Abstract
Background
A phase III clinical trial has already shown the survival benefits of postoperative chemotherapy in gastric cancer. However, there are limited published data concerning the elderly. This study aims to investigate the use of adjuvant chemotherapy for gastric cancer after D2 gastrectomy among the elderly and identify its impact on survival.
Methods
We retrospectively reviewed 360 patients who had undergone D2 gastrectomy, aged 65 years or older, with non-metastatic gastric cancer in a single institution. We analyzed the predictors and survival benefits of adjuvant chemotherapy use in the elderly. Further, we analyzed the survival benefits of adjuvant chemotherapy by dividing the patients into groups according to disease stages and chemotherapeutic regimens.
Results
Among the 360 patients, only 34.7% of patients received adjuvant chemotherapy. Age, tumor location, lymph node involvement and tumor invasion were associated with the receipt of adjuvant chemotherapy. Adjuvant chemotherapy improved the overall survival for non-metastatic elderly patients (HR 0.60, 95%CI 0.42–0.83, P = 0.003). Significant survival benefits were found with adjuvant chemotherapy in stage III patients (HR 0.67, 95%CI 0.47–0.97, P = 0.033), but not in stage I patients or in stage II patients (HR 0.52, 95%CI 0.21–1.30 P = 0.161). Compared to adjuvant chemotherapy without platinum, no significant survival benefits were observed with platinum-containing chemotherapy (HR 0.84, 95%CI 0.49–1.45, P = 0.530). Besides adjuvant chemotherapy, other independent prognostic factors of survival included tumor location, tumor size, histologic grade, depth of tumor invasion, and lymph node status.
Conclusions
This study demonstrated the survival benefits of adjuvant fluoropyrimidine-based chemotherapy among the elderly patients with non-metastatic gastric cancer after D2 gastrectomy. However, due to the limitations of this study, further well-designed prospective studies with large populations are needed to confirm these findings and identify the patients that can tolerate and benefit from adjuvant chemotherapy.
Introduction
As the second leading cause of cancer-related death worldwide, gastric cancer is a health threat. The only available treatment to cure gastric cancer is surgical resection, but the prognosis of the patients that have received radical resection is still poor. Therefore, adjuvant therapy is considered to improve the survival for patients with curative gastric resection. Several meta-analyses suggested that adjuvant therapy resulted in a small but significant advantage [1], [2], [3]. The U.S. Intergroup study(INT-0116) and the United Kingdom Medical Council MAGIC trial showed survival benefits from postoperative chemoradiotherapy and perioperative chemotherapy, respectively [4], [5]. However, most of the gastrectomy procedures in both studies were limited to D0 or D1 resections, while D2 resection only comprised about 10% of the cases. Therefore, the evidence cannot be generalized to patients in East Asia where D2 gastrectomy is the standard treatment. The recently reported five-year outcome of the Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer (ACTS-GC) study and the Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer (CLASSIC) study confirmed the efficacy of the S-1 and XELOX regimen in adjuvant chemotherapy after D2 surgery compared with surgery alone [6], [7].
As the population continues to age, gastric cancer in the elderly will become an increasing clinical challenge. However, the elderly are less likely to receive the recommended treatment because of their shorter life expectancy, higher incidence of comorbidities, and higher risk of complications [8], [9]. Thus the effectiveness of postoperative chemotherapy for elderly patients (aged 65 or older) should be carefully assessed to avoid overtreatment or undertreatment. However, there are limited published data concerning the elderly. The elderly are underrepresented in clinical trials, and detailed clinicopathological data are limited.
This study aims to investigate the use of adjuvant chemotherapy among patients aged 65 years or older with non-metastatic gastric cancer after D2 gastrectomy in a single center and explore the survival benefits from chemotherapy.
Materials and Methods
Patients
We retrospectively analyzed the medical records of 360 patients who were pathologically proved and diagnosed with non-metastatic staged I through IV(M0) gastric adenocarcinoma according to American Joint Committee on Cancer (AJCC, seventh edition). All of the patients received curative gastrectomy with D2 nodal dissection by experienced surgeons in the Cancer Center of Sun Yat-Sen University between 1999 and 2007. We excluded 162 patients because of the presence of residual tumors or palliative surgery. There were 216 patients excluded because of distal metastasis, refusal of surgery, intolerance to surgery, death within 1 month of surgery, primary or secondary tumor history, neoadjuvant chemotherapy or adjuvant radiotherapy.
The adjuvant chemotherapy was mainly based on fluoropyrimidine, with or without a combination of other agents that included oxaliplatin, cisplatin, lobaplatin, paclitaxel, docetaxal, etoposide, doxorubicin, epirubicin, leucovorin, mitomycin. We recorded the cycles and regimens of chemotherapy of all patients in detail.
