Abstract
Rab20 is a member of the Rab GTPase family, but its implication in macropinocytosis is unclear. We examined the spatiotemporal localization of Rab20 in RAW264 macrophages by the live-cell imaging of fluorescent protein-fused Rab20. It was shown that Rab20 was transiently associated with macropinosomal membranes. During the early stage of macropinosome formation, Rab20 was slightly localized on the circular ruffles (macropinocytic cups), the precursor forms of macropinosomes, and was increasingly recruited to the newly formed macropinosomes. Although Rab20 was colocalized with Rab5 and Rab21 on macropinosomal membranes, the association of Rab20 with macropinosomes persisted even after the dissociations of Rab5 and Rab21 from macropinosomal membranes. Rab20 was then colocalized with Rab7 and Lamp1, late endosomal/lysosomal markers, on macropinosomes for a while. Our data indicate that Rab20 is a novel component of macropinocytic pathway and functions at long-standing stages from early to late macropinosome maturation.
Keywords: Rab20, macropinocytosis, macrophage, live-cell imaging, endocytosis
I. Introduction
Macropinocytosis is a clathrin-independent endocytosis that accounts for the bulk fluid-phase uptake from the extracellular environment, and is an essential aspect of normal cell function. In macrophages and dendritic cells, which display high degrees of constitutive macropinocytosis, macropinocytosis plays an important role in antigen presentation [12, 14, 21, 22, 30]. In epithelial cells and fibroblasts, macropinocytosis is rarely seen, is but markedly induced after stimulation by epidermal growth factor (EGF) and platelet-derived growth factor (PDGF), respectively [12, 13], although the physiological role of macropinocytosis in these cell types is largely unknown. Moreover, some pathogenic bacteria and viruses exploit macropinocytosis to invade their host [1, 18, 19, 25]. Owing to this critical physiopathological relevance of macropinocytosis, the regulatory mechanisms of macropinosome formation and maturation have much attracted attention in recent years.
Macropinosome formation is initiated by actin-dependent cell-surface membrane ruffling [4, 14]. Subsequently, some membrane ruffles form circular ruffles (macropinocytic cups), and close into macropinosomes, which are large endocytic vacuoles (0.2–5 µm), by fission from the plasma membrane [3, 33]. Usually, newly formed macropinosomes gradually mature and finally fuse with lysosomes [23]. Phosphoinositides and small GTPases, such as Cdc42, Rac, ARF6, and Rab5 are known to regulate actin polymerization and remodeling in membrane ruffling and macropinocytosis [5, 8, 16, 21, 24, 32, 36]. Although the actin-dependent process of macropinosome formation is more similar in appearance and regulation to phagocytosis than to clathrin-dependent endocytosis, some of the mechanisms in macropinosome maturation are shared with those of the clathrin-dependent endocytic pathway. Knowledge about macropinocytosis has increased; nevertheless, the complex regulatory molecular components and signaling pathways of macropinocytosis still require detailed investigation.
Rab GTPases are key regulators of membrane trafficking in the endocytic pathways [12, 23, 35]. To date, more than 60 Rab members have been identified in the human genome [31]. Many of the Rab proteins that are localized on distinct intracellular vesicles have been reported to coordinate sequential steps of membrane transport [27, 29]. In the receptor-mediated, clathrin-dependent endocytic pathway, Rab5, Rab7 and Rab11 are localized on distinct subcellular compartments (i.e. early endosomes, late endosomes/lysosomes and recycling endosomes) [27, 29, 31, 35]. Rab5, the best-characterized member of Rab family, is involved in membrane traffic into and between early endosomes in the clathrin-dependent endocytosis [35]. Rab7 regulates transport from early to late endosomes and lysosomes [11, 20]. Rab11 participates in interactions between endosomal and plasma membrane by controlling membrane traffic through recycling endosomes [34].
