43, 53.1%) | 90 controls (48, 53.3%) | None | -- |
| Komiya Y 2005 (41) | Japan | East Asians | 186 prostate cancer cases (112, 60.2%) | 288 controls (149, 51.7%) | None | -- |
| Joseph MA 2004 (45) | USA | Caucasians | 177 prostate cancer cases (55, 31.1%) | 265 controls (61, 23.0%) | None | -- |
| Mittal RD 2004 (43) | India | Indians | 103 prostate cancer cases (35, 34.0%) | 117 controls (13, 11.1%) | None | -- |
| Medeiros R 2004 (44) | Portugal | Caucasians | 145 prostate cancer cases (31, 21.7%) | 184 controls (44, 23.9%) | None | -- |
| Nakazato H 2003 (46) | Japan | East Asians | 81 prostate cancer cases (40, 49.4%) | 105 controls (44, 41.9%) | None | -- |
| Kidd LC 2003 (47) | Finland | Caucasians | 202 prostate cancer cases (24, 11.9%) | 189 controls (29, 15.3%) | None | -- |
| Beer TM 2002 (48) | USA | Caucasians | 111 prostate cancer cases (28, 25.2%) | 146 controls (33, 22.6%) | Age | 1.0 (0.48–2.08) |
| Kote-Jarai Z 2001 (50) | UK | Caucasians | 273 prostate cancer cases (67, 24.5%) | 278 controls (66, 23.7%) | None | -- |
| Murata M 2001 (49) | Japan | East Asians | 115 prostate cancer cases (68, 59.1%) | 200 controls (96, 48.0%) | None | -- |
| Gsur A 2001 (51) | Austria | Caucasians | 166 prostate cancer cases (27, 16.3%) | 166 BPH patients as controls (33, 19.9%) | None | -- |
| Kelada SN 2000 (53) | USA | Mixed | 256 prostate cancer cases (60, 23.4%) | 469 controls (155, 33.0%) | None | -- |
| Steinhoff C 2000 (52) | Germany | Caucasians | 91 prostate cancer cases (23, 25.3%) | 127 controls (17, 13.4%) | None | -- |
| Autrup JL 1999 (55) | Denmark | Caucasians | 153 prostate cancer cases (29, 19.0%) | 288 controls (44, 15.3%) | Age at diagnosis | 1.31 (0.77–2.19) |
| Rebbeck TR 1999 (54) | USA | Mixed | 232 prostate cancer cases (186, 80.2%) | 231 controls (159, 68.8%) | Age and race | 1.83 (1.19–2.80) |
Meta-Analysis
The summary of meta-analysis for GSTT1 null genotype with prostate cancer risk was shown in Table 2.
Table 2. Summary of meta-analysis for GSTT1 null genotype with prostate cancer risk.
| Groups | Studies | Subjects (Cases/Controls) | OR (95%CI) | POR | I2 | P heterogeneity |
| Total studies | 43 | 9934/16012 | 1.14(1.01–1.29) | 0.034 | 67.2% | <0.001 |
| Subgroup analyses | ||||||
| Adjusted ORs | 13 | 4343/5387 | 1.34(1.09–1.64) | 0.006 | 72.8% | <0.001 |
| BPH controls | 7 | 713/918 | 1.15(0.73–1.80) | 0.549 | 71.1% | 0.002 |
| Controls without BPH | 4 | 447/937 | 1.41(1.06–1.88) | 0.020 | 37.3% | 0.189 |
| Caucasians | 21 | 4763/9463 | 1.17(1.01–1.35) | 0.044 | 50.2% | 0.005 |
| East Asians | 6 | 792/1212 | 1.28(1.07–1.54) | 0.007 | 0.0% | 0.727 |
| Africans | 2 | 220/596 | 0.72(0.23–2.34) | 0.571 | 65.6% | 0.088 |
| Indians | 6 | 542/718 | 2.09(1.60–2.74) | <0.001 | 27.6% | 0.228 |
(GSTT1, Glutathione S-transferase T1; 95%CI, 95% confidence interval; OR, odds ratio; BPH, benign prostate hyperplasia).