We retrospectively reviewed the clinicopathological features, including age at diagnosis, gender, comorbidities, tumor size, tumor location, depth of tumor invasion, number of retrieved lymph nodes, number of metastatic lymph nodes, histological grade, and tumor stage. We also collected the follow-up data of tumor recurrence or metastases and survival. The last follow-up data were collected prior to April 30th, 2012.
Statistical analysis
All statistical analyses were performed using SPSS software, version 16.0. The clinicopathologic characteristics of the cohort were described, and the differences of these characteristics between the treatment groups were compared. Ordinal data were compared using the chi-square test, and continuous data were compared using a Mann-Whitney U test. A logistic regression model was created, using the treatment group as a dependent variable and potential clinicopathologic factors as covariables. Unadjusted Kaplan-Meier survival curves with log rank testing were generated to compare the survival benefits between treatment groups of all patients, and for each stage. The disease-specific survival would be analyzed by the Cox proportional hazards regression once the survival curves of the treatment groups separated. All potential predictors were taken into consideration for overall survival analysis. The hazard ratio and 95% confidence interval were used to estimate the role of each independent predictor of survival.
Results
Clinicopathologic characteristics
The median age of the 360 elderly patients with non-metastatic resectable gastric carcinoma was 69 years (range 65 to 83). The male to female ratio was 2.71∶1. Most patients in this study were more likely to have a Charlson comorbidity score [10] of 1 or less (96.1%). The patients were likely to have tumors in the proximal third of the stomach (60.6%), tumors smaller than 5 cm (60.3%), tumors that were poorly differentiated (65.0%), and tumors corresponding to AJCC stage II to III (33.1% and 55.8%, respectively). Among the 360 patients, 5.6% had T1 disease, 10.6% had T2 disease, 10.0% had T3 disease, and 73.9% had T4 disease. In this study, 22.5% of patients had N1 lymph node involvement, 20.0% had N2, and 18.1% had N3 (Table 1). Only 34.7% of patients (n = 125) received adjuvant chemotherapy, 6.9% (n = 25) received monochemotherapy and 27.8% (n = 100) received polychemotherapy. Among those 125 patients who received chemotherapy, 98.4% (n = 123) received fluoropyrimidine-based chemotherapy, 64.8% (n = 81) received chemotherapy included platinum, 9.6% (n = 12) received chemotherapy included paclitaxel or docetaxal, 5.6% (n = 7) received chemotherapy included etoposide, 4% (n = 5) received chemotherapy included doxorubicin or epirubicin, and 4% (n = 5) received chemotherapy included mitomycin. The number of the cycles ranged from 1 to 10, and there was a median of 3 cycles.
Table 1. Clinicopathological characteristics of patients aged 65 or older with non-metastatic resected gastric cancer.
| Characteristic | Total | Surgery only | Surgery+chemotherapy | P |
| No. of patients | 360 | 235 | 125 | |
| Age at diagnosis | 0.003* | |||
| 65–70 years | 189(52.5) | 110(46.9) | 79(63.2) | |
| >70 years | 171(47.5) | 125(53.1) | 46(36.8) | |
| Gender | 0.535 | |||
| Male | 263(73.1) | 169(71.9) | 94(75.2) | |
| Female | 97(26.9) | 66(28.1) | 31(24.8) | |
| Charlson score | 0.144 | |||
| 0 | 270(75) | 171(72.7) | 99(79.2) | |
| 1 | 76(21.1) | 52(22.1) | 24(19.2) | |
| 2+ | 14(3.9) | 12(5.1) | 2(1.6) | |
| Time of diagnosis | 0.276 | |||
| 1999–2003 | 161(44.7) | 110(46.8) | 51(40.8) | |
| 2004–2007 | 199(55.3) | 125(53.2) | 74(59.2) | |
| Location of tumor | <0.001* | |||
| Proximal | 218(60.6) | 165(70.2) | 53(42.4) | |
| Distal | 142(39.4) | 70(29.8) | 72(57.6) | |
| Size | 0.862 | |||
| ≤5 cm | 217(60.3) | 144(61.3) | 75(60.0) | |
| >5 cm | 141(39.2) | 91(38.7) | 50(40.0) | |
| Grade | 0.001* | |||
| Well or moderately differentiated | 126(35.0) | 96(40.9) | 30(24.0) | |
| Poorly differentiated or undifferentiated | 234(65.0) | 139(59.1) | 95(76.0) | |
| T category | 0.012* | |||
| T1 | 20(5.6) | 18(7.7) | 2(1.6) | |
| T2 | 38(10.6) | 27(11.5) | 11(8.8) | |
| T3 | 36(10.0) | 24(10.2) | 12(9.6) | |
| T4 | 266(73.9) | 166(70.6) | 100(80.0) | |
| N category | <0.001* | |||
| N0 | 142(39.4) | 116(49.4) | 26(20.8) | |
| N1 | 81(22.5) | 50(21.3) | 31(24.8) | |
| N2 | 72(20.0) | 42(17.9) | 30(24.0) | |
| N3 | 65(18.1) | 27(11.5) | 38(30.4) | |
| AJCC stage(7th) | <0.001* | |||
| I | 40(11.1) | 34(14.5) | 6(4.8) | |
| II | 119(33.1) | 91(38.7) | 28(22.4) | |
| III | 201(55.8) | 110(46.8) | 91(72.8) |
Abbreviations: CI, confidence interval; T, tumor; N, lymph node; AJCC, American Joint Committee on Cancer.
