Abstract
FGF1 is highly expressed in neurons and it has been proposed to play a role in the neuroprotection and in regeneration. Low FGF1 expression in neurons has been linked to increased vulnerability in cholinergic neurons. Previous reports have shown that the expression of FGF1 in rat brain is localized to the cholinergic nuclei of the medulla oblongata, with low ratio of neurons positive for FGF1 in the dorsal motor nucleus of the vagus (DMNV). The role of FGF1 in the primate brain has yet to be clarified. In this study, we mapped FGF1 immunoreactivity in the medulla oblongata of cynomolgus monkey brainstems. Our results demonstrated that FGF1 immunoreactivity follows the pattern of distribution of cholinergic nuclei in the medulla oblongata; with strong localization of FGF1 to cholinergic neurons of the hypoglossal nucleus, the facial nucleus and the nucleus ambiguus. In contrast, the DMNV shows markedly lower FGF1 immunoreactivity. Localization of FGF1 to cholinergic neurons was only observed in the lateral region of the DMNV, with higher immunoreactivity in the rostral ventral-lateral region of the DMNV. These findings are consistent with the distribution of FGF1 immunoreactivity in previous studies of the rat brain.
Keywords: FGF1, DMNV, cynomolgus monkey, medulla oblongata, cholinergic neurons
I. Introduction
Acidic fibroblast growth factor (FGF1, also abbreviated as aFGF or FGF-1) is a member of the fibroblast growth factor (fgf) family. It was first discovered alongside basic fibroblast growth factor (FGF2) by Thomas et al. [40]. FGF1 was isolated from bovine brain, and was named for its mitogenic activity on fibroblast proliferation. In the nervous system, it is predominantly expressed in neuronal cells, with little to no expression in glial cells [11, 37], and expressed particularly in subpopulations of cholinergic neurons [9, 10]. FGF1 is secreted through a non-classical pathway [26], and shows potent neurotrophic and neuroprotective functions [17, 28]. Previous studies have pointed to the importance of FGF1 in maturation, maintaining plasticity, and long-term potentiation of neurons, indicating further essential roles in memory and learning [27]. The widespread expression of FGF1 throughout the neural tissue from the early developmental stage to high levels of expression in the adult CNS, points to essential roles in both the development and maintenance of neuronal function [6, 11, 17, 18, 32, 36].
Studies of cholinergic neurons in senescence-accelerated mice models demonstrated that the application of FGF1 or a fragment analog of FGF1 prolongs the period of latency in behavioral studies and preserves septal cholinergic neurons [34, 41]. Amyotrophic lateral sclerosis (ALS) is a disease characterized by progressive loss of cholinergic motor neurons in the spinal cord. A greater loss of FGF1 in anterior horn cholinergic motor neurons was observed in ALS cases compared to control cases [15]. These observations taken together suggest that FGF1 plays an important neuroprotective and regenerative role in cholinergic neurons.
Neurons in the dorsal motor nucleus of the vagus (DMNV) of the medulla oblongata are more vulnerable to injury and have a lower recovery rate when injured [2, 22]. In studies using rodents, high expression of FGF1 was observed in cholinergic neurons in the hypoglossal nucleus, the nucleus ambiguus, and the facial nucleus, while low expression was observed in the lateral region of the DMNV [23, 33]. The laryngeal nerve, which is partly innervated by the DMNV, is notoriously slow to recover from nerve damage for reasons that remain unclear. Interestingly, studies in rats have shown that neurons projecting their axons to the laryngeal nerve from the DMNV have low levels of FGF1 expression [42]. These observations suggest that low FGF1 expression may partly explain the poor recovery of the laryngeal nerve.
While extensive studies of FGF1 have been undertaken in rodents, information on non-human primate animal models is not yet available. Such data would be highly informative in determining the role of FGF1 in the survival of cholinergic neurons. In this study, we sought to elucidate the distribution of FGF1 in the cranial nuclei of the medulla oblongata of the cynomolgus monkey (Macaca fascicularis), using immunohistochemical techniques.
II. Materials and Methods
Sample preparation
Brain stem samples were obtained from four cynomolgus monkeys (Macaca fascicularis), euthanized under deep pentobarbital anaesthesia, after use for separate research purposes by other researchers. The protocols for the use of animals in the current study were assessed and approved by the Institutional Animal Care and Use Committee (IACUC) of Shiga University of Medical Science. For immunohistochemistry, the brains were removed from two male (5 years and 3 months old; weighing 3.38–4.68 kg) and one female (5 years old; weighing 3.28 kg) cynomolgus monkeys, perfused with 500 ml of saline followed with 500 ml 4% formaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) under deep pentobarbital anaesthesia. The brains were immediately post-fixed in 4% formaldehyde in 0.1 M PB at 4°C for 2 days. Samples were immersed in 15% sucrose in 0.1 M PB with 0.1% sodium azide, with daily change of sucrose solution for 4 days and were then stored in sucrose solution until processing. The samples were cryosectioned at –20°C into 20 µm serial coronal sections which were floated in 0.1 M phosphate buffered saline (PBS) containing 0.3% Triton X-100 (PBST, pH 7.4) and then maintained in PBST at 4°C.