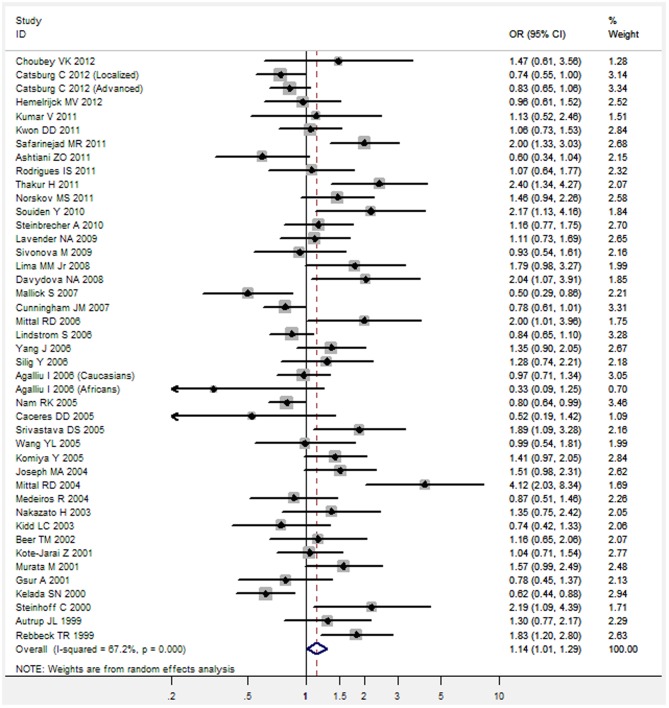
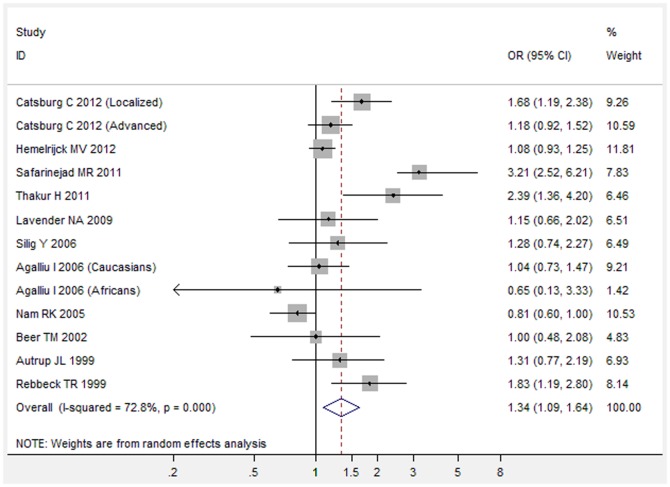
Overall, there was a significant association between GSTT1 null genotype and increased risk of prostate cancer (OR = 1.14, 95%CI 1.01–1.29, P = 0.034) (Figure 1). Meta-analysis of adjusted ORs also showed a significant association between GSTT1 null genotype and increased risk of prostate cancer (OR = 1.34, 95%CI 1.09–1.64, P = 0.006) (Figure 2).
Figure 1. Unadjusted OR with its 95%CI for the association between GSTT1 null genotype and risk of prostate cancer.
Figure 2. Adjusted OR with its 95%CI for the association between GSTT1 null genotype and risk of prostate cancer.
In the subgroup analyses were firstly performed by ethnicity (Caucasians, East Asians, Africans, and Indians). There was an obvious association between GSTT1 null genotype and increased risk of prostate cancer in Caucasians (OR = 1.17, 95%CI 1.01–1.35, P = 0.044), East Asians (OR = 1.28, 95%CI 1.07–1.54, P = 0.007), and Indians (OR = 2.09, 95%CI 1.60–2.74, P<0.001), but not in Africans (OR = 0.72, 95%CI 0.23–2.34, P = 0.571).
In the subgroup analysis of BPH controls, there was no obvious association between GSTT1 null genotype and increased risk of prostate cancer (OR = 1.15, 95%CI 0.73–1.80, P = 0.549). In the subgroup analysis of controls without BPH, there was an obvious association between GSTT1 null genotype and increased risk of prostate cancer (OR = 1.41, 95%CI 1.06–1.88, P = 0.020).