Statistically significant.
Predictors of receiving adjuvant chemotherapy
By univariate logistic regression analysis, age (reference age 65–70 years: age>70 years, odd ratio (OR) 0.51, 95% confidence interval (CI) 0.33–0.80, P = 0.003), tumor location (reference location proximal: distal, OR 3.20, 95% CI 2.04–5.03, P<0.001), depth of tumor invasion (reference depth of tumor invasion T1:T2, OR 3.67, 95%CI 0.73–18.54, P = 0.116; T3, OR 4.50, 95%CI 0.89–22.67, P = 0.068; T4, OR 5.42, 95%CI 1.23–23.86, P = 0.025), and lymph node involvement (reference lymph node status N0:N1, OR 2.77, 95%CI 1.49–5.13, P = 0.001; N2, OR 3.19, 95%CI 1.69–6.00, P<0.001; N3, OR 6.28, 95%CI 3.27–12.04, P<0.001) were associated with receiving adjuvant chemotherapy after D2 resection. The patient's gender (reference gender male: female, OR 0.84, 95% CI 0.51–1.39, P = 0.504), Charlson index (reference score 0: 1, OR 0.80, 95%CI 0.46–1.37, P = 0.414; 2+, OR 0.29, 95%CI 0.06–1.31, P = 0.108), tumor size (reference size ≤5 cm: >5 cm, OR 1.06, 95%CI 0.68–1.64, P = 0.813), and diagnosis time(reference time 1999–2003: 2004–2007, OR 1.28, 95%CI 0.82–1.98, P = 0.275) were not associated with the receipt of adjuvant therapy(Table 2). Age (reference age 65–70 years: age>70 years, adjusted OR 0.51, 95%CI 0.31–0.83, P = 0.007), tumor location (reference location proximal: distal, OR 3.96, 95% CI 2.40–6.58, P<0.001),lymph node involvement (reference lymph node status N0:N1, OR 2.36, 95%CI 1.22–4.57, P = 0.011; N2, OR 3.24, 95%CI 1.63–6.44, P = 0.001; N3, OR 5.19, 95%CI 2.56–10.50, P<0.001), and depth of tumor invasion (reference depth of tumor invasion T1:T2, OR 3.58, 95%CI 0.65–19.62, P = 0.142; T3, OR 5.12, 95%CI 0.91–28.67, P = 0.063; T4, OR 5.29, 95%CI 1.11–25.27, P = 0.037) were still associated with the receipt of adjuvant therapy by multivariate logistic regression analysis (Table 3).
Table 2. Univariate predictors of receiving adjuvant chemotherapy among the 360 patients aged 65 or older with non-metastatic resected gastric cancer.
| Covariate | Odd Ratio | 95% Cl | P |
| Age at diagnosis | 0.007* | ||
| 65–70 years | 1.00 | Referent | - |
| >70 years | 0.51 | 0.33–0.80 | 0.003* |
| Gender | |||
| Male | 1.00 | Referent | - |
| Female | 0.84 | 0.51–1.39 | 0.504 |
| Time of diagnosis | |||
| 1999–2003 | 1.00 | Referent | - |
| 2004–2007 | 1.28 | 0.82–1.98 | 0.275 |
| Chalson index | |||
| 0 | 1.00 | Referent | - |
| 1 | 0.80 | 0.46–1.37 | 0.414 |
| 2+ | 0.29 | 0.06–1.31 | 0.108 |
| Location of tumor | |||
| Proximal | 1.00 | Referent | - |
| Distal | 3.20 | 2.04–5.03 | <0.001* |
| Size | |||
| ≤5 cm | 1.00 | Referent | - |
| >5 cm | 1.06 | 0.68–1.64 | 0.813 |
| Grade | |||
| Well or moderately differentiated | 1.00 | Referent | - |
| Poorly differentiated or undifferentiated | 2.19 | 1.35–3.56 | 0.002* |
| T category | |||
| T1 | 1.00 | Referent | - |
| T2 | 3.67 | 0.73–18.54 | 0.116 |
| T3 | 4.50 | 0.89–22.67 | 0.068 |
| T4 | 5.42 | 1.23–23.86 | 0.025* |
| N category | |||
| N0 | 1.00 | Referent | - |
| N1 | 2.77 | 1.49–5.13 | 0.001* |
| N2 | 3.19 | 1.69–6.00 | <0.001* |
| N3 | 6.28 | 3.27–12.04 | <0.001* |
| AJCC stage | |||
| I | 1.00 | Referent | - |
| II | 1.74 | 0.66–4.58 | 0.259 |
| III | 4.69 | 1.89–11.66 | 0.001* |
Abbreviations: CI, confidence interval; T, tumor; N, lymph node; AJCC, American Joint Committee on Cancer.