Immunohistochemistry
Immunohistochemical analyses were performed as described previously [23, 33, 39, 42] with slight modifications. Briefly, serial sections were washed in PBST and immersed in 0.1 M hydrochloric acid (HCl) in Milli-Q® water for 1 min for antigen retrieval, and then washed in PBST. Endogenous peroxidase was quenched by incubation in 0.5% hydrogen peroxide (H2O2) in PBST for 30 min. The sections were then washed in PBST and blocked using 4% normal horse serum in PBST for 1 hr. The sections were incubated for 3 days at 4°C with goat anti-choline acetyltransferase (ChAT) antibody (diluted 1:500, Chemicon, Temecula, CA, USA) or with mouse monoclonal anti-FGF1 antibody (diluted 1:500) in PBST containing 0.4% normal horse serum. The sections were then washed in PBST and incubated with biotinylated anti-goat IgG or anti-mouse IgG (diluted 1:500, Vector Laboratories, Burlingame, CA, USA) in PBST containing 0.4% normal horse serum for 2 hr at room temperature. The samples were washed in PBST and incubated in avidin-biotin-peroxidase complex at 1:4000 dilution for 1 hr. The peroxidase-labeled sections were developed using 0.02% 3,3-diaminebenzine-tetra-hydrochloride (DAB) in 50 mM Tris-HCl, containing 0.07% nickel ammonium sulphate and 0.005% H2O2.
FGF1 monoclonal antibody used in this study was raised against human recombinant FGF1 [14]. An immunoabsorption test reported previously showed abolished staining [33, 42]. Western blot analysis was performed in this study to confirm the antibody specificity for FGF1.
Western blot analysis
Medulla oblongata was obtained from one female cynomolgus monkey (10 years and 4 months old; weighing 3.17 kg) euthanized under deep pentobarbital anaesthesia, after use for separate research purposes by other researchers, was used for western blot analysis, performed as described previously [23, 33] with slight modifications. The brainstem was homogenized in 5 volumes of ice-cold 50 mM Tris-HCl (pH 7.4) containing 0.5% Triton X-100 and protease inhibitors (Complete Mini, Roche Diagnostics, Mannheim, Germany; 1 tablet/10 ml). The homogenate was centrifuged at 20,600 g for 20 min at 4°C. The supernatant was collected as a crude protein fraction. Protein concentration was assayed using Tecan readers Infinite M200 (Tecan, Männedorf, Switzerland). Fifty micrograms of the crude extracted protein, and prestained precision protein standards (BioRad, Hercules, CA, USA), were electrophoresed on a 5–20% sodium dodecyl sulfate-polyacrylamide gel under reducing conditions, and then transferred to polyvinylidene difluoride membrane (Immobilon-P; Millipore, Billerica, MA, USA). The membrane was incubated for 1 hr with 5% skimmed milk powder in 25 mM Tris-buffered saline (TBS, pH 7.4) at room temperature, and further incubated overnight with the monoclonal anti-FGF1 antibody at a dilution of 1:500 in 25 mM TBS containing 0.5% skimmed milk powder at 4°C. The membrane was then washed with 25 mM TBS containing 0.1% Tween-20 (BioRad), followed by incubation for 1 hr with a peroxidase-labeled anti-mouse IgG (diluted 1:20,000, Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The peroxidase labeling was detected by chemiluminescence using the SuperSignal West Pico Chemiluminescent Substrate (ThermoFisher Scientific, Rockford, IL, USA).
Mapping
Mapping of FGF-1 distribution in the medulla oblongata of monkey brainstem was performed as described previously [1] using a camera lucida.
Double fluorescence immunohistochemistry
Sections were washed in PBST and immersed in 0.1 M HCl in Milli-Q® water for 1 min for antigen retrieval, then washed in PBST. Blocking was performed by soaking in 4% normal horse serum in PBST for 1 hr. The sections were then incubated with a mixture of mouse monoclonal anti-FGF1 antibody (diluted 1:500) and goat anti-ChAT antibody (diluted 1:500, Chemicon) or rabbit anti-tyrosine hydroxylase (TH) antibody (diluted 1:2000, Biosensis, Australia) in PBST containing 0.4% normal horse serum for 3 days at 4°C. The sections were then washed in PBST and incubated with a mixture of fluorescent-labeled chicken anti-mouse IgG (diluted 1:1000, Molecular Probes, Eugene, OR, USA) with chicken anti-goat IgG or horse anti-rabbit IgG (diluted 1:1000, Molecular Probes) in PBST at 4°C for 4 hr. The sections were then washed three times in PBST, once in 50 mM Tris-HCl and mounted on coated glass slides. Fluorescence was detected using a scanning laser confocal microscope (TE2000-E, Nikon, Japan).
Quantification of FGF-1 immunoreactivity in cholinergic nuclei of medulla oblongata
The number of ChAT-positive, FGF1-positive and, double-immunopositive neurons was determined by cell counting using rostral to caudal representative sections of the medulla oblongata processed for double fluorescence immunohistochemistry. Confocal microscopy was employed to capture images of fluorescent labeled neurons in the hypoglossal nucleus, the DMNV, the nucleus ambiguus, and the facial nucleus. The images were then processed using Adobe®Photoshop®CS2, with adjustments only made to levels, brightness, and contrast. A cell count of fluorescent-labeled neurons was then performed by eye, and the percentage colocalization of FGF1-positive to ChAT-positive neurons relative to the total number of fluorescent labeled neurons was calculated for each nucleus.
