Abstract
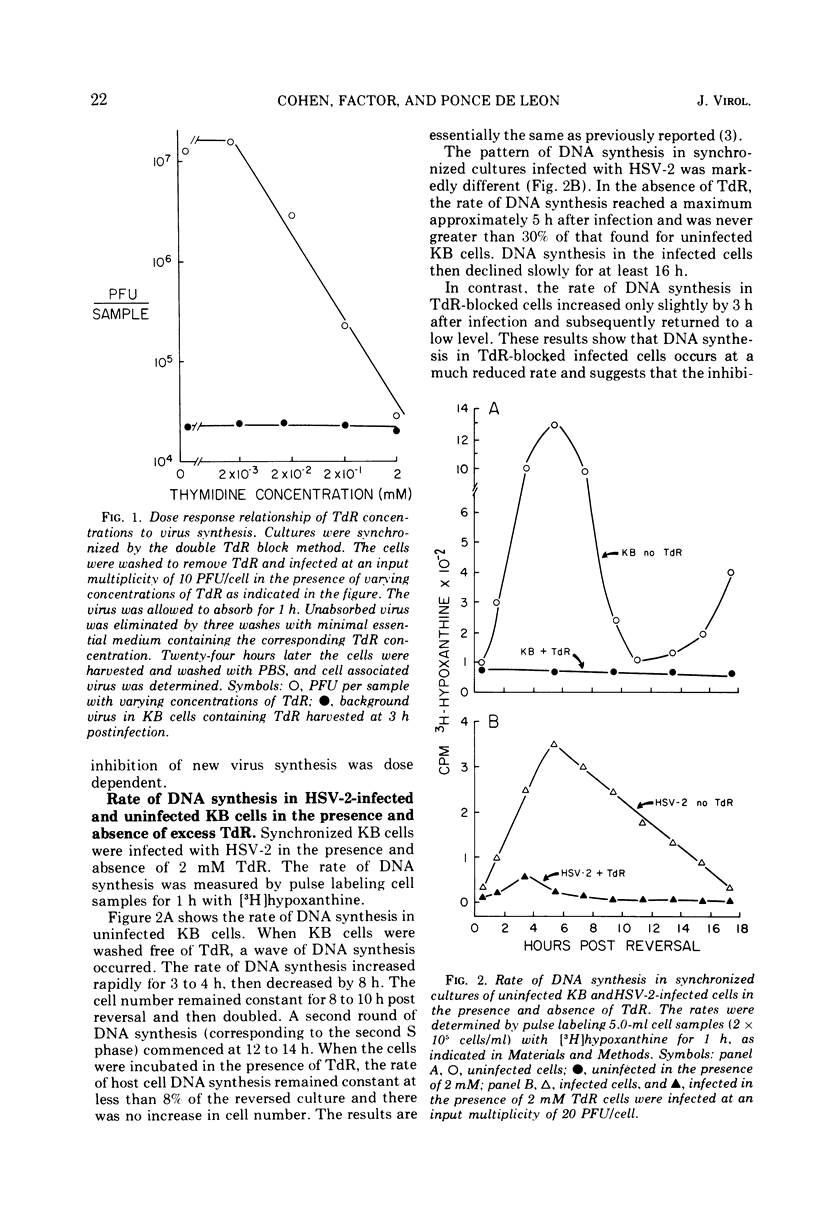
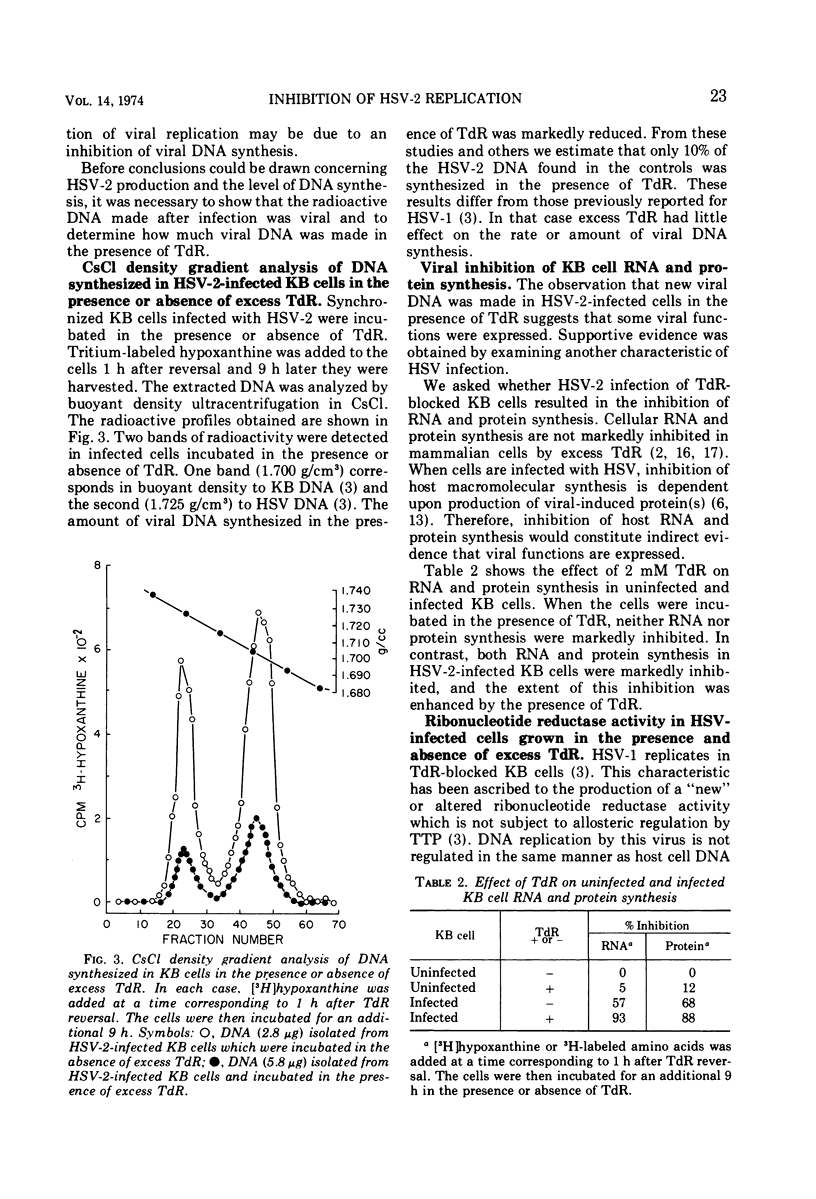
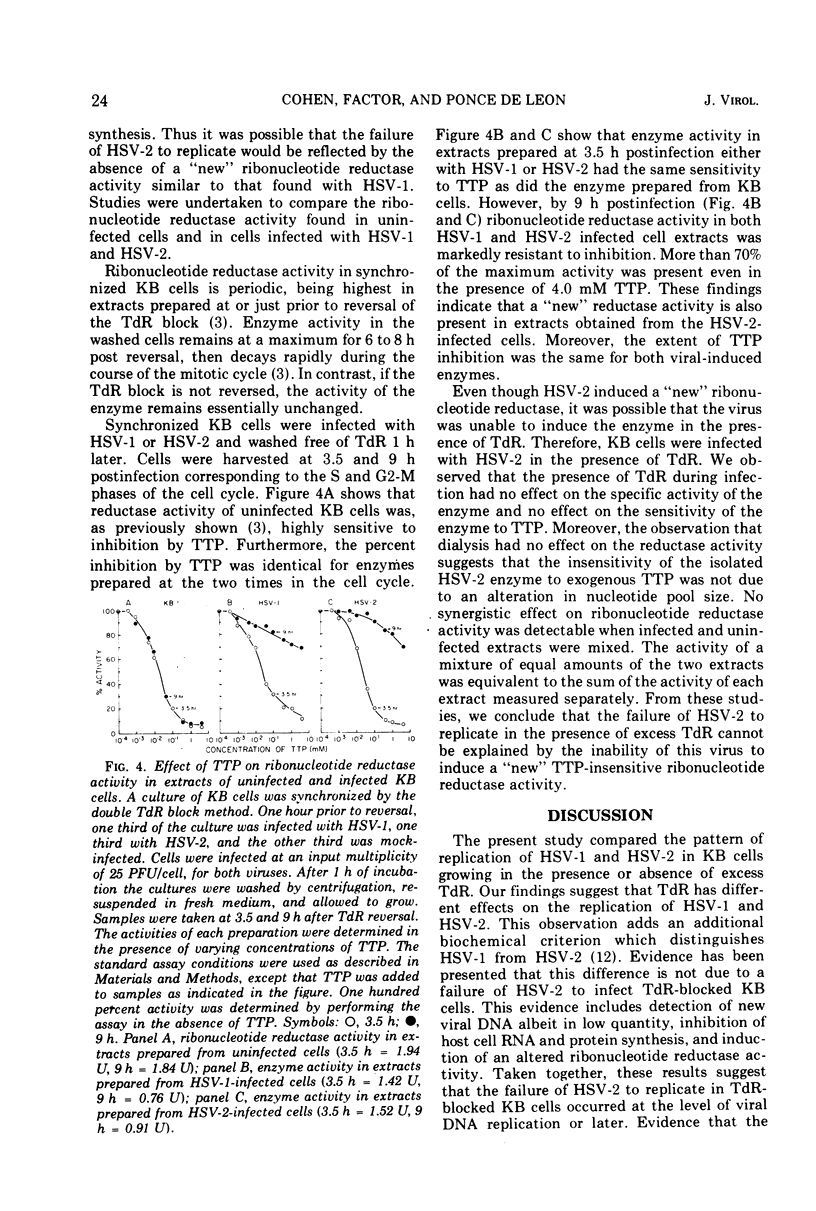
Replication of herpes simplex virus type 2 (HSV-2) was impeded in KB cells which were blocked in their capacity to synthesize DNA by 2 mM thymidine (TdR). The degree of inhibition was dependent upon the concentration of TdR. In marked contrast, HSV-1 is able to replicate under these conditions. The failure of HSV-2 to replicate is probably due to the inhibition of viral DNA synthesis; there was a marked reduction in the rate of DNA synthesis as well as the total amount of HSV-2 DNA made in the presence of 2 mM TdR. We postulated that the effect of TdR on viral replication occurs at the level of ribonucleotide reductase in a manner similar to KB cells. However, unlike KB cells, an altered ribonucleotide reductase activity, highly resistant to thymidine triphosphate inhibition, was found in extracts of HSV-2-infected KB cells. This activity was present in HSV-2-infected cells incubated in the presence or absence of TdR. Ribonucleotide reductase activity in extracts of HSV-1-infected KB cells showed a similar resistance to thymidine triphosphate inhibition. These results suggest that the effect of TdR on HSV-2 replication occurs at a stage of DNA synthesis other than reduction of cytidine nucleotides to deoxycytidine nucleotides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjursell G., Reichard P. Effects of thymidine on deoxyribonucleoside triphosphate pools and deoxyribonucleic acid synthesis in Chinese hamster ovary cells. J Biol Chem. 1973 Jun 10;248(11):3904–3909. [PubMed] [Google Scholar]
- Cohen G. H. Ribonucleotide reductase activity of synchronized KB cells infected with herpes simplex virus. J Virol. 1972 Mar;9(3):408–418. doi: 10.1128/jvi.9.3.408-418.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Vaughan R. K., Lawrence W. C. Deoxyribonucleic acid synthesis in synchronized mammalian KB cells infected with herpes simplex virus. J Virol. 1971 Jun;7(6):783–791. doi: 10.1128/jvi.7.6.783-791.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M. Phosphorylation of 5-bromodeoxycytidine in cells infected with herpes simplex virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3788–3792. doi: 10.1073/pnas.70.12.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. S. A brief review of the biochemistry of herpesvirus-host cell interaction. Cancer Res. 1973 Jun;33(6):1393–1398. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence W. C. Evidence for a relationship between equine abortion (herpes) virus deoxyribonucleic acid synthesis and the S phase of the KB cell mitotic cycle. J Virol. 1971 Jun;7(6):736–748. doi: 10.1128/jvi.7.6.736-748.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T., Shiman R., Rapp F. Deoxythymidine kinase from rabbit kidney cells infected with herpes simplex virus types 1 and 2. Intervirology. 1973;1(2):80–95. doi: 10.1159/000148835. [DOI] [PubMed] [Google Scholar]
