Abstract
Mutation of Escherichia coli K12 HfrH to resistance to fluorophenylalanine resulted in changes in the plaque morphology of bacteriophage MS2 on this strain and led to an increased efficiency of propagation of the phage in liquid cultures. Evidence was obtained that the mutation resulted in inhibition of early lysis in infected cells and that lysis involved the production of a lysozyme. Genetic studies suggested that the observed pleiotropy of the resistance mutation was due to informational suppression.
Full text
PDF
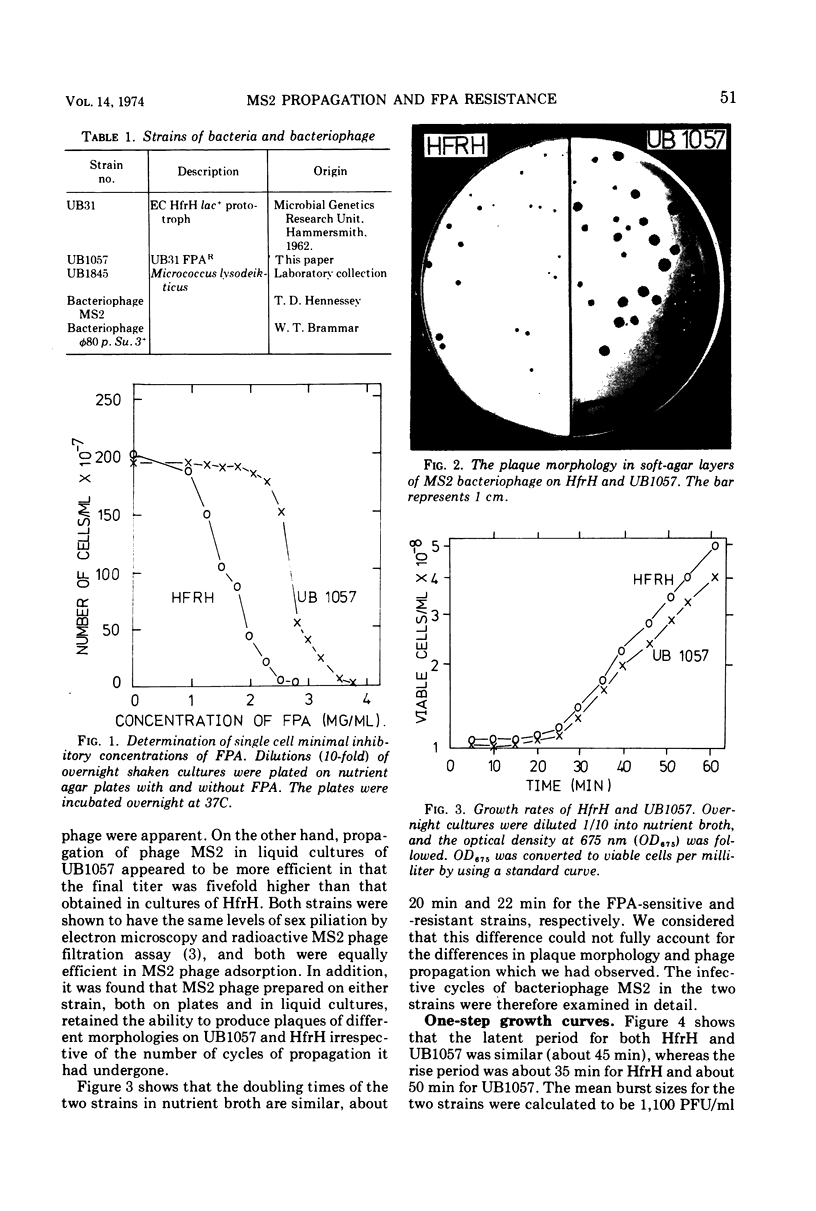
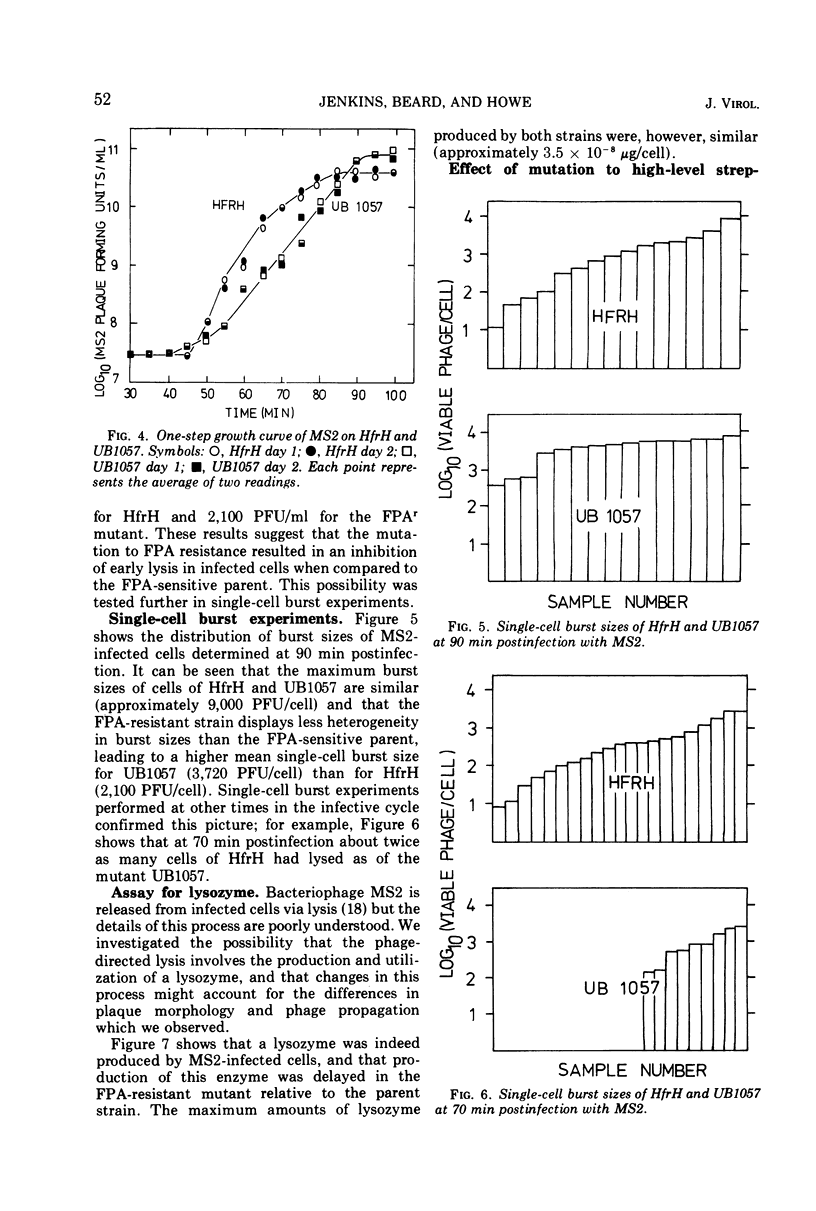
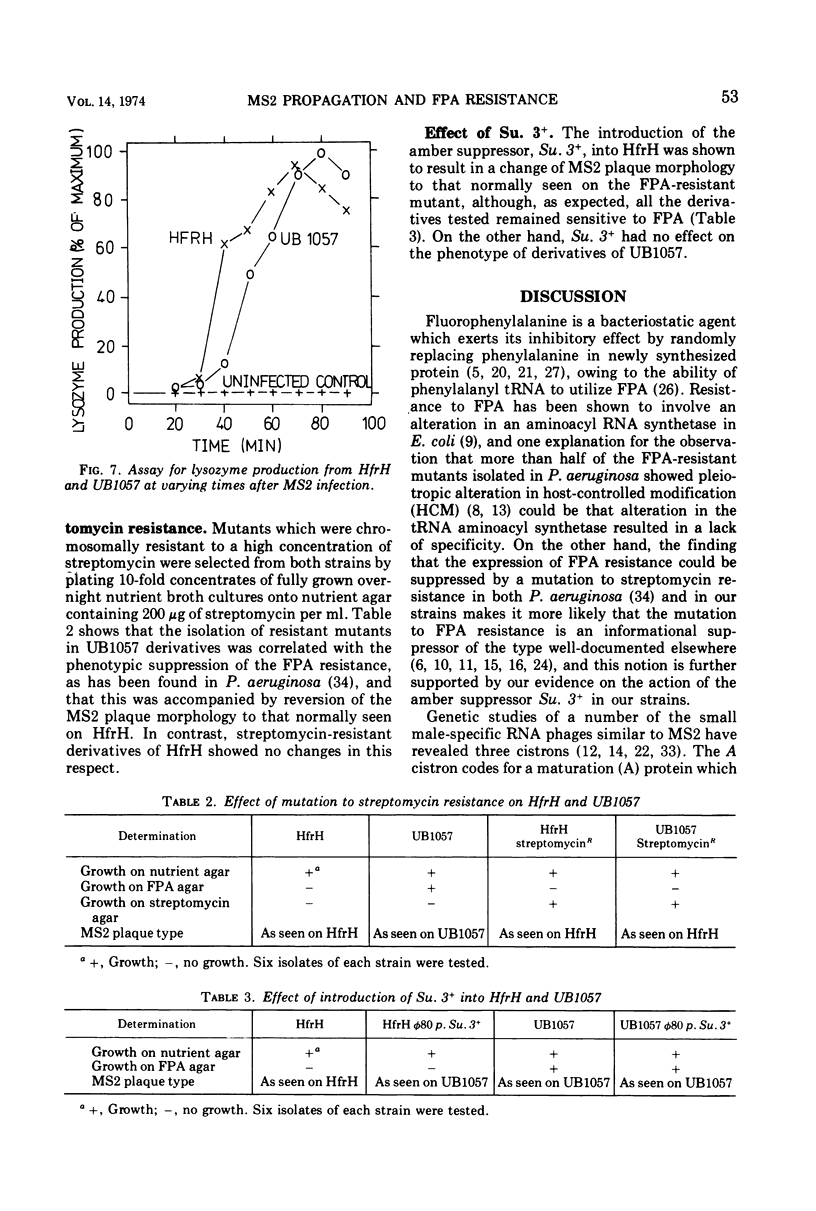


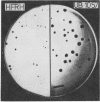
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard J. P., Howe T. G., Richmond M. H. Purification of sex pili from Escherichia coli carrying a derepressed F-like R factor. J Bacteriol. 1972 Sep;111(3):814–820. doi: 10.1128/jb.111.3.814-820.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., MUNIER R. Effets des analogues structuraux d'aminoacides sur la croissance, la synthèse de protéines et la synthèse d'enzymes chez Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):347–356. doi: 10.1016/0006-3002(59)90007-1. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MUNIER R. Incorporation d'analogues structuraux d'aminoacides dans les protéines bactériennes. Biochim Biophys Acta. 1956 Sep;21(3):592–593. doi: 10.1016/0006-3002(56)90207-4. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Polarity in vitro. J Mol Biol. 1967 Nov 28;30(1):213–217. doi: 10.1016/0022-2836(67)90254-9. [DOI] [PubMed] [Google Scholar]
- Davies J. Streptomycin and the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:665–670. doi: 10.1101/sqb.1966.031.01.085. [DOI] [PubMed] [Google Scholar]