III. Results
Specificity of the FGF1 monoclonal antibody
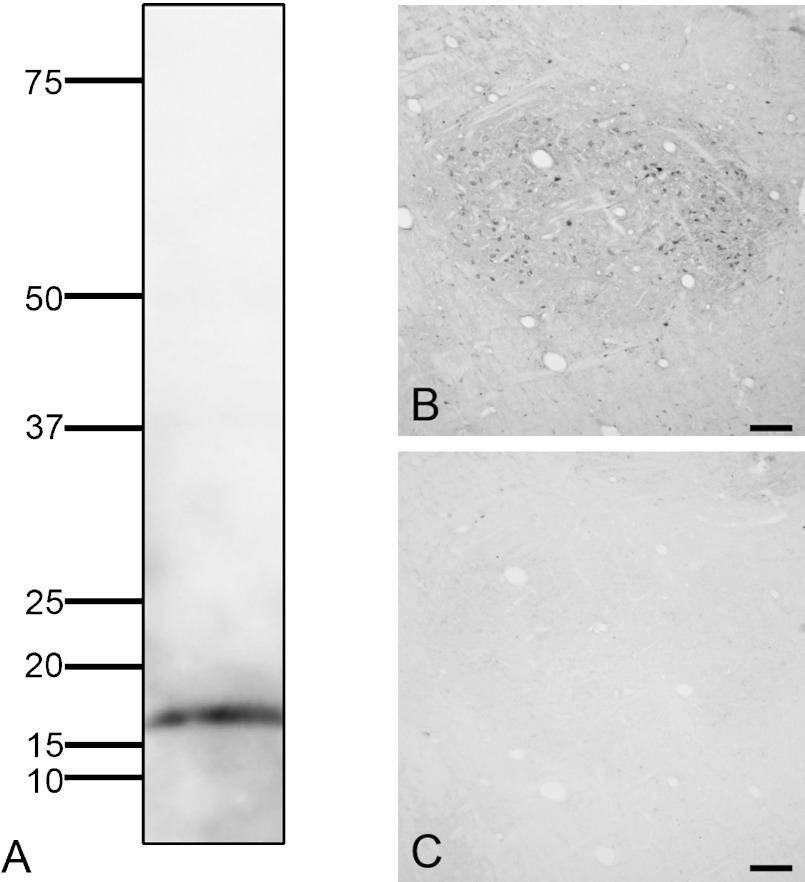
Western blots showed that the monoclonal anti-FGF1 antibody detection of a single band with a molecular weight of about 17 kDa (Fig. 1A). The size is consistent with the molecular weight of a native form of FGF1 [23, 33, 37]. FGF1 immunoreactivity was observed in neuronal structures (Fig. 1B). These staining signals were not seen when the FGF1 antibody was omitted (Fig. 1C).
Fig. 1.
Antibody specificity for FGF1. A; Western blot analysis of crude protein extract from monkey medulla oblongata using mouse monoclonal anti-FGF1 antibody. The antibody recognized a single band at approximately 17 kDa. B; Immunostaining for FGF1 in the monkey medulla oblongata with mouse monoclonal anti-FGF1 antibody. C; Immunostaining using no primary antibody in the monkey medulla oblongata showed no staining. Bar=200 µm.
Distribution of FGF1 immunoreactivity in the medulla oblongata of cynomolgus monkeys
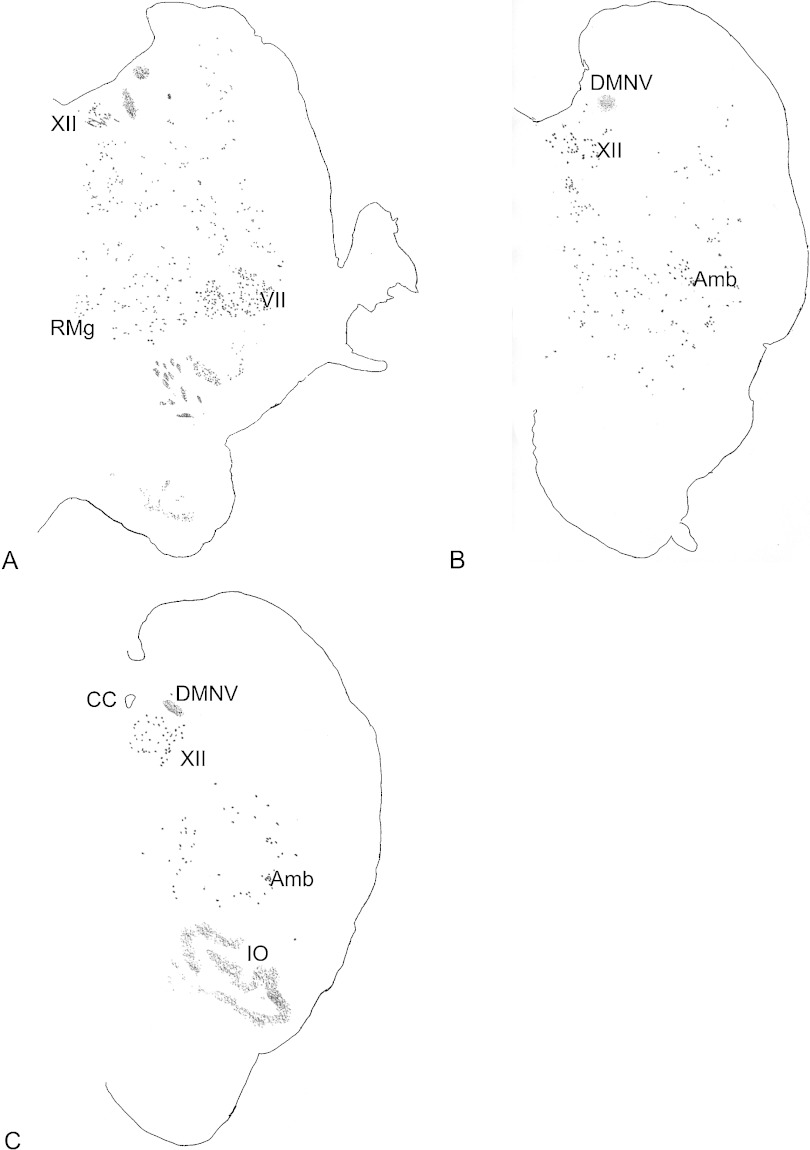
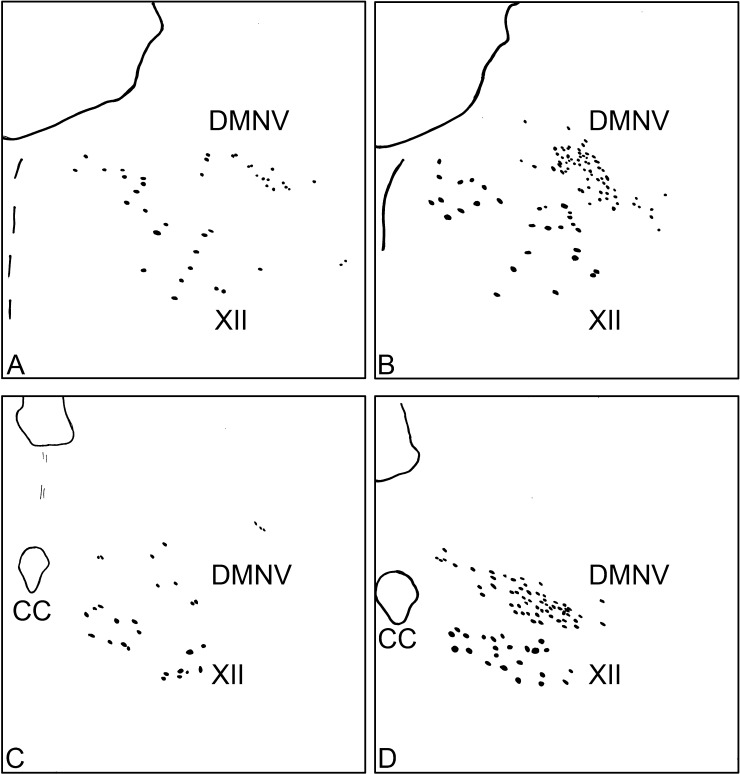
FGF1 immunoreactivity was detected in neuronal structures in the medulla oblongata, with staining of neuronal cell bodies and neural processes but no staining was observed in glia. The FGF1-immunoreactive neuronal structures observed in several regions of the medulla are shown in drawings prepared using a camera lucida (Fig. 2A–2C). There was no difference in the distribution pattern of FGF1 immunoreactivity among the three samples of monkey brain examined in this study. In the medulla oblongata, FGF1-immunoreactive neurons were observed in the raphe magnus nucleus, the inferior olive, the