In the macropinocytic pathway, Rab5 has been shown to be involved in macropinosome formation [16, 26]. Also, Rab34 is associated with membrane ruffles and is involved in macropinosome formation [32]. Furthermore, we have recently reported that Rab21, a close homolog of Rab5, localizes on macropinosomes in macrophages [9]. To date, a growing number of Rab GTPases have been identified on macropinosomal membranes [9, 12]. However, the involvements of many other members including Rab20 in macropinocytosis are not yet fully examined. Rab20, which is expressed in the apical side of kidney tubule, intestine epithelial cells and macrophages [6, 10, 17], is localized in the Golgi, ER region and endocytic vesicles, but its localization and function in the macropinocytic pathway remains uncharacterized [2, 7, 17].
In this study, we revealed that Rab20 is transiently associated with macropinosomes in RAW264 macrophages. This study provides the first evidence that Rab20 is a novel component of the macropinocytic pathway.
II. Materials and Methods
Reagents
Bovine serum albumin (BSA) and Dulbecco’s modified Eagle’s medium (DMEM) were obtained from Sigma Chemical (St Louis, MO). All other reagents were purchased from Wako Pure Chemicals (Osaka, Japan) or Nacalai Tesque (Kyoto, Japan), unless otherwise indicated.
Cell culture
Mouse macrophage RAW264 cells were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin, as described in the manuals of the cell line bank. Before the experiments, the culture medium was replaced with Ringer’s buffer (RB) consisting of 155 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 2 mM Na2HPO4, 10 mM glucose, 10 mM HEPES pH 7.2 and 0.5 mg/ml BSA.
DNA constructs and transfection
The cDNA fragment comprising the entire coding region for human Rab20 was generated by PCR amplification of human cDNAs. The primers used were TCTCGA GCTATGAGGAAGCCCGACAGCAA and AGAATTCTC AGGCACAACACCCAGATC. The fragment was cloned into the XhoI and EcoRI restriction sites of the pEGFP-C1 vector (Clontech, Palo Alto, CA). pEGFP-Rab21 was kindly provided by Dr. Arwyn T. Jones. pECFP-Rab20 and pECFP-Rab21 were generated by the replacement of GFP with cyan fluorescent protein (CFP). pEYFP-Rab5, pEYFP-Rab7 and pEYFP-Lamp1 were generous gifts from Dr. Joel A. Swanson. pECFP-Lamp1 and pECFP-Rab5 were generated by the replacement of yellow fluorescent protein (YFP) with CFP. All constructs were verified by sequencing prior to use. The RAW264 cells were transfected by electroporation and suspended at 5×106 cells/ml in growth medium. Four hundred µl of the cell suspension were mixed with 10 µg of plasmid DNA in a 4-mm-gap electroporation cuvette. Electroporation was performed using an ECM 630 Electroporation System (BTX Harvard Apparatus, Inc., MA) at 300 V, 1000 µf and 25 Ω, to yield a time constant of ~12 msec. Cells were then seeded onto 25-mm coverslips and maintained in the culture medium. Experiments were performed 24–48 hr after transfection.
Live-cell imaging
RAW264 cells were cultured on 25-mm circular coverslips and assembled in an RB-filled chamber on the thermo-controlled stage (Tokai Hit INU-ONI, Shizuoka, Japan) of an inverted epifluorescence microscope (Nikon TE300). Phase-contrast and fluorescence images of live cells were sequentially taken through a digital cooled CCD camera (Retiga Exi, QImaging, Surrey, BC, Canada) using a Uniblitz VMM-D1 shutter (Vincent Associates, Rochester, NY) and Lambda 10-2 filter wheels (Sutter Instruments, Novato, CA). These microscopic devices were controlled in an integrated manner by the MetaMorph imaging system (Molecular Devices, Downingtown, PA). Images were acquired by positioning a JP-4 filter set (Chroma Technology, Rockingham, VT) to visualize GFP or YFP: excitation at 500±10 nm and emission at 535±15 nm; CFP: excitation at 430±12.5 nm and emission at 470±15 nm. We used the narrow band YFP filter set for visualizing GFP signal to minimize signal crossover in the cells co-expressing GFP- and CFP-fusion proteins. We have confirmed there is no detectable level of fluorescence crossover of GFP into the CFP channel and vice versa under these experimental conditions. Time-lapse images of phase-contrast and fluorescence microscopy were sequentially taken at 10 sec intervals by the MetaMorph imaging system, as previously described [5]. At least 5 examples were observed in each experiment.