- Dennert G., Hertel R., Deppe G., Henning U. Action of an amber suppressor gene carried by phage phi 80. Z Vererbungsl. 1965;97(3):243–254. doi: 10.1007/BF02035930. [DOI] [PubMed] [Google Scholar]
- Dunn N. W., Holloway B. W. Pleiotrophy of p-fluorophenylalanine-resistant and antibiotic hypersensitive mutants of Pseudomonas aeruginosa. Genet Res. 1971 Oct;18(2):185–197. doi: 10.1017/s0016672300012593. [DOI] [PubMed] [Google Scholar]
- FANGMAN W. L., NEIDHARDT F. C. DEMONSTRATION OF AN ALTERED AMINOACYL RIBONUCLEIC ACID SYNTHETASE IN A MUTANT OF ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1839–1843. [PubMed] [Google Scholar]
- Gartner T. K., Orias E. Effects of mutations to streptomycin resistance on the rate of translation of mutant genetic information. J Bacteriol. 1966 Mar;91(3):1021–1028. doi: 10.1128/jb.91.3.1021-1028.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner T. K., Orias E., Lannan J. E., Beeson J., Reid P. J. The molecular basis of suppression in an ochre suppressor strain possessing altered ribosomes. Proc Natl Acad Sci U S A. 1969 Mar;62(3):946–951. doi: 10.1073/pnas.62.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N. Site of synthesis of viral ribonucleic acid and phage assembly in MS2-infected Escherichia coli. J Mol Biol. 1968 May 28;34(1):149–163. doi: 10.1016/0022-2836(68)90241-6. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Stanisich V. Pseudomonas genetics. Annu Rev Genet. 1971;5:425–446. doi: 10.1146/annurev.ge.05.120171.002233. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Ishizawa M., Endo H. Su-II-specific restriction of amber suppression by mutation to streptomycin resistance. J Mol Biol. 1968 Apr 28;33(2):513–516. doi: 10.1016/0022-2836(68)90209-x. [DOI] [PubMed] [Google Scholar]