hypoglossal nucleus, the nucleus ambiguus and the DMNV. FGF1 immunoreactivity was also observed in scattered neurons of the raphe magnus nucleus (Fig. 2A) and inferior olive (Fig. 2C), whereas there was a defined distribution pattern in the hypoglossal nucleus (Fig. 2A–C) and the nucleus ambiguus (Fig. 2B and 2C). A densely packed population of FGF1-positive neurons was also observed in the facial nucleus (Fig. 2A). Figure 3 shows camera lucida drawings of FGF1 and ChAT immunostaining at a high magnification in the regions containing the DMNV and the hypoglossal nucleus. In the DMNV, FGF1-positive neurons were relatively rich in the lateral region (Fig. 3A and 3C). More FGF1-positive neurons were observed in the rostral region of the DMNV (Fig. 3A), than in the caudal region of the DMNV (Fig. 3C). ChAT-positive neurons were similarly distributed in the nucleus in the DMNV (Fig. 3B and 3D).
Fig. 2.
Camera lucida drawing of FGF1 immunoreactivity in the medulla oblongata of cynomolgus monkey brainstems, prepared from representative coronal sections of the medulla. FGF1 immunoreactivity is observed in rostral to caudal regions of the medulla in the hypoglossal nucleus (XII) (A–C), the dorsal motor nucleus of the vagus (DMNV) (A–C), the nucleus ambiguus (Amb) (B and C), the facial nucleus (VII) (A), the raphe magnus nucleus (RMg) (A), and the inferior olive (IO) (C). CC indicates the central canal.
Fig. 3.
Camera lucida drawing of FGF1 (A and C) and ChAT (B and D) immunoreactivity in the rostral (A and B) and caudal (C and D) regions of the dorsal motor nucleus of vagus (DMNV) and the hypoglossal nucleus (XII). CC indicates the central canal.