III. Results
Rab20 is transiently associated with macropinosomal membranes in macrophages
Rab20 shows the highest homology with Rab21, as compared to all the other Rab family members [2]. In addition, we have recently demonstrated that Rab21 localizes on macropinosomes in macrophages [9]. These findings prompted us to examine whether or not Rab20 also localizes on macropinosomal membranes. We investigated the association of Rab20 with macropinosomes in live RAW264 macrophages expressing GFP-Rab20 using phase-contrast and fluorescence microscopy. Time-lapse image analysis showed that GFP-Rab20 was localized on circular ruffles (macropinocytic cups) to a small extent (Fig. 1, t=0 sec). After the closure of macropinocytic cups into intracellular macropinosomes, GFP-Rab20 gradually and clearly accumulated at newly formed macropinosomes. The levels of Rab20 on macropinosomal membrane peaked at around 4 min after the beginning of circular ruffle formation. The association of Rab20 with macropinosomes was observed for up to ~10 min. Thereafter, Rab20 dissociated from macropinosomal membranes over time. These findings indicate that Rab20 is transiently recruited to newly formed macropinosomes in macrophages.
Fig. 1.

Live-cell imaging of GFP-Rab20 in RAW264 cells during macropinocytosis. Live RAW264 cells expressing GFP-Rab20 were observed by fluorescence microscopy. Time-lapse images were acquired using the MetaMorph imaging system. Phase-contrast images are shown in the upper panels. The elapsed time is indicated at the top. The beginning of circular ruffle formation from curved ruffles was set as time 0. It is noteworthy that Rab20 is associated with a formed macropinosome (inset, t=100 sec). At least fifteen cells were examined in five independent experiments. Bar=5 µm.
The association of Rab20 with macropinosomes persists even after the dissociation of Rab5 and Rab21 from macropinosomal membranes
It has been demonstrated that Rab5 is associated with circular ruffles (macropinocytic cups) and internalized macropinosomes [9, 16]. To clarify the stage of Rab20 recruitment to nascent macropinosomes, we transiently co-transfected RAW264 cells with GFP-Rab20 and CFP-Rab5, and examined the localizations of Rab20 and Rab5 during macropinocytosis. The time-lapse imaging showed that Rab20 was colocalized with Rab5 on circular ruffles (Fig. 2, t=0 sec). The localization of Rab20 on macropinosomal membranes was somewhat similar to that of Rab5 after the closure of macropinocytic cups into macropinosomes (Fig. 2, t=180 sec). However, the association of Rab20 with macropinosomes persisted even after the dissociation of Rab5 (Fig. 2, t=410 sec).
Fig. 2.

The association of Rab20 with macropinosomes persists even after the loss of Rab5. Live RAW264 cells co-expressing GFP-Rab20 (green) and CFP- Rab5 (red) were observed by digital fluorescence microscopy. The elapsed time is indicated at the top. Representative images from five independent experiments are shown. The insets show higher-magnification images of the indicated regions of the cells. Bar=5 µm. The beginning of circular ruffle formation from curved ruffles was set as time 0.
We have previously reported that Rab21 remains associated with macropinosomal membranes even after the dissociation of Rab5 from macropinosomes [9]. Hence, we next co-expressed GFP-Rab20 with CFP-Rab21, and compared their localizations during macropinocytosis. As expected, Rab20 and Rab21 were largely colocalized on macropinosomes in RAW264 cells (Fig. 3, t=200 sec). Interestingly, the dissociation of Rab20 from macropinosomal membranes was slightly preceded by that of Rab21 (Fig. 3, t=460 sec). These observations indicate that the association of Rab20 with macropinosomes persists even when Rab5 and Rab21 are dissociated from them.