- LEDERBERG E. M., CAVALLI-SFORZA L., LEDERBERG J. INTERACTION OF STREPTOMYCIN AND A SUPPRESSOR FOR GALACTOSE FERMENTATION IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Apr;51:678–682. doi: 10.1073/pnas.51.4.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEB T., ZINDER N. D. A bacteriophage containing RNA. Proc Natl Acad Sci U S A. 1961 Mar 15;47:282–289. doi: 10.1073/pnas.47.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E. Genetics of bacteriophage. Annu Rev Microbiol. 1962;16:205–240. doi: 10.1146/annurev.mi.16.100162.001225. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Zinder N. D. Replication of the RNA of Bacteriophage f2. Science. 1966 Apr 15;152(3720):372–377. doi: 10.1126/science.152.3720.372. [DOI] [PubMed] [Google Scholar]
- MUNIER R., COHEN G. N. Incorporation d'analogues structuraux d'aminoacides dans les protéines bactériennes au cours de leur synthèse in vivo. Biochim Biophys Acta. 1959 Feb;31(2):378–391. doi: 10.1016/0006-3002(59)90011-3. [DOI] [PubMed] [Google Scholar]
- Nathans D., Oeschger M. P., Eggen K., Shimura Y. Bacteriophage-specific proteins in e. Coli infected with an RNA bacteriophage. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1844–1851. doi: 10.1073/pnas.56.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuji N., Aono H. Effect of mutation to streptomycin resistance on amber suppressor genes. J Bacteriol. 1968 Jul;96(1):43–50. doi: 10.1128/jb.96.1.43-50.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. Random replacement of phenylalanine by p-fluorophenylalanine in alkaline phosphatase(s) formed during biosynthesis by E. coli. J Mol Biol. 1963 Apr;6:284–294. doi: 10.1016/s0022-2836(63)80089-3. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. The effect of amino acid analogues on growth and protein synthesis in microorganisms. Bacteriol Rev. 1962 Dec;26:398–420. doi: 10.1128/br.26.4.398-420.