Statistically significant.
Table 3. Factors associated with the receipt of adjuvant chemotherapy by multivariate analysis for patients aged 65 or older with non-metastatic resected gastric cancer.
| Covariate | Adjusted Odd Ratio | 95% Cl | P |
| Age at diagnosis | |||
| 65–70 years | 1.00 | Referent | - |
| >70 years | 0.51 | 0.31–0.83 | 0.007* |
| Location of tumor | |||
| Proximal | 1.00 | Referent | - |
| Distal | 3.96 | 2.40–6.58 | <0.001* |
| T category | |||
| T1 | 1.00 | Referent | - |
| T2 | 3.58 | 0.65–19.62 | 0.142 |
| T3 | 5.12 | 0.91–28.67 | 0.063 |
| T4 | 5.29 | 1.11–25.27 | 0.037* |
| N category | |||
| N0 | 1.00 | Referent | - |
| N1 | 2.36 | 1.22–4.57 | 0.011* |
| N2 | 3.24 | 1.63–6.44 | 0.001* |
| N3 | 5.19 | 2.56–10.50 | <0.001* |
Abbreviations: CI, confidence interval; T, tumor; N, lymph node.
Statistically significant.
Survival analysis
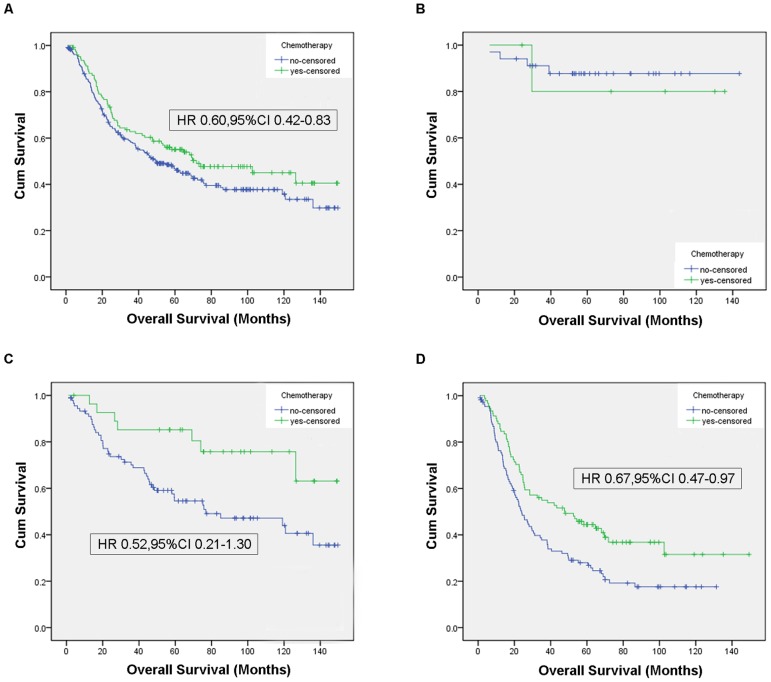
During the follow-up period, 195(55%) patient died from tumors, and 6 died from other diseases. The median followed-up was 46.7 months after gastectomy (range 1.1 to 149.3). Unadjusted Kaplan-Meier survival curves were constructed for all patients, and for each stage (Figure 1). The median survival in this study was 55.9 months among the patient who received surgery plus adjuvant chemotherapy and 39.6 months among the patients who received surgery alone (P = 0.083, Figure 1A). The median months of survival for surgery alone group vs. surgery/adjuvant chemotherapy group was 57.4 months vs. 88.0 months for stage I (P = 0.72, Figure 1B), 49.9 vs. 77.6 for stage II (P = 0.026, Figure 1C), 22.4 vs. 46.5 for stage III (P = 0.004, Figure 1D). The median months of survival for platinum-containing chemotherapy group vs. chemotherapy without platinum group was 53.2 months vs. 62.8 months (P = 0.314, Figure 2).