Fig. 3.
The association of Rab20 with macropinosomal membranes persists even after the loss of Rab21. The time-lapse images of RAW264 cells co-expressing GFP-Rab20 (green) and CFP-Rab21 (red) were acquired in the same way as for Figure 2. The time-lapse images are representative of five independent experiments. Bar=5 µm.
Rab20 is colocalized with Rab7 and Lamp1 on maturating macropinosomes
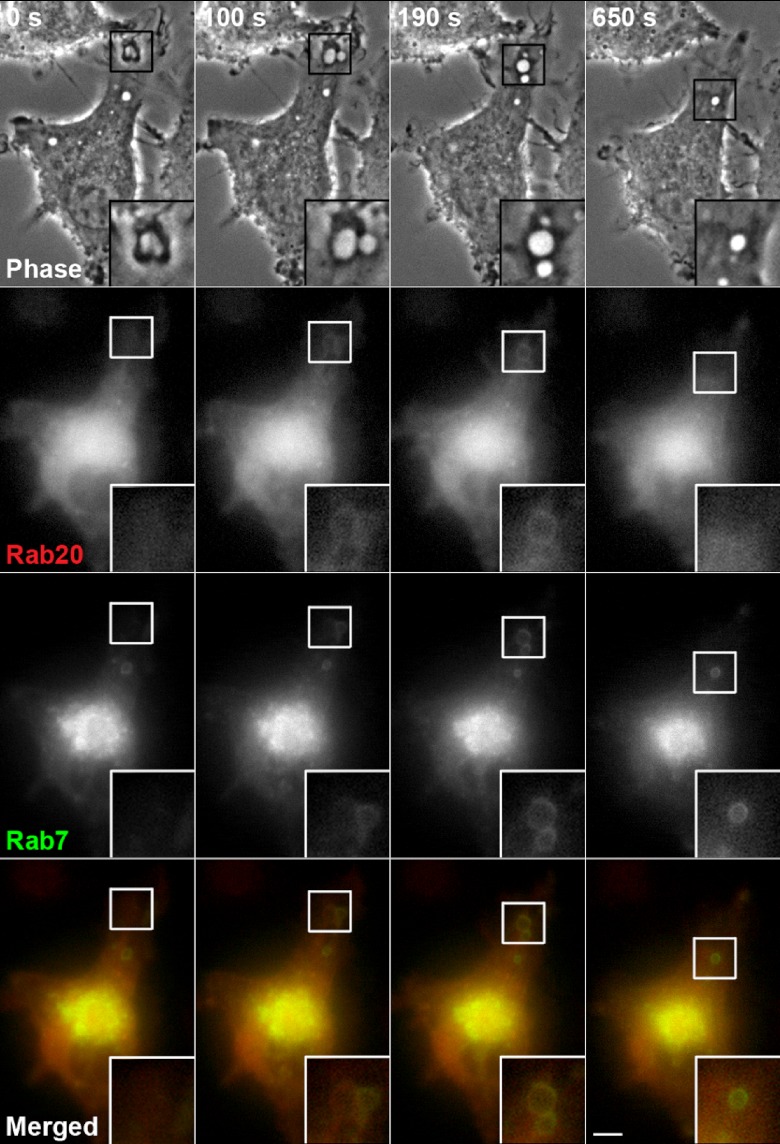
It has been shown that macropinosomes sequentially acquire Rab5 (an early endosomal marker), Rab21 (an early and late endosomal marker), and Rab7 (a late endosomal and lysosomal marker) [9]. In addition, Rab21, a close homolog of Rab20, is transiently colocalized with Rab7 on macropinosomes in macrophages. Then, we co-transfected RAW264 cells with CFP-Rab20 and YFP-Rab7, and examined whether or not Rab20 was also colocalized with Rab7 on macropinosomal membranes. The time-lapse images in Figure 4 show that the recruitment of Rab20 to macropinosomes occurred earlier than that of Rab7 during the early stage of macropinosome maturation. Although increased levels of Rab7 on macropinosomal membranes accompanied the reduction in Rab20 levels (Fig. 4, t=190 sec and 650 sec), Rab20 was colocalized with Rab7 on macropinosomes during the intermediate/late stage of macropinosome maturation.