Evaluation of Publication Bias
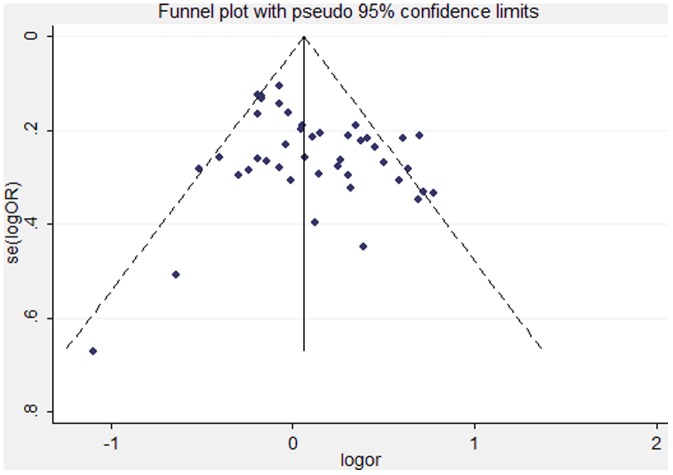
Both funnel plot and Egger's test were performed to assess the publication bias of the studies. The shape of the funnel plots did not reveal any evidence of obvious asymmetry for any genetic model in the overall and subgroup meta-analysis (Figure 3). Next, Egger's test was used to provide statistical evidence of the funnel plot symmetry. The results still did not suggest any obvious evidence of publication bias for any genetic model (P Egger's test = 0.117). Thus, there was no obvious risk of bias in this meta-analysis.
Figure 3. Funnel plot to assess the publication bias of the studies in this meta-analysis.
Discussion
Genetic susceptibility to cancer has been a research focus and many genetic association meta-analyses have been published to find some possible susceptibility polymorphisms [3], [10]–[20], [26]–[55]. Previous studies assessing the association between GSTT1 null genotype and prostate cancer risk reported inconclusive and inconsistent findings. Therefore, to get a reliable conclusion for the association of GSTT1 null genotype and prostate risk, we conducted the present meta-analysis of 43 independent studies including a total of 26, 393 subjects (9, 934 cases and 16, 459 controls). Overall, there was a significant association between GSTT1 null genotype and increased risk of prostate cancer (Table 2). Meta-analysis of adjusted ORs also showed a significant association between GSTT1 null genotype and increased risk of prostate cancer (Table 2). Similar association was also found in the subgroup analyses by ethnicity and types of controls (Table 2). Therefore, our meta-analysis demonstrates that GSTT1 null genotype is associated with prostate cancer susceptibility, and GSTT1 null genotype contributes to increased risk of prostate cancer.
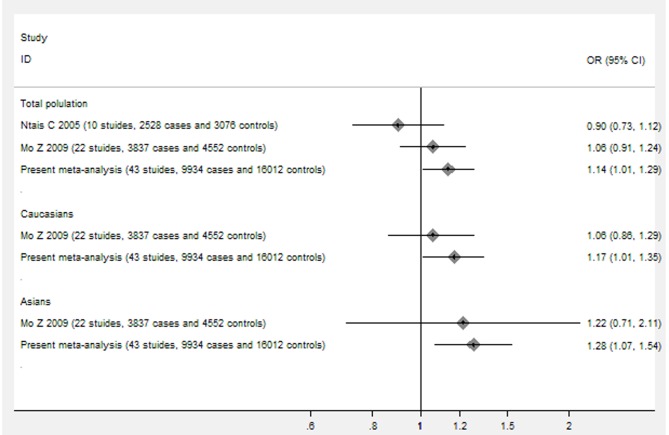
Previous literature didn't provide a comprehensive assessment on the association between GSTT1 null genotype and prostate cancer risk, but a trend for potential genetic effects was suggested in early data for the association between GSTT1 null genotype and prostate cancer risk. Postulated genetic associations for prostate cancer need to be carefully validated, because early and small genetic association studies may come up with spurious findings. Two previous meta-analyses were published to assess the association between GSTT1 null genotype and prostate cancer risk, but both failed to find a significant association [9], [62] (Figure 4). Compared with those two meta-analyses, our meta-analysis provides several new findings. Our meta-analysis includes much larger participants and more new studies (43 studies, 9, 934 cases and 16, 459 controls) and is the largest meta-analysis of the association between GSTT1 null genotype and prostate cancer risk. The present meta-analysis has much greater power to detect the real association, and draw a more precise and reliable conclusion. The pooled results in our meta-analysis suggests a significant association between GSTT1 null genotype and increased risk of prostate cancer, which provides a comprehensive evidence and reliable conclusion for the association above (Figure 4).
Figure 4. The main differences in the findings between present meta-analysis and previous published meta-analyses.