Figure 1. Unadjusted Kaplan-Meier curves, overall and by stages.
Figure 1A shows the unadjusted Kaplan-Meier curves of the 360 elderly patients. Figure 1B shows the unadjusted Kaplan-Meier curves of the elderly patients with stage I gastric adenocarcinoma. Figure 1C shows the unadjusted Kaplan-Meier curves of the elderly patients with stage II gastric adenocarcinoma. Figure 1D shows the unadjusted Kaplan-Meier curves of the elderly patients with stage III gastric adenocarcinoma. The Hazard ratio were generated from a multivariate Cox regression model adjusted for age, gender, Chalson index, tumor site, histologic grade, size, and treatment group.
Figure 2. Unadjusted Kaplan-Meier curves, by chemotherapeutic regimens.
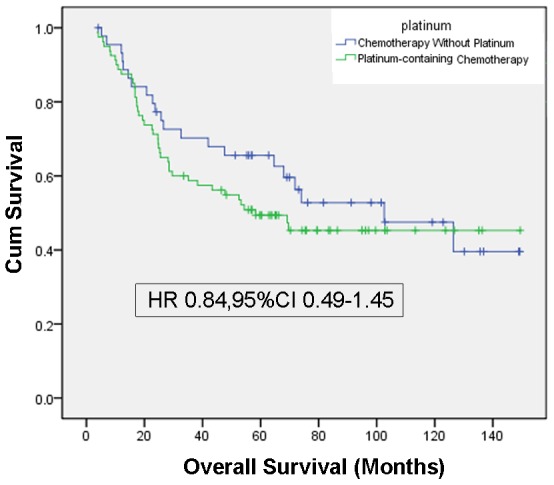
Figure 2 shows the unadjusted Kaplan-Meier curves of the elderly patients who received platinum-containing chemotherapy and chemotherapy without platinum. The Hazard ratio were generated from a multivariate Cox regression model adjusted for age, gender, Chalson index, tumor site, histologic grade, size, depth of tumor invasion, and lymph node involvement.
Using Cox proportional hazards multivariate analysis, proximal tumor (P = 0.001), larger tumor (P = 0.004), higher histologic grade (P = 0.004), deeper tumor invasion (P = 0.001), and lymph node involvement (P<0.001) were negative independent prognostic factors. On multivariate analysis, adjuvant chemotherapy was associated with a significantly reduced risk of death (HR 0.60, 95%CI 0.42–0.83, P = 0.003) among this elderly population (Table 4). Significant survival benefits were found with adjuvant chemotherapy in stage III patients (HR 0.67, 95%CI 0.47–0.97, P = 0.033), but not in stage I patients or in stage II patients (HR 0.52, 95%CI 0.21–1.30 P = 0.161)(Figure 1). Compared to adjuvant chemotherapy without platinum, no significant survival benefits were observed with platinum-containing chemotherapy (HR 0.84, 95%CI 0.49–1.45)(Figure 2)
Table 4. Multivariate prognostic factors for overall mortality among the 360 patients with non-metastatic resected gastric cancer.
| Variate | HR | 95% Cl | P |
| Age at diagnosis | |||
| 65–70 years | 1.00 | Referent | - |
| >70 years | 1.22 | 0.91–1.62 | 0.19 |
| Gender | |||
| Male | 1.00 | Referent | - |
| Female | 0.87 | 0.62–1.22 | 0.421 |
| Chalson index | |||
| 0 | 1.00 | Referent | - |
| 1 | 1.12 | 0.79–1.58 | 0.521 |
| 2+ | 0.68 | 0.28–1.69 | 0.409 |
| Time of diagnosis | |||
| 1999–2003 | 1.00 | Referent | - |
| 2004–2007 | 0.98 | 0.72–1.33 | 0.886 |
| Location of tumor | |||
| Proximal | 1.00 | Referent | - |
| Distal | 0.55 | 0.39–0.77 | 0.001* |
| Size | |||
| ≤5 cm | 1.00 | Referent | - |
| >5 cm | 1.54 | 1.14–2.07 | 0.004* |
| Grade | |||
| Well or moderately differentiated | 1.00 | Referent | - |
| Poorly differentiated or undifferentiated | 1.64 | 1.18–2.30 | 0.004* |
| T category | |||
| T1/T2 | 1.00 | Referent | - |
| T3 | 1.65 | 0.75–3.65 | 0.217 |
| T4 | 2.90 | 1.54–5.45 | 0.001* |
| N category | |||
| N0 | 1.00 | Referent | - |
| N1 | 2.03 | 1.34–3.07 | 0.001* |
| N2 | 2.22 | 1.46–3.37 | <0.001* |
| N3 | 3.49 | 2.25–5.39 | <0.001* |
| Treatment | |||
| Surgery alone | 1.00 | Referent | - |
| Adjuvant chemotherapy | 0.60 | 0.42–0.83 | 0.003* |
Abbreviations: CI, confidence interval; T, tumor; N, lymph node.