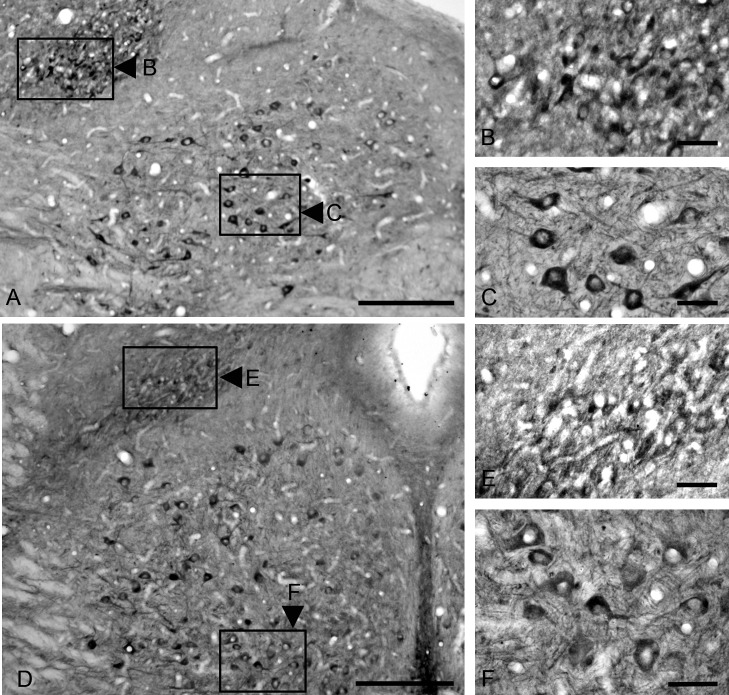
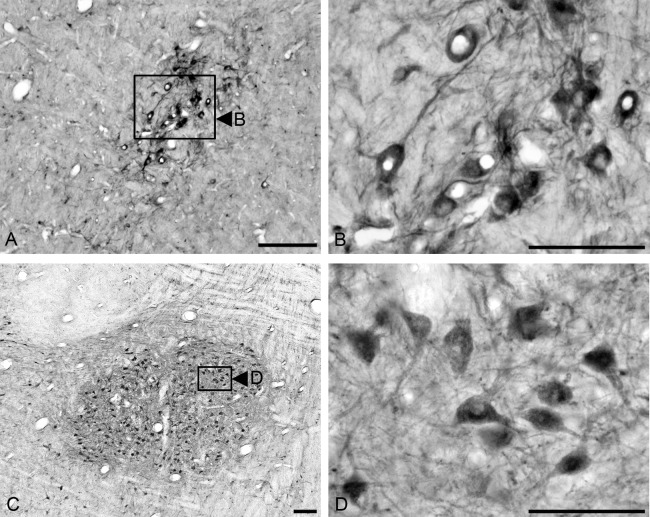
We found FGF1-immunoreactive neurons in the DMNV (Fig. 4A, 4B, 4D, and 4E), the hypoglossal nucleus, (Fig. 4A, 4C, 4D, and 4F), the nucleus ambiguus (Fig. 5A and 5B) and the facial nucleus (Fig. 5C and 5D). FGF1 immunoreactivity was observed in the cell bodies in the cytoplasm, and in the neural processes, but not in the nucleus. Immunoreactive neurons in the DMNV varied in distribution from the rostral to caudal region of the medulla oblongata, and were mainly observed in the lateral portion of the DMNV. A comparison of the FGF1 distribution in the hypoglossal nucleus and the DMNV in the caudal and rostral regions of the medulla oblongata is shown in Fig. 4. We observed a few small-sized neurons (10–15 µm in diameter) [20] that were immunoreactive for FGF1 in the DMNV. In addition, the caudal region (Fig. 4D and 4E) showed a reduced number of FGF1-positive neurons in the DMNV compared to the rostral region (Fig. 4A and 4B). FGF1 immunoreactivity in the hypoglossal nucleus was similar in both rostral (Fig. 4A and 4C) and caudal regions (Fig. 4D and 4F) of the medulla oblongata, with medium-sized, oval-shaped neurons (30–50 µm in diameter) that showed clear staining of FGF1 in the cytoplasm and neural processes.
Fig. 4.
Immunohistochemical staining for FGF1 in the medulla oblongata is observed in the dorsal motor nucleus of the vagus and the hypoglossal nucleus in rostral and caudal sections of the medulla. Strong FGF1 immunoreactivity is observed in the neurons of the hypoglossal nucleus in both rostral and caudal areas (A, D). Higher magnification of areas in boxes (C and F) show that neural bodies and neural projections are positive for FGF1 in the hypoglossal nucleus. The dorsal motor nucleus of the vagus shows FGF1 immunoreactivity only in the lateral area (A) of the rostral area of medulla, and only weakly stained fibers in the caudal region (D). Higher magnification of the dorsal motor nucleus of the vagus shows few FGF1-positive neural bodies in the rostral area (B) and in the caudal area (E). Bar=300 µm (A and D); 50 µm (B, C, E); 100 µm (F).
Fig. 5.
FGF1 immunoreactivity in the facial nucleus (A and B) and the nucleus ambiguus (C and D). At high magnification (B and D), both neural bodies and neural processes are stained for FGF1 in the nucleus ambiguus (B) and the facial nucleus (D). Bar=100 µm.
In the nucleus ambiguus, (Fig. 5A and 5B), clear FGF1 staining was present in medium-sized neurons (25–40 µm in diameter) [21] in the cytoplasm and neural processes. In the facial nucleus (Fig. 5C and 5D), a large population of medium-sized, fusiform-shaped neurons were moderately immunoreactive for FGF1.
Double immunofluorescence for FGF1 and ChAT
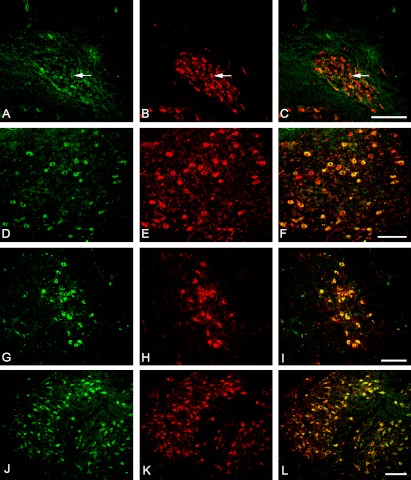
The distribution pattern of FGF1-positive neurons was similar to that observed for some cholinergic neurons. In order to clarify the localization of FGF1 in cholinergic neurons, double immunohistochemistry was employed. Our results showed considerably strong colocalization of FGF1- and ChAT-positive neurons in the hypoglossal nucleus (Fig. 6D–6F), the nucleus ambiguus (Fig. 6G–6I), and the facial nucleus (Fig. 6J–6L). In contrast, the DMNV (Fig. 6A–6C) showed markedly less colocalization of FGF1- and ChAT-positive neurons, and it was mainly in the lateral region. Higher immunoreactivity for FGF1 was apparent in the rostral lateral region of the DMNV.