In our meta-analysis, the cases and controls have been recruited through different sources. The control subjects in our meta-analysis are defined as cancer-free, and the BPH patients are also enrolled in many included studies in the meta-analysis. Though there is no obvious association between BPH and prostate cancer, there is also a significant association between GSTT1 polymorphism and BPH and the GSTT1 null genotype frequency is higher in the BPH patients than that in the healthy controls [10]. Our meta-analysis suggest there is no obvious association between GSTT1 null genotype and prostate cancer risk in the subgroup analysis of studies with BPH controls, but there is an obvious association between GSTT1 null genotype and increased risk of prostate cancer in the subgroup analysis of studies with non-BPH controls (Table 2), which indicates this discrepancy in the GSTT1 null genotype frequency between BPH patients and healthy controls may affect the association between GSTT1 null genotype and risk of prostate cancer. Since there is also an obvious association between GSTT1 null genotype and increased risk of BPH, the frequency of GSTT1 null genotype is much higher in the BPH patients than that in the healthy controls [10]. When one case-control study selects the BPH patients as the controls to assess the association between GSTT1 null genotype and prostate cancer risk, the higher frequency of GSTT1 null genotype in the BPH patients may become a major confounding factor and could bias the real estimation of the association between GSTT1 null genotype and prostate cancer risk [10].
GSTs are the most important family of phase II isoenzymes which are known to detoxify a variety of electrophilic compounds including carcinogens, chemotherapeutic drugs, environmental toxins, and DNA products generated by reactive oxygen species damage to intracellular molecules [4], [6]. GSTs also play a major role in cellular antimutagen and antioxidant defense mechanisms, and these enzymes may regulate pathways that prevent damage from several carcinogens [4], [6]. The null genotype of GSTT1 gene causes complete absence of GST enzymes activity, decreases the ability of detoxifying electrophilic compounds, and may increase the susceptibility to various cancers [7]. Thus, there is obvious biochemical evidence for the relationship of GSTT1 null genotype with prostate cancer risk. Besides, GSTT1 null genotype has also been studied extensively in terms of susceptibility for other malignancies. Previous meta-analyses have yielded significant associations of GSTT1 null genotype with colorectal cancer [63], breast cancer [64], lung cancer [65] and hepatocellular carcinoma [66], which further suggest GSTT1 null genotype plays an important role the carcinogenesis and can affect the host susceptibility to common malignancies.
Some limitations of this study should be acknowledged. Firstly, significant between-study heterogeneity was detected in overall analysis, and subgroup analyses in Caucasians and Africans. There are several aspects could explain the significant heterogeneity: the different proportion of BPH patients in the controls, different definition of control group and ethnicity. In addition, it is known that a shorter androgen signaling pathway exist in these individuals from African population, which contributes to prostate cancer risk and may bias the real estimate of the gene-cancer associations in Africans [67]. Therefore, more studies with estimates adjusting for those known risk factors are needed. Secondly, meta-analysis remains retrospective research that is subject to the methodological deficiencies of the included studies. We minimized the likelihood of bias by developing a detailed protocol before initiating the study, by performing a meticulous search for published studies, and by using explicit methods for study selection, data extraction, and data analysis. Thirdly, some misclassification bias is possible. Most studies could not exclude latent prostate cancer cases in the control group. Finally, we could not address gene-gene and gene-environmental interactions. The latter may be important for genes that code proteins with detoxifying function, but would require detailed information on exposures to various potential carcinogens and individual-level data and would be most meaningful only for common exposures that are found to be strong risk factors for the disease.
In conclusion, this study is, to the best our knowledge, the largest meta-analysis of the association between GSTT1 null genotype and prostate cancer risk. This meta-analysis demonstrates that GSTT1 null genotype is associated with prostate cancer susceptibility, and GSTT1 null genotype contributes to increased risk of prostate cancer.
Supporting Information
PRISMA 2009 flow diagram in this meta-analysis.
(TIF)
PRISMA Checklist.