Fig. 4.
Rab20 dissociates from macropinosomes before Rab7. RAW264 cells co-expressing CFP-Rab20 (red) and YFP-Rab7 (green) were observed by digital fluorescence microscopy. The association of Rab20 with macropinosomes was transient and followed by that of Rab7, which remained associated with the later stage of macropinosome maturation. The insets show higher-magnification images of the indicated regions of the cells. Bar=5 µm.
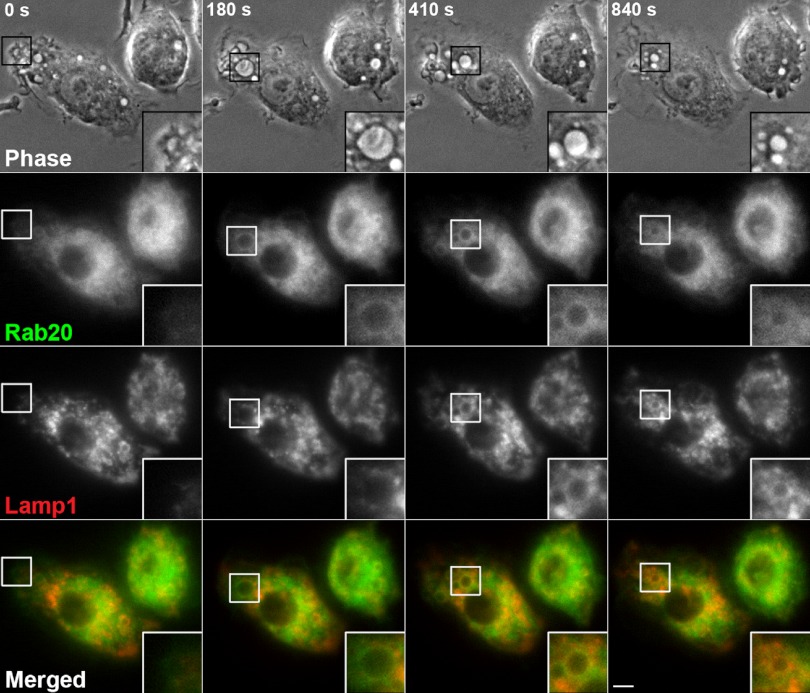
During the late stages of macropinosome maturation, it has been shown that Rab21 dissociates from macropinosomal membranes prior to the accumulation of Lamp1 [9]. In this study, we observed that the association of Rab20 with macropinosomes persists even after the loss of Rab21 from macropinosomal membranes (Fig. 3). Taking these facts into account, we predicted that Rab20 colocalized with Lamp1 at the late stage of macropinosome maturation. In order to verify this prediction, we compared the localization of GFP-Rab20 with that of CFP- Lamp1 in co-transfected RAW264 cells to clarify the distinct distribution of Rab20 and Rab21 during macropinocytosis. The time-lapse microscopic observations revealed that, during the early stage of macropinosome maturation, moderate levels of Rab20 were present on the macropinosomes in which Lamp1 was less seen (Fig. 5, t=180 sec). Thereafter, Rab20 and Lamp1 were found to be colocalized on macropinosomes (Fig. 5, t=410 sec). While Lamp1 remained associated with macropinosomal membranes, Rab20 dissociated from the macropinosomes over time. These results suggest that, although the localization of Rab20 on macropinosomal membranes resembles that of Rab21, the association of Rab20 with macropinosomes persists even after the dissociation of Rab21 from macropinosomal membranes.
Fig. 5.