- Pages J., Manteuil S., Stehelin D., Fiszman M., Marx M., Girard M. Relationship between replication of simian virus 40 DNA and specific events of the host cell cycle. J Virol. 1973 Jul;12(1):99–107. doi: 10.1128/jvi.12.1.99-107.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Duff R. In vitro cell transformation by herpesviruses. Fed Proc. 1972 Nov-Dec;31(6):1660–1668. [PubMed] [Google Scholar]
- Setlow P. Deoxyribonucleic acid synthesis and deoxynucleotide metabolism during bacterial spore germination. J Bacteriol. 1973 Jun;114(3):1099–1107. doi: 10.1128/jb.114.3.1099-1107.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog K. L., Nordenskjöld B. A., Bjursell K. G. Deoxyribonucleoside-triphosphate pools and DNA synthesis in synchronized hamster cells. Eur J Biochem. 1973 Mar 15;33(3):428–432. doi: 10.1111/j.1432-1033.1973.tb02699.x. [DOI] [PubMed] [Google Scholar]
- Studzinski G. P., Lambert W. C. Thymidine as a synchronizing agent. I. Nucleic acid and protein formation in synchronous HeLa cultures treated with excess thymidine. J Cell Physiol. 1969 Apr;73(2):109–117. doi: 10.1002/jcp.1040730204. [DOI] [PubMed] [Google Scholar]
- XEROS N. Deoxyriboside control and synchronization of mitosis. Nature. 1962 May 19;194:682–683. doi: 10.1038/194682a0. [DOI] [PubMed] [Google Scholar]