Fig. 6.
Double immunofluorescent staining for FGF1 (green) and ChAT (red) showing colocalization of FGF1-positive and ChAT-positive neurons in the dorsal motor nucleus of the vagus (A–C), the hypoglossal nucleus (D–F), the nucleus ambiguus (G–I), and the facial nucleus (J–L). Few neurons in the DMNV showed colocalization (indicated by arrows) of FGF1 immunoreactivity to ChAT (A–C). Almost all of the ChAT-positive neurons in the hypoglossal nucleus (D–F), nucleus ambiguus (G–I) and facial nucleus (J–L) were colocalized with FGF1 immunoreactivity. Bar=100 µm.
Table 1 shows the percentage of colocalization for FGF1 and ChAT immunoreactivity. There was a high percentage of doubly stained neurons relative to the total of ChAT-positive neurons in the facial nucleus (70.5%), the nucleus ambiguus (83.9%), and the hypoglossal nucleus (71.7%), but only a low percentage in the DMNV (16.3%). There was also a low percentage of cholinergic neurons among FGF1-positive neurons in the DMNV, with only 48.4% in the caudal part of the DMNV, and 34.1% in the rostral region of the DMNV, whereas a higher percentage of cholinergic neurons was present among FGF1-positive neurons in the facial nucleus (87.8%), the nucleus ambiguus (66.2%), and the hypoglossal nucleus (65.5%).
Table 1.
Percentage of neurons stained for ChAT and FGF1 relative to the total ChAT-positive neurons (upper row) and total FGF1-positive neurons (lower row) in cranial nuclei in the medulla oblongata of cynomolgus monkey brainstem
| Region | Facial nucleus | Nucleus ambiguus | Hypoglossal nucleus | DMNV (Total) | DMNV (Rostral) | DMNV (Caudal) |
|---|---|---|---|---|---|---|
| Percentage of double-positive neurons to total cholinergic neurons | 70.5% | 83.9% | 71.7% | 16.3% | 20.1% | 11.9% |
| Percentage of double-positive neurons to total FGF1-positive neurons | 87.8% | 66.2% | 65.5% | 37.9% | 34.1% | 48.4% |
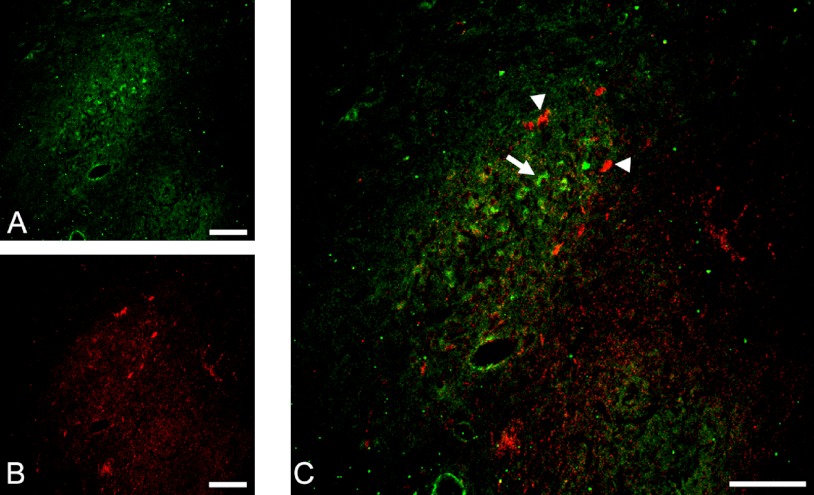
Dopaminergic and noradrenergic neurons have previously been localized in the rostral region of the DMNV [3, 24]. We observed TH immunoreactivity in the DMNV (Fig. 7A); however, FGF1 immunoreactivity in the DMNV did not colocalize with TH-positive neurons (Fig. 7C).
Fig. 7.
Double immunofluorescent staining for FGF1 (green) (A) and TH (red) (B) in the rostral portion of the dorsal motor nucleus of the vagus. No colocalization of FGF1-positive neurons (indicated by arrow) to TH-positive neurons (indicated by arrowheads) was observed in the DMNV (C). Bar=200 µm.
IV. Discussion
In the current study, we successfully mapped the distribution of FGF1 in the medulla oblongata of Macaca fascicularis. We demonstrated that FGF1 immunoreactivity was restricted to neuronal structures, where it was observed in the cytoplasm of the somata and neuronal processes, with no immunoreactivity observed in glial cells. These results are in good agreement with previous studies in normal rat brains [10, 37]. A recent study by Hiruma et al. [13] reported that neurotrophin NT-3 is colocalized with FGF1 in lung alveolar macrophages. Although we did not observe FGF1-positive macrophage/microglia in the medulla of the normal monkey brain, FGF1 expression may be induced in microglia under a pathological condition.
FGF1 immunoreactivity was found extensively in ChAT-positive neurons in the hypoglossal nucleus, the nucleus ambiguus, the facial nucleus, and in scattered neurons in the raphe magnus nucleus and inferior olive. The FGF1 stained neurons ranged in size from small to medium and in shape from fusiform to oval and ovoid-like structure. FGF1 immunoreactivity was also observed in the inferior olive and in axons extending from the hypoglossal nucleus. Low immunoreactivity was observed in the DMNV, with only a few FGF1-positive neurons found in the caudal-lateral and rostral ventral-lateral regions. These observations are in agreement with previous studies in the rat, where there is strong expression of FGF1 in motor neurons of the hypoglossal nucleus and nucleus ambiguus [37]. Furthermore, FGF1-positive cells were observed previously in the DMNV of the rat, localized predominantly in the lateral region of the DMNV [23, 33].