Localizations of Rab20 and Lamp1 partially overlap each other in late macropinosomal membranes. The dynamics of GFP-Rab20 (green) on macropinosomes formed in RAW264 cells expressing CFP-Lamp1 (red) was examined by live-cell imaging. Shown is a representative series of images demonstrating the colocalization of Rab20 and Lamp1 on macropinosomes during the late stage of macropinosome maturation. Bar=5 µm.
IV. Discussion
Our study provides the first evidence that Rab20 is associated with macropinocytosis in RAW264 macrophages. In our time-lapse observation, Rab20 is found to be recruited to circular ruffles (macropinocytic cups) during the initial stage of macropinosome formation. Subsequently, Rab20 is transiently colocalized with Rab21 on internalized macropinosomes. We have previously reported that Rab21 is hardly observed on circular ruffles and is accumulated with nascent macropinosomes [9]. Therefore, it is likely that the onset of Rab20 recruitment occurs earlier than that of Rab21.
In this study, we revealed that Rab20 is colocalized with Rab5 on circular ruffles (macropinocytic cups). Then, Rab20 is increasingly accumulated with newly formed macropinosomes. Curiously, the association of Rab20 temporarily overlaps with that of Rab5, Rab21, Rab7 and Lamp1 on macropinosomal membranes (Fig. 6), suggesting its involvement at broad stages of the macropinocytic pathway from macropinosome formation to maturation. Meanwhile, we observed that the onset of Rab20 recruitment occurs earlier than that of Rab7, and that Rab20 is predominantly accumulated with maturing macropinosomes labeled with Rab7 and Lamp1. Recently, it has been demonstrated that Rab7 regulates the fusion of formed macropinosomes with late endosomes/lysosomes [15]. In addition, Rab20 is recruited to newly formed phagosomes and regulates phagosome maturation [10, 28]. The kinetics of macropinosome maturation seem to be similar to those of phagosome maturation [23]. Accordingly, Rab20 might regulate the recruitment of Rab7 to accelerate macropinosome maturation, although we cannot exclude the possibility that Rab20 is involved in the macropinosome formation. Moreover, Rab20 has been shown to be colocalized with vacuolar H+-ATPase (V-ATPase) [6]. So, Rab20 might alternatively modulate V-ATPase traffic during macropinosome maturation.
Fig. 6.
Time series of association of Rab proteins with macropinocytosis. Rab5 is localized on circular ruffles and newly formed macropinosomes during the early stage of macropinocytosis. Rab21 is mainly accumulated with internalized macropinosomes during the intermediate stage of macropinocytosis. During the late stage of macropinocytosis, Rab7 and Lamp1 are recruited to maturating macropinosomes. It is noteworthy that Rab20 is transiently associated with macropinosomes at broad stages overlapping Rab5-, Rab21-, Rab7- and Lamp1-recruitment period.
To date, the functional molecules regulating Rab20 translocation to/from macropinosomes have remained to be clarified. Rab20 shows the highest homology with Rab21 [2] and colocalizes with Rab21-positive macropinosomes (Fig. 3). Interestingly, our microscopic observations revealed that Rab20 and Rab21 showed similar but not identical spatiotemporal dynamics during macropinocytosis. As described above, the association of Rab20 with macropinosomes precedes that of Rab21. Moreover, our live-cell imaging showed that the association of Rab20 persists even after Rab21 dissociation. These facts imply that different guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) are involved in the regulation of Rab20 and Rab21 translocation. In further studies, the identification of the GEFs and GAPs for Rab20 would be important for understanding the physiological consequences of Rab20 in the transient activation of Rab GTPases during macropinosome maturation.
In conclusion, our study demonstrated that Rab20 is temporarily associated with macropinosomes at broad stages overlapping Rab5-, Rab21-, Rab7- and Lamp1-association. Although the function of Rab20 remains to be clarified in future studies, the macropinocytic pathway is coordinately regulated by some Rab GTPases, which create transient Rab-effector recruitments that mediate the progressive maturation of macropinosomes.