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelson E., Nathans D. Association of bacteriophage proteins and RNA in E. coli infected with MS2. Biochem Biophys Res Commun. 1967 Dec 29;29(6):842–849. doi: 10.1016/0006-291x(67)90296-3. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Steitz J. E. The reconstitution of infective bacteriophage R17. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1416–1421. doi: 10.1073/pnas.58.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe B., Holloway B. W. Genetic control of DNA specificity in Pseudomonas aeruginosa. Genet Res. 1968 Aug;12(1):99–102. doi: 10.1017/s0016672300011678. [DOI] [PubMed] [Google Scholar]
- SECHAUD J., KELLENBERGER E. Lyse précoce, provoquée par le chloroforme, chez les bactéries infectées par du bactériophage. Ann Inst Pasteur (Paris) 1956 Jan;90(1):102–106. [PubMed] [Google Scholar]
- Steitz J. A. Identification of the A protein as a structural component of bacteriophage R17. J Mol Biol. 1968 May 14;33(3):923–936. doi: 10.1016/0022-2836(68)90328-8. [DOI] [PubMed] [Google Scholar]
- Tooze J., Weber K. Isolation and characterization of amber mutants of bacteriophage R17. J Mol Biol. 1967 Sep 14;28(2):311–330. doi: 10.1016/s0022-2836(67)80012-3. [DOI] [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Ochoa S. Synthesis of virus-specific proteins in Escherichia coli infected with the RNA bacteriophage MS2. Eur J Biochem. 1967 Mar;1(1):3–11. doi: 10.1007/978-3-662-25813-2_2. [DOI] [PubMed] [Google Scholar]
- Waltho J. A., Holloway B. W. Suppression of fluorophenylalanine resistance by mutation to streptomycin resistance in Pseudomonas aeruginosa. J Bacteriol. 1966 Jul;92(1):35–42. doi: 10.1128/jb.92.1.35-42.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder N. D., Lyons L. B. Cell lysis: another function of the coat protein of the bacteriophage f2. Science. 1968 Jan 5;159(3810):84–86. [PubMed] [Google Scholar]