(DOC)
Acknowledgments
We thank all the people who give technical support and useful discussion of the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61 2: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Damber JE, Aus G (2008) Prostate cancer. Lancet 371 9625: 1710–21. [DOI] [PubMed] [Google Scholar]
- 3. Dong LM, Potter JD, White E, Ulrich CM, Cardon LR, et al. (2008) Genetic susceptibility to cancer: the role of polymorphisms in candidate genes. JAMA 299 20: 2423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45: 51–88. [DOI] [PubMed] [Google Scholar]
- 5. Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC (2004) Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal 6 2: 289–300. [DOI] [PubMed] [Google Scholar]
- 6. Strange RC, Spiteri MA, Ramachandran S, Fryer AA (2001) Glutathione-S-transferase family of enzymes. Mutat Res 482 1–2: 21–6. [DOI] [PubMed] [Google Scholar]
- 7. Hayes JD, Strange RC (2000) Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology 61 3: 154–66. [DOI] [PubMed] [Google Scholar]
- 8. Katoh T, Yamano Y, Tsuji M, Watanabe M (2008) Genetic polymorphisms of human cytosol glutathione S-transferases and prostate cancer. Pharmacogenomics 9 1: 93–104. [DOI] [PubMed] [Google Scholar]
- 9. Mo Z, Gao Y, Cao Y, Gao F, Jian L (2009) An updating meta-analysis of the GSTM1, GSTT1, and GSTP1 polymorphisms and prostate cancer: a HuGE review. Prostate 69 6: 662–88. [DOI] [PubMed] [Google Scholar]
- 10. Choubey VK, Sankhwar SN, Tewari R, Sankhwar P, Singh BP, et al. (2012) Null genotypes at the GSTM1 and GSTT1 genes and the risk of benign prostatic hyperplasia: A case-control study and a meta-analysis. Prostate [DOI] [PubMed] [Google Scholar]
- 11. Catsburg C, Joshi AD, Corral R, Lewinger JP, Koo J, et al. (2012) Polymorphisms in carcinogen metabolism enzymes, fish intake, and risk of prostate cancer. Carcinogenesis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thakur H, Gupta L, Sobti RC, Janmeja AK, Seth A, et al. (2011) Association of GSTM1T1 genes with COPD and prostate cancer in north Indian population. Mol Biol Rep 38 3: 1733–9. [DOI] [PubMed] [Google Scholar]
- 13. Safarinejad MR, Shafiei N, Safarinejad SH (2011) Glutathione S-transferase gene polymorphisms (GSTM1, GSTT1, GSTP1) and prostate cancer: a case-control study in Tehran, Iran. Prostate Cancer Prostatic Dis 14 2: 105–13. [DOI] [PubMed] [Google Scholar]
- 14. Rodrigues IS, Kuasne H, Losi-Guembarovski R, Fuganti PE, Gregorio EP, et al. (2011) Evaluation of the influence of polymorphic variants CYP1A1 2B, CYP1B1 2, CYP3A4 1B, GSTM1 0, and GSTT1 0 in prostate cancer. Urol Oncol 29 6: 654–63. [DOI] [PubMed] [Google Scholar]
- 15. Norskov MS, Frikke-Schmidt R, Bojesen SE, Nordestgaard BG, Loft S, et al. (2011) Copy number variation in glutathione-S-transferase T1 and M1 predicts incidence and 5-year survival from prostate and bladder cancer, and incidence of corpus uteri cancer in the general population. Pharmacogenomics J 11 4: 292–9. [DOI] [PubMed] [Google Scholar]
- 16. Kwon DD, Lee JW, Han DY, Seo IY, Park SC, et al. (2011) Relationship between the Glutathione-S-Transferase P1, M1, and T1 Genotypes and Prostate Cancer Risk in Korean Subjects. Korean J Urol 52 4: 247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar V, Yadav CS, Datta SK, Singh S, Ahmed RS, et al. (2011) Association of GSTM1 and GSTT1 polymorphism with lipid peroxidation in benign prostate hyperplasia and prostate cancer: a pilot study. Dis Markers 30 4: 163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ashtiani ZO, Hasheminasab SM, Ayati M, Goulian BS, Modarressi MH (2011) Are GSTM1, GSTT1 and CAG repeat length of androgen receptor gene polymorphisms associated with risk of prostate cancer in Iranian patients? Pathol Oncol Res 17 2: 269–75. [DOI] [PubMed] [Google Scholar]