Statistically significant.
Discussion
Although the 5-year outcome of a randomized phase III ACTS-GC study showed that postoperative adjuvant treatment could improve overall survival in patients with gastric cancer who had undergone D2 gastrectomy, the subgroup analysis indicated that the survival benefits decrease as patient age increases [7]. Moreover, there were no statistically significant effects of postoperative chemotherapy for patients aged older than 70(HR 0.779, 95%CI 0.527–1.151). In the CLASSIC study, for which the collection of the overall survival data are not yet completed, the subgroup analysis of the 3-year disease-free survival showed more benefits of chemotherapy for patients 65 or older [6]. It was reported that disease-free survival was strongly correlated with overall survival based on the GASTRIC data. Therefore, the delivery of adjuvant chemotherapy to elderly patients with gastric cancer after D2 gastrectomy remains a dilemma for physicians.
In this single center study, we examined the clinicopathological characteristics associated with delivery of adjuvant chemotherapy to 360 elderly patients with non-metastatic gastric cancer after D2 gastrectomy, providing insight into the current treatment recommendations for the elderly in China. We also evaluated the efficacy of delivering adjuvant chemotherapy to elderly patients in China and observed that patients had a significant survival benefit from postoperative chemotherapy in this study cohort.
In our study, only approximately one third of the patients received postoperative chemotherapy. Within this population, the patients aged older than 70 were less likely to receive chemotherapy compared to younger patients. This finding is similar to several other cancers, including prostate cancer, ovarian cancer, breast cancer and colorectal cancer [11], [12], [13], [14], where elderly patients are less likely to receive adjuvant therapy even if the treatments are known to be effective and tolerable. There may be various reasons of this result. Older patients may have more comorbid diseases, they might be less tolerant to chemotherapy, and they may prefer to undergo less treatment in their relatively limited lifetime [15], [16]. In the CLASSIC study, 56% of patients who received the XELOX chemotherapy regimen experienced grade 3 or 4 adverse events, such as neutropenia, thrombocytopenia, vomiting, nausea [6]. In the ACT-GC study, 22.8% of patients with monochemotherapy experienced grade 3 or 4 adverse events [7]. The possible treatment toxicity may be an important barrier for the elderly to receive adjuvant therapy, especially when the patient has a decrease in physical status due to gastectomy.
Hoffman et al. [17]analyzed 1,023 elderly patients with resected gastric cancer in the linked Surveillance, Epidemiology, and End Result-Medicare database (SEER), and they reported that patients diagnosed during the later months of the study were more likely to receive adjuvant chemoradiation therapy. This was mainly due to the report of the INT-0116 trial in 2000. Strauss et al. reported similar results in a study including 1,993 elderly patients with non-metastatic gastric adenocarcinoma. However, the diagnosis time did not significantly influence the administration of adjuvant chemotherapy to patients during the study period in our study. The possible explanation may be that chemotherapy was not considered as a standard or recommended adjuvant treatment until the 3-year result of ACTS-GC study published in 2007.
It was reported in a previous study of the elderly that having fewer comorbidities was an independent factor associated with receipt of adjuvant treatment [17], [18]. However, most of the patients in our study had a Charlson comorbidity score of 0 or 1, because they were required to be able to tolerate the surgery. Thus, the comorbidity score was not a statistically significant predictor of receiving chemotherapy in our study cohort. The size, location, and histologic grade of the tumor are potential prognostic factors of the survival of resectable gastric cancer, so that they are associated with the use of adjuvant chemotherapy. However, the prognostic importance of these parameters is inconsistent in different studies [18], [19], [20], influencing the predictive value of receipt of adjuvant chemotherapy. In our study, the location and histologic grade of the tumor was significantly associated with the receipt of adjuvant chemotherapy of the elderly.