FGF1 expression has been linked in various studies to neuroprotection, rescue after injury and regeneration of nerve tissue. The application of exogenous FGF1 rescued neurons after ischemic injury [6], and promoted the regeneration of sensory axons after severing of adult dorsal roots [17]. In addition, subcutaneous injection of FGF1 was shown to delay senescence and preserve cholinergic neurons in the medial septum of senescence-accelerated mice [41].
The present study showed that abundant FGF1 expression in motor nuclei such as the facial nucleus, the ambiguus nucleus, and the hypoglossal nucleus, although, the function of such high expression of FGF1 in motor nuclei remains unclear. Previous studies have demonstrated that FGF1 may play an important role in motor neurons. Motor neurons possess Ca2+ permeable AMPA receptor (AMPAR) lacking the GluR2 subunit and are selectively vulnerable to AMPAR-mediated glutamate excitotoxicity [4, 7, 43]. However, FGF1 interactions with FGF1R protect neurons from glutamate excitotoxicity via GSK3β inactivation by the PI3K-Akt involved pathway [12], implying a neuroprotective function for FGF1 against glutamate excitotoxicity in motor neurons. Previous studies have shown that glutamate excitotoxicity is involved in motor neuron death in ALS [25, 29, 30, 35], and motor neuron degeneration can be triggered by induced glutamate transport defect [31]. Low FGF1 expression has also been shown in anterior horn in ALS [15]. Together, these data suggest that the low levels of FGF1 in motor neurons play a role in motor neuron death in ALS, and it could be postulated that FGF1 expression correlates to the differential resistance to injury seen in cholinergic neurons in motor nuclei.
Cholinergic neurons of the DMNV are more vulnerable to nerve damage and notoriously difficult to recover after injury, compared to neurons of other motor cranial nerves [2]. In a study in rats by Navaratnam et al. [22], marked neuronal loss with very limited recovery of neurons in the DMNV was observed after axonal crush of the vagus nerve. This vulnerability of neurons in the DMNV could be linked to the low number of cells positive for FGF1, which we observed in this study.
The DMNV shows a distinct functional somatotopic distribution from the rostral to the caudal region [5, 8, 16, 19]. The caudal lateral region of the DMNV innervates the trachea and esophagus, while inhibitory cardiovascular efferents extend from the mid to rostral lateral region of the DMNV [8]. Innervations of the subdiaphragmatic viscera extend bilaterally from the rostral to the caudal region of the DMNV, with efferents projecting to the stomach, intestines and pancreas [16, 19, 24]. We observed limited colocalization of FGF1 to cholinergic neurons in the lateral region of the DMNV in this study. In addition, we observed non-cholinergic FGF1-immunoreactive neurons, with 28% of FGF1-positive neurons not colocalized with ChAT-positive neurons in the rostral region. Previous studies have reported that the rostral region of the DMNV also contains noradrenergic and dopaminergic neurons [24, 38], although our immunohistochemical analysis revealed no colocalization of FGF1-positive neurons with TH-immunoreactive neurons in the DMNV.
Our results demonstrated that the pattern of distribution and localization of FGF1 immunoreactivity in the medulla oblongata of the cynomolgus monkey are similar to those reported in rodents. This suggests that FGF1 may have similar function in neuroprotection and neuroregeneration to that observed in the DMNV of non-primate animal models.
V. Conclusion
This study has successfully mapped the distribution of FGF1 immunoreactivity in the medulla oblongata of the cynomolgus monkey. Our results demonstrate that (1) FGF1 colocalized to cholinergic neurons of cranial nuclei in the medulla; (2) The DMNV has markedly lower FGF1 immunoreactivity compared to the hypoglossal nucleus, the facial nucleus, and the nucleus ambiguus; and, (3) FGF1 immunoreactivity is observed only in the lateral region of the DMNV. These observations are in good agreement with previous studies and suggest a potentially similar function for FGF1 in cranial nerve neurons to that seen in rodent models.
VI. Acknowledgments
This study was supported by Grants-in-Aid from the Ministry of Education, Culture, Science, Sports and Technology of Japan. The authors would like to thank Mr. T. Yamamoto of the Central Research Laboratory, Shiga University of Medical Science, for his excellent technical assistance in confocal microscopy.