- 19. Steinbrecher A, Rohrmann S, Timofeeva M, Risch A, Jansen E, et al. (2010) Dietary glucosinolate intake, polymorphisms in selected biotransformation enzymes, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 19 1: 135–43. [DOI] [PubMed] [Google Scholar]
- 20. Souiden Y, Mahdouani M, Chaieb K, Elkamel R, Mahdouani K (2010) Polymorphisms of glutathione-S-transferase M1 and T1 and prostate cancer risk in a Tunisian population. Cancer Epidemiol 34 5: 598–603. [DOI] [PubMed] [Google Scholar]
- 21. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22 4: 719–48. [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7 3: 177–88. [DOI] [PubMed] [Google Scholar]
- 23. Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10 1: 101–29. [Google Scholar]
- 24. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327 7414: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315 7109: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hemelrijck MV, Rohrmann S, Steinbrecher A, Kaaks R, Teucher B, et al. (2012) Heterocyclic Aromatic Amine [HCA] Intake and Prostate Cancer Risk: Effect Modification by Genetic Variants. Nutr Cancer 64 5: 704–13. [DOI] [PubMed] [Google Scholar]
- 27. Sivonova M, Waczulikova I, Dobrota D, Matakova T, Hatok J, et al. (2009) Polymorphisms of glutathione-S-transferase M1, T1, P1 and the risk of prostate cancer: a case-control study. J Exp Clin Cancer Res 28: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lavender NA, Benford ML, VanCleave TT, Brock GN, Kittles RA, et al. (2009) Examination of polymorphic glutathione S-transferase (GST) genes, tobacco smoking and prostate cancer risk among men of African descent: a case-control study. BMC Cancer 9: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lima MM Jr, Oliveira MN, Granja F, Trindade AC, De Castro Santos LE, et al. (2008) Lack of association of GSTT1, GSTM1, GSTO1, GSTP1 and CYP1A1 polymorphisms for susceptibility and outcome in Brazilian prostate cancer patients. Folia Biol (Praha) 54 3: 102–8. [PubMed] [Google Scholar]
- 30. Davydova NA, Dmitrieva AI, Selivanov SP, Tkachenko SB, Kovalik TA, et al. (2008) [Polymorphism of glutathion-S-transferase genes in patients with prostatic cancer]. Urologiia 2: 26–9. [PubMed] [Google Scholar]
- 31. Mallick S, Romana M, Blanchet P, Multigner L (2007) GSTM1 and GSTT1 polymorphisms and the risk of prostate cancer in a Caribbean population of African descent. Urology 69 6: 1165–9. [DOI] [PubMed] [Google Scholar]
- 32. Cunningham JM, Hebbring SJ, McDonnell SK, Cicek MS, Christensen GB, et al. (2007) Evaluation of genetic variations in the androgen and estrogen metabolic pathways as risk factors for sporadic and familial prostate cancer. Cancer Epidemiol Biomarkers Prev 16 5: 969–78. [DOI] [PubMed] [Google Scholar]
- 33. Yang J, Wu HF, Zhang W, Gu M, Hua LX, et al. (2006) Polymorphisms of metabolic enzyme genes, living habits and prostate cancer susceptibility. Front Biosci 11: 2052–60. [DOI] [PubMed] [Google Scholar]
- 34. Silig Y, Pinarbasi H, Gunes S, Ayan S, Bagci H, et al. (2006) Polymorphisms of CYP1A1, GSTM1, GSTT1, and prostate cancer risk in Turkish population. Cancer Invest 24 1: 41–5. [DOI] [PubMed] [Google Scholar]
- 35. Mittal RD, Mishra DK, Mandhani A (2006) Evaluating polymorphic status of glutathione-S-transferase genes in blood and tissue samples of prostate cancer patients. Asian Pac J Cancer Prev 7 3: 444–6. [PubMed] [Google Scholar]
- 36. Lindstrom S, Zheng SL, Wiklund F, Jonsson BA, Adami HO, et al. (2006) Systematic replication study of reported genetic associations in prostate cancer: Strong support for genetic variation in the androgen pathway. Prostate 66 16: 1729–43. [DOI] [PubMed] [Google Scholar]