In our study cohort, we found that adjuvant chemotherapy significantly improved overall survival for non-metastatic elderly patients with a median survival of 16.3 months longer (HR 0.60, 95%CI 0.42–0.83). In subgroup analysis stratified by disease stages, treatment was associated with an HR for tumor-specific death of 0.67 and a median survival that was 24.1 months longer compared to surgery alone for stage III patients. For stage II patients, there was no significant benefit for group that received the surgery plus postoperative chemotherapy. However, the P value of the log-rank test for stage II patients between treatment groups was 0.026, and the survival curves of two treatment groups were separate. What's more, the patients with stage II disease trended toward survival improvement with adjuvant chemotherapy with an HR smaller than 1 and a longer median survival. We suggest that this result may be caused by the small proportion of patients (n = 28) who received chemotherapy with stage II disease. Similarly, the analysis of the role of adjuvant chemotherapy in stage I disease in this cohort was limited by small numbers of patients. The survival benefits of adjuvant chemotherapy reported based on the data of the phase III trial ACTS-GC study were similar (HR 0.669, CI 0.540–0.828) [7]. Several meta-analyses also reported that chemotherapy could reduce the risk of death in gastric cancer after curative resection (HR ranged from 0.72 to 0.90) [1], [3], [21], [22]. After evaluating the results from 20 randomized clinical trials, Mari, et al. reported that chemotherapy reduced the risk of death by 18% (HR 0.82, 95% CI 0.75–0.89) [1]. Liu, et al. evaluated 19 qualified clinical randomized trials and reporded that adjuvant chemotherapy could improve the survival rate after curative resection(RR 0.85, 95%CI 0.80–0.90) [22]. Paoletti, et al. recently published the result of a meta-analysis of survival data from 17 trials involving 3,838 patients and demonstrated that adjuvant chemotherapy was associated with a statistically significant benefit for overall survival (HR 0.82, 95% CI 0.76∼0.90) [2]. Similar studies with opposite conclusion also reported recently. Hoffman et al. reported that elderly patients may not gain a survival benefit from the administration of adjuvant chemoradiation after analyzing elderly patients with resected gastric cancer in the SEER-Medicare database [17]. But most of the gastrectomy procedures in these studies were limited to D0 or D1 resections, and the postoperative treatment is adjuvant chemoradiation. Dittmar et al. recently reported that there was a trend toward longer survival for the elderly patients who underwent gastric resection plus chemotherapy [23]. However, the gastric resection in this retrospective study included both radical and palliative resection.
So far, there is no standard regimen for adjuvant chemotherapy. Some suggested that patients would benefit from adjuvant chemotherapy and should not be influenced by different schemes, including monotherapy, double therapy and triple therapy [1], [22]. Some also reported that statistically significant benefits could be detected from a fluoropyrimidine-based monochemotherapy regimen and a fluoropyrimidine-based polychemotherapy regimen but not from chemotherapy regimens without fluoropyrimidines [2]. In our study, 123 out of 125(98.4%) patients received fluoropyrimidine-based monochemotherapy or polychemotherapy. We examined the monochemotherapy group and the polychemotherapy group versus surgery alone and only detected a significant survival benefit of polychemotherapy over surgery alone. The absence of a survival benefit in monochemotherapy group may be due to the small proportion of patients (n = 25) that received monochemotherapy. We examined the overall survival between the patients received platinum-containing chemotherapy and chemotherapy without platinum, and did not detect significant survival differences between groups. The number of patients who received chemotherapy included taxane (n = 12), etoposide (n = 7), anthracyclines (n = 5), or mitomycin (n = 5) was small to undergo further analysis. We suggested that elderly patients with resected gastric cancer may gain a survival benefit from the fluoropyrimidine-based chemotherapy, with or without platinum.
In addition to adjuvant chemotherapy, there were other independent negative predictors of survival for the elderly, including a more proximal site, larger tumor, poorer differentiation, deeper tumor invasion, and lymph node involvement. These prognostic factors have been reported in previous contexts as well [20], [24].
Our study has various limitations. First, this study is based on retrospective data. However, the bias may be reduced by the fact that these data were collected from a single institution. Second, the patients received various chemotherapy regimens as new regimens were developed during the period of this study. Thus, we cannot conclude a specific regimen recommended from this study. Third, the proportion of patients with early disease who received chemotherapy or those who received monochemotherapy was small, which makes it difficult to stratify the patients for further analysis.
In this retrospective, single institution study, we demonstrated the survival benefits of adjuvant fluoropyrimidine-based chemotherapy present among the elderly with non-metastatic gastric cancer after D2 gastrectomy. Further well-designed prospective studies with larger populations are needed to confirm these findings. Elderly patients are highly variable in their functional status, reserve capacity, and comorbidity. Thus further studies are needed to identify the patients that can tolerate and gain benefit from adjuvant chemotherapy.
Acknowledgments
We gratefully thank the staff members of the Department of Medical Oncology at Sun Yat-sen University Cancer Center for their suggestions and assistance. The study was conducted in the Medical Oncology department, Sun Yat-sen University, Cancer Center.
Funding Statement
The authors have no support or funding to report.