V. Acknowledgments
The authors would like to thank Dr. Arwyn T. Jones for pEGFP-Rab21 and Dr. Joel A. Swanson for pEYFP-Rab5, pEYFP-Rab7 and pEYFP-Lamp1. We also thank Dr. Katsuya Miyake and Dr. Makoto Fujii for their helpful discussion, Ms. Akane Fukuda for proof reading, and Mr. Kazuhiro Yokoi and Ms. Yukiko Iwabu for their skillful help. This study was supported by a Grant-in-Aid for Young Scientists (B) #22790188 and partially by a Grant-in-Aid for Scientific Research (B) #23390039 from the Japan Society for the Promotion of Science. Y. Egami was supported by the fund for Kagawa University Young Scientists 2012.
VI. References
- 1.Alpuche-Aranda C. M., Racoosin E. L., Swanson J. A., Miller S. I. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J. Exp. Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amillet J. M., Ferbus D., Real F. X., Antony C., Muleris M., Gress T. M., Goubin G. Characterization of human Rab20 overexpressed in exocrine pancreatic carcinoma. Hum. Pathol. 2006;37:256–263. doi: 10.1016/j.humpath.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Araki N., Johnson M. T., Swanson J. A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araki N., Hatae T., Yamada T., Hirohashi S. Actinin-4 is preferentially involved in circular ruffling and macropinocytosis in mouse macrophages: analysis by fluorescence ratio imaging. J. Cell Sci. 2000;113:3329–3340. doi: 10.1242/jcs.113.18.3329. [DOI] [PubMed] [Google Scholar]
- 5.Araki N., Egami Y., Watanabe Y., Hatae T. Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp. Cell Res. 2007;313:1496–1507. doi: 10.1016/j.yexcr.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Curtis L. M., Gluck S. Distribution of Rab GTPases in mouse kidney and comparison with vacuolar H+-ATPase. Nephron Physiol. 2005;100:p31–42. doi: 10.1159/000085114. [DOI] [PubMed] [Google Scholar]
- 7.Das Sarma J., Kaplan B. E., Willemsen D., Koval M. Identification of rab20 as a potential regulator of connexin 43 trafficking. Cell Commun. Adhes. 2008;15:65–74. doi: 10.1080/15419060802014305. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson J. G., Porat-Shliom N., Cohen L. A. Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal. 2009;21:1–6. doi: 10.1016/j.cellsig.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egami Y., Araki N. Dynamic changes in the spatiotemporal localization of Rab21 in live RAW264 cells during macropinocytosis. PLoS One. 2009;4:e6689. doi: 10.1371/journal.pone.0006689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egami Y., Araki N. Rab20 regulates phagosome maturation in RAW264 macrophages during Fc gamma receptor-mediated phagocytosis. PLoS One. 2012;7:e35663. doi: 10.1371/journal.pone.0035663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Y., Press B., Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J. Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones A. T. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J. Cell. Mol. Med. 2007;11:670–684. doi: 10.1111/j.1582-4934.2007.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr M. C., Lindsay M. R., Luetterforst R., Hamilton N., Simpson F., Parton R. G., Gleeson P. A., Teasdale R. D. Visualisation of macropinosome maturation by the recruitment of sorting nexins. J. Cell Sci. 2006;119:3967–3980. doi: 10.1242/jcs.03167. [DOI] [PubMed] [Google Scholar]
- 14.Kerr M. C., Teasdale R. D. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 15.Kerr M. C., Wang J. T., Castro N. A., Hamilton N. A., Town L., Brown D. L., Meunier F. A., Brown N. F., Stow J. L., Teasdale R. D. Inhibition of the PtdIns(5) kinase PIKfyve disrupts intracellular replication of Salmonella. EMBO J. 2010;29:1331–1347. doi: 10.1038/emboj.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanzetti L., Palamidessi A., Areces L., Scita G., Di Fiore P. P. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 2004;429:309–314. doi: 10.1038/nature02542. [DOI] [PubMed] [Google Scholar]