- 37. Agalliu I, Langeberg WJ, Lampe JW, Salinas CA, Stanford JL (2006) Glutathione S-transferase M1, T1, and P1 polymorphisms and prostate cancer risk in middle-aged men. Prostate 66 2: 146–56. [DOI] [PubMed] [Google Scholar]
- 38. Wang YL, Jiang J, Wang LF, Liu YF (2005) Polymorphisms of glutathione-S-transferase genes GSTMl and GSTTl and prostate cancer risk in Chinese population. ACTA ACADEMIAE MEDICINAE MILITARIS TERTIAE 27 10: 1039–41. [Google Scholar]
- 39. Srivastava DS, Mandhani A, Mittal B, Mittal RD (2005) Genetic polymorphism of glutathione S-transferase genes (GSTM1, GSTT1 and GSTP1) and susceptibility to prostate cancer in Northern India. BJU Int 95 1: 170–3. [DOI] [PubMed] [Google Scholar]
- 40. Nam RK, Zhang WW, Jewett MA, Trachtenberg J, Klotz LH, et al. (2005) The use of genetic markers to determine risk for prostate cancer at prostate biopsy. Clin Cancer Res 11 23: 8391–7. [DOI] [PubMed] [Google Scholar]
- 41. Komiya Y, Tsukino H, Nakao H, Kuroda Y, Imai H, et al. (2005) Human glutathione S-transferase A1, T1, M1, and P1 polymorphisms and susceptibility to prostate cancer in the Japanese population. J Cancer Res Clin Oncol 131 4: 238–42. [DOI] [PubMed] [Google Scholar]
- 42. Caceres DD, Iturrieta J, Acevedo C, Huidobro C, Varela N, et al. (2005) Relationship among metabolizing genes, smoking and alcohol used as modifier factors on prostate cancer risk: exploring some gene-gene and gene-environment interactions. Eur J Epidemiol 20 1: 79–88. [DOI] [PubMed] [Google Scholar]
- 43. Mittal RD, Srivastava DS, Mandhani A, Kumar A, Mittal B (2004) Polymorphism of GSTM1 and GSTT1 genes in prostate cancer: a study from North India. Indian J Cancer 41 3: 115–9. [PubMed] [Google Scholar]
- 44. Medeiros R, Vasconcelos A, Costa S, Pinto D, Ferreira P, et al. (2004) Metabolic susceptibility genes and prostate cancer risk in a southern European population: the role of glutathione S-transferases GSTM1, GSTM3, and GSTT1 genetic polymorphisms. Prostate 58 4: 414–20. [DOI] [PubMed] [Google Scholar]
- 45. Joseph MA, Moysich KB, Freudenheim JL, Shields PG, Bowman ED, et al. (2004) Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr Cancer 50 2: 206–13. [DOI] [PubMed] [Google Scholar]
- 46. Nakazato H, Suzuki K, Matsui H, Koike H, Okugi H, et al. (2003) Association of genetic polymorphisms of glutathione-S-transferase genes (GSTM1, GSTT1 and GSTP1) with familial prostate cancer risk in a Japanese population. Anticancer Res 23 3C: 2897–902. [PubMed] [Google Scholar]
- 47. Kidd LC, Woodson K, Taylor PR, Albanes D, Virtamo J, et al. (2003) Polymorphisms in glutathione-S-transferase genes (GST-M1, GST-T1 and GST-P1) and susceptibility to prostate cancer among male smokers of the ATBC cancer prevention study. Eur J Cancer Prev 12 4: 317–20. [DOI] [PubMed] [Google Scholar]
- 48. Beer TM, Evans AJ, Hough KM, Lowe BA, McWilliams JE, et al. (2002) Polymorphisms of GSTP1 and related genes and prostate cancer risk. Prostate Cancer Prostatic Dis 5 1: 22–7. [DOI] [PubMed] [Google Scholar]
- 49. Murata M, Watanabe M, Yamanaka M, Kubota Y, Ito H, et al. (2001) Genetic polymorphisms in cytochrome P450 (CYP) 1A1, CYP1A2, CYP2E1, glutathione S-transferase (GST) M1 and GSTT1 and susceptibility to prostate cancer in the Japanese population. Cancer Lett 165 2: 171–7. [DOI] [PubMed] [Google Scholar]
- 50. Kote-Jarai Z, Easton D, Edwards SM, Jefferies S, Durocher F, et al. (2001) Relationship between glutathione S-transferase M1, P1 and T1 polymorphisms and early onset prostate cancer. Pharmacogenetics 11 4: 325–30. [DOI] [PubMed] [Google Scholar]
- 51. Gsur A, Haidinger G, Hinteregger S, Bernhofer G, Schatzl G, et al. (2001) Polymorphisms of glutathione-S-transferase genes (GSTP1, GSTM1 and GSTT1) and prostate-cancer risk. Int J Cancer 95 3: 152–5. [DOI] [PubMed] [Google Scholar]