References
- 1. Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, et al. (2000) Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato Digerente). Ann Oncol 11: 837–843. [DOI] [PubMed] [Google Scholar]
- 2. Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, et al. (2010) Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA 303: 1729–1737. [DOI] [PubMed] [Google Scholar]
- 3. Panzini I, Gianni L, Fattori PP, Tassinari D, Imola M, et al. (2002) Adjuvant chemotherapy in gastric cancer: a meta-analysis of randomized trials and a comparison with previous meta-analyses. Tumori 88: 21–27. [PubMed] [Google Scholar]
- 4. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, et al. (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345: 725–730. [DOI] [PubMed] [Google Scholar]
- 5. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, et al. (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355: 11–20. [DOI] [PubMed] [Google Scholar]
- 6. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, et al. (2012) Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 379: 315–321. [DOI] [PubMed] [Google Scholar]
- 7. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, et al. (2011) Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29: 4387–4393. [DOI] [PubMed] [Google Scholar]
- 8. Saif MW, Makrilia N, Zalonis A, Merikas M, Syrigos K (2010) Gastric cancer in the elderly: an overview. Eur J Surg Oncol 36: 709–717. [DOI] [PubMed] [Google Scholar]
- 9. Goodwin JS, Hunt WC, Samet JM (1993) Determinants of cancer therapy in elderly patients. Cancer 72: 594–601. [DOI] [PubMed] [Google Scholar]
- 10. Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 11. Cohen SM, Neugut AI (2004) Adjuvant therapy for rectal cancer in the elderly. Drugs Aging 21: 437–451. [DOI] [PubMed] [Google Scholar]
- 12. Bouvier A-M, Jooste V, Bonnetain F, Cottet V, Bizollon M-H, et al. (2008) Adjuvant treatments do not alter the quality of life in elderly patients with colorectal cancer. Cancer 113: 879–886. [DOI] [PubMed] [Google Scholar]
- 13. Barthelemy P, Heitz D, Mathelin C, Polesi H, Asmane I, et al. (2011) Adjuvant chemotherapy in elderly patients with early breast cancer. Impact of age and comprehensive geriatric assessment on tumor board proposals. Crit Rev Oncol Hematol 79: 196–204. [DOI] [PubMed] [Google Scholar]
- 14. Samet J, Hunt WC, Key C, Humble CG, Goodwin JS (1986) Choice of cancer therapy varies with age of patient. JAMA 255: 3385–3390. [PubMed] [Google Scholar]
- 15. Sakuramoto S, Yamashita K, Watanabe M (2009) Newly emerging standard chemotherapies for gastric cancer and clinical potential in elderly patients. World J Gastrointest Oncol 1: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dudeja V, Habermann EB, Zhong W, Tuttle TM, Vickers SM, et al. (2011) Guideline recommended gastric cancer care in the elderly: insights into the applicability of cancer trials to real world. Ann Surg Oncol 18: 26–33. [DOI] [PubMed] [Google Scholar]
- 17. Hoffman KE, Neville BA, Mamon HJ, Kachnic LA, Katz MS, et al. (2012) Adjuvant therapy for elderly patients with resected gastric adenocarcinoma: population-based practices and treatment effectiveness. Cancer 118: 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strauss J, Hershman DL, Buono D, McBride R, Clark-Garvey S, et al. (2010) Use of Adjuvant 5-Fluorouracil and Radiation Therapy After Gastric Cancer Resection Among the Elderly and Impact on Survival. International Journal of Radiation Oncology*Biology*Physics 76: 1404–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu CC, Levison DA, Dunn JA, Ward LC, Demonakou M, et al. (1995) Pathological prognostic factors in the second British Stomach Cancer Group trial of adjuvant therapy in resectable gastric cancer. Br J Cancer 71: 1106–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Msika S, Benhamiche AM, Jouve JL, Rat P, Faivre J (2000) Prognostic factors after curative resection for gastric cancer. A population-based study. Eur J Cancer 36: 390–396. [DOI] [PubMed] [Google Scholar]
- 21. Zhao SL, Fang JY (2008) The role of postoperative adjuvant chemotherapy following curative resection for gastric cancer: a meta-analysis. Cancer Invest 26: 317–325. [DOI] [PubMed] [Google Scholar]
- 22. Liu TS, Wang Y, Chen SY, Sun YH (2008) An updated meta-analysis of adjuvant chemotherapy after curative resection for gastric cancer. Eur J Surg Oncol 34: 1208–1216. [DOI] [PubMed] [Google Scholar]
- 23. Dittmar Y, Rauchfuss F, Gotz M, Scheuerlein H, Jandt K, et al. (2012) Impact of clinical and pathohistological characteristics on the incidence of recurrence and survival in elderly patients with gastric cancer. World journal of surgery 36: 338–345. [DOI] [PubMed] [Google Scholar]
- 24. Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK (1998) Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer 1: 125–133. [DOI] [PubMed] [Google Scholar]