- 17.Lutcke A., Parton R. G., Murphy C., Olkkonen V. M., Dupree P., Valencia A., Simons K., Zerial M. Cloning and subcellular localization of novel rab proteins reveals polarized and cell type-specific expression. J. Cell Sci. 1994;107(Pt 12):3437–3448. doi: 10.1242/jcs.107.12.3437. [DOI] [PubMed] [Google Scholar]
- 18.Mayor S., Pagano R. E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercer J., Helenius A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 20.Meresse S., Gorvel J. P., Chavrier P. The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J. Cell Sci. 1995;108(Pt 11):3349–3358. doi: 10.1242/jcs.108.11.3349. [DOI] [PubMed] [Google Scholar]
- 21.Nobes C., Marsh M. Dendritic cells: new roles for Cdc42 and Rac in antigen uptake? Curr. Biol. 2000;10:R739–741. doi: 10.1016/s0960-9822(00)00736-3. [DOI] [PubMed] [Google Scholar]
- 22.Norbury C. C. Drinking a lot is good for dendritic cells. Immunology. 2006;117:443–451. doi: 10.1111/j.1365-2567.2006.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Racoosin E. L., Swanson J. A. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J. Cell Biol. 1993;121:1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radhakrishna H., Klausner R. D., Donaldson J. G. Aluminum fluoride stimulates surface protrusions in cells overexpressing the ARF6 GTPase. J. Cell Biol. 1996;134:935–947. doi: 10.1083/jcb.134.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sansonetti P. J. Microbes and microbial toxins: paradigms for microbial-mucosal interactions III. Shigellosis: from symptoms to molecular pathogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G319–323. doi: 10.1152/ajpgi.2001.280.3.G319. [DOI] [PubMed] [Google Scholar]
- 26.Schnatwinkel C., Christoforidis S., Lindsay M. R., Uttenweiler-Joseph S., Wilm M., Parton R. G., Zerial M. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol. 2004;2:E261. doi: 10.1371/journal.pbio.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz S. L., Cao C., Pylypenko O., Rak A., Wandinger-Ness A. Rab GTPases at a glance. J. Cell Sci. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- 28.Seto S., Tsujimura K., Koide Y. Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes. Traffic. 2011;12:407–420. doi: 10.1111/j.1600-0854.2011.01165.x. [DOI] [PubMed] [Google Scholar]
- 29.Somsel Rodman J., Wandinger-Ness A. Rab GTPases coordinate endocytosis. J. Cell Sci. 2000;113 Pt 2:183–192. doi: 10.1242/jcs.113.2.183. [DOI] [PubMed] [Google Scholar]
- 30.Steinman R. M., Swanson J. The endocytic activity of dendritic cells. J. Exp. Med. 1995;182:283–288. doi: 10.1084/jem.182.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stenmark H., Olkkonen V. M. The Rab GTPase family. Genome Biol. 2001;2:REVIEWS3007. doi: 10.1186/gb-2001-2-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun P., Yamamoto H., Suetsugu S., Miki H., Takenawa T., Endo T. Small GTPase Rah/Rab34 is associated with membrane ruffles and macropinosomes and promotes macropinosome formation. J. Biol. Chem. 2003;278:4063–4071. doi: 10.1074/jbc.M208699200. [DOI] [PubMed] [Google Scholar]
- 33.Swanson J. A. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X., Kumar R., Navarre J., Casanova J. E., Goldenring J. R. Regulation of vesicle trafficking in madin-darby canine kidney cells by Rab11a and Rab25. J. Biol. Chem. 2000;275:29138–29146. doi: 10.1074/jbc.M004410200. [DOI] [PubMed] [Google Scholar]
- 35.Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q., Calafat J., Janssen H., Greenberg S. ARF6 is required for growth factor- and rac-mediated membrane ruffling in macrophages at a stage distal to rac membrane targeting. Mol. Cell. Biol. 1999;19:8158–8168. doi: 10.1128/mcb.19.12.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]