- 52. Steinhoff C, Franke KH, Golka K, Thier R, Romer HC, et al. (2000) Glutathione transferase isozyme genotypes in patients with prostate and bladder carcinoma. Arch Toxicol 74 9: 521–6. [DOI] [PubMed] [Google Scholar]
- 53. Kelada SN, Kardia SL, Walker AH, Wein AJ, Malkowicz SB, et al. (2000) The glutathione S-transferase-mu and -theta genotypes in the etiology of prostate cancer: genotype-environment interactions with smoking. Cancer Epidemiol Biomarkers Prev 9 12: 1329–34. [PubMed] [Google Scholar]
- 54. Rebbeck TR, Walker AH, Jaffe JM, White DL, Wein AJ, et al. (1999) Glutathione S-transferase-mu (GSTM1) and -theta (GSTT1) genotypes in the etiology of prostate cancer. Cancer Epidemiol Biomarkers Prev 8 4 Pt 1: 283–7. [PubMed] [Google Scholar]
- 55. Autrup JL, Thomassen LH, Olsen JH, Wolf H, Autrup H (1999) Glutathione S-transferases as risk factors in prostate cancer. Eur J Cancer Prev 8 6: 525–32. [DOI] [PubMed] [Google Scholar]
- 56. Taioli E, Flores-Obando RE, Agalliu I, Blanchet P, Bunker CH, et al. (2011) Multi-institutional prostate cancer study of genetic susceptibility in populations of African descent. Carcinogenesis 32 9: 1361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nock NL, Bock C, Neslund-Dudas C, Beebe-Dimmer J, Rundle A, et al. (2009) Polymorphisms in glutathione S-transferase genes increase risk of prostate cancer biochemical recurrence differentially by ethnicity and disease severity. Cancer Causes Control 20 10: 1915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nock NL, Liu X, Cicek MS, Li L, Macarie F, et al. (2006) Polymorphisms in polycyclic aromatic hydrocarbon metabolism and conjugation genes, interactions with smoking and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 15 4: 756–61. [DOI] [PubMed] [Google Scholar]
- 59. Dong JT (2006) Prevalent mutations in prostate cancer. J Cell Biochem 97 3: 433–47. [DOI] [PubMed] [Google Scholar]
- 60. Nam RK, Zhang WW, Trachtenberg J, Jewett MA, Emami M, et al. (2003) Comprehensive assessment of candidate genes and serological markers for the detection of prostate cancer. Cancer Epidemiol Biomarkers Prev 12 12: 1429–37. [PubMed] [Google Scholar]
- 61. Figer A, Friedman T, Manguoglu AE, Flex D, Vazina A, et al. (2003) Analysis of polymorphic patterns in candidate genes in Israeli patients with prostate cancer. Isr Med Assoc J 5 10: 741–5. [PubMed] [Google Scholar]
- 62. Ntais C, Polycarpou A, Ioannidis JP (2005) Association of GSTM1, GSTT1, and GSTP1 gene polymorphisms with the risk of prostate cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 14 1: 176–81. [PubMed] [Google Scholar]
- 63. Xu D, Yan S, Yin J, Zhang P (2011) Null genotype of GSTT1 contributes to colorectal cancer risk in Asian populations: evidence from a meta-analysis. Asian Pac J Cancer Prev 12 9: 2279–84. [PubMed] [Google Scholar]
- 64. Chen XX, Zhao RP, Qiu LX, Yuan H, Mao C, et al. (2011) Glutathione S-transferase T1 polymorphism is associated with breast cancer susceptibility. Cytokine 56 2: 477–80. [DOI] [PubMed] [Google Scholar]
- 65. Ye Z, Song H, Higgins JP, Pharoah P, Danesh J (2006) Five glutathione s-transferase gene variants in 23,452 cases of lung cancer and 30,397 controls: meta-analysis of 130 studies. PLoS Med 3 4: e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang B, Huang G, Wang D, Li A, Xu Z, et al. (2010) Null genotypes of GSTM1 and GSTT1 contribute to hepatocellular carcinoma risk: evidence from an updated meta-analysis. J Hepatol 53 3: 508–18. [DOI] [PubMed] [Google Scholar]
- 67. Gilligan T, Manola J, Sartor O, Weinrich SP, Moul JW, et al. (2010) Absence of a correlation of androgen receptor gene CAG repeat length and prostate cancer risk in an African-American population. Clin Prostate Cancer 3 2: 98–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 flow diagram in this meta-analysis.
(TIF)
PRISMA Checklist.
(DOC)