VII. References
- 1.Abdelalim E. M., Tooyama I. Mapping of NPR-B immunoreactivity in the brainstem of Macaca fascicularis. Brain Struct. Funct. 2011;216:387–402. doi: 10.1007/s00429-011-0313-1. [DOI] [PubMed] [Google Scholar]
- 2.Aldskogius H., Barron K. D., Regal R. Axon reaction in dorsal motor vagal and hypoglossal neurons of the adult rat. Light microscopy and RNA-cytochemistry. J Comp. Neurol. 1980;193:165–177. doi: 10.1002/cne.901930111. [DOI] [PubMed] [Google Scholar]
- 3.Björklund A., Hökfelt T. vol. 2, Classical Transmitters in the CNS, Part 1. Elsevier; Amsterdam: 1984. Handbook of Chemical Neuroanatomy. [Google Scholar]
- 4.Carriedo S. G., Yin H. Z., Weiss J. H. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J. Neurosci. 1996;16:4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contreras R. J., Gomez M. M., Norgren R. Central origins of cranial nerve parasympathetic neurons in the rat. J. Comp. Neurol. 1980;190:373–394. doi: 10.1002/cne.901900211. [DOI] [PubMed] [Google Scholar]
- 6.Cuevas P., Carceller F., Redondo-Horcajo M., Lozano R. M., Giménez-Gallego G. Systemic administration of acidic fibroblast growth factor ameliorates the ischemic injury of the retina in rats. Neurosci. Lett. 1998;255:1–4. doi: 10.1016/s0304-3940(98)00672-7. [DOI] [PubMed] [Google Scholar]
- 7.Cull-Candy S., Kelly L., Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr. Opin. Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Dennison S. J., O’Connor B. L., Aprison M. H., Merritt V. E., Felten D. L. Viscerotopic localization of preganglionic parasympathetic cell bodies of origin of the anterior and posterior subdiaphragmatic vagus nerves. J. Comp. Neurol. 1981;197:259–269. doi: 10.1002/cne.901970207. [DOI] [PubMed] [Google Scholar]
- 9.Eckenstein F. P., Kuzis K., Nishi R., Woodward W. R., Meshul C., Sherman L., Ciment G. Cellular distribution, subcellular localization and possible functions of basic and acidic fibroblast growth factors. Biochem. Pharmacol. 1994;47:103–110. doi: 10.1016/0006-2952(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 10.Elde R., Cao Y., Cintra A., Brelje T. C., Pelto-Huikko M., Junttila T., Fuxe K., Pettersson R. F., Hökfelt T. Prominent expression of acidic fibroblast growth factor in motor and sensory neurons. Neuron. 1991;7:349–364. doi: 10.1016/0896-6273(91)90288-b. [DOI] [PubMed] [Google Scholar]
- 11.Ford-Perriss M., Abud H., Murphy M. Fibroblast growth factors in the developing central nervous system. Clin. Exp. Pharma. Physiol. 2001;28:493–503. doi: 10.1046/j.1440-1681.2001.03477.x. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto M., Sagara Y., Langford D., Everall I. P., Mallory M., Everson A., Digicaylioglu M., Masliah E. Fibroblast growth factor 1 regulates signaling via the glycogen synthase kinase-3β pathway: implications in neuroprotection. J. Biol. Chem. 2002;277:32985–32991. doi: 10.1074/jbc.M202803200. [DOI] [PubMed] [Google Scholar]
- 13.Hiruma H., Hikawa S., Kawakami T. Immunocytochemical colocalization of fibroblast growth factor-1 with neurotrophin-3 in mouse alveolar macrophages. Acta Histochem. Cytochem. 2012;45:131–137. doi: 10.1267/ahc.11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imamura T., Oka S., Tanahashi T., Okita Y. Cell cycle-dependent nuclear localization of exogenously added fibroblast growth factor-1 in BALB/c 3T3 and human vascular endothelial cells. Exp. Cell. Res. 1994;215:363–372. doi: 10.1006/excr.1994.1353. [DOI] [PubMed] [Google Scholar]
- 15.Kage M., Yang Q., Sato H., Matsumoto S., Kaji R., Akiguchi I., Kimura H., Tooyama I. Acidic fibroblast growth factor (FGF-1) in the anterior horn cells of ALS and control cases. Clin. Neurosci. Neuropathol. 2001;174:3799–3803. doi: 10.1097/00001756-200112040-00040. [DOI] [PubMed] [Google Scholar]
- 16.Kalia M., Mesulam M. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J. Comp. Neurol. 1980;193:435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- 17.Lee L. M., Huang M. C., Chuang T. Y., Lee L. S., Cheng H., Lee I. H. Acidic FGF enhances functional regeneration of adult dorsal roots. Life Sci. 2004;74:1937–1943. doi: 10.1016/j.lfs.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 18.Lee M. J., Chen C. J., Cheng C. H., Huang W. C., Kuo H. S., Wu J. C., Tsai M. J., Huang M. C., Chang W. C., Cheng H. Combined treatment using peripheral nerve graft and FGF-1: Changes to the glial environment and differential macrophage reaction in a complete transected spinal cord. Neurosci. Lett. 2008;433:163–169. doi: 10.1016/j.neulet.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 19.Leslie R. A., Gwyn D. G., Hopkins D. A. The central distribution of the cervical vagus nerve and gastric afferent and efferent projections in the rat. Brain Res. Bull. 1981;8:37–43. doi: 10.1016/0361-9230(82)90025-9. [DOI] [PubMed] [Google Scholar]
- 20.McLean J. H., Hopkins D. A. Ultrastructure of the dorsal motor nucleus of the vagus nerve in monkey with a comparison of synaptology in monkey and cat. J. Comp. Neurol. 1985;231:162–174. doi: 10.1002/cne.902310204. [DOI] [PubMed] [Google Scholar]
- 21.McLean J. H., Hopkins D. A. Ultrastructural studies of the nucleus ambiguus in cat and monkey following injection of HRP into the vagus nerve. J. Neurocytol. 1985;14:961–979. doi: 10.1007/BF01224807. [DOI] [PubMed] [Google Scholar]
- 22.Navaratnam V., Jacques T. S., Skepper J. N. Ultrastructural and cytochemical study of neurones in the rat dorsal motor nucleus of the vagus after axon crush. Microsc. Res. Techniq. 1998;42:334–344. doi: 10.1002/(SICI)1097-0029(19980901)42:5<334::AID-JEMT4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Okano H., Toyoda K., Bamba H., Hisa Y., Oomura Y., Imamura T., Furukawa S., Kimura H., Tooyama I. Localization of fibroblast growth factor-1 in cholinergic neurons innervating the rat larynx. J. Histochem. Cytochem. 2006;54:1061–1071. doi: 10.1369/jhc.5A6843.2006. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G. Vol. 2: Hindbrain and Spinal Cord. Academic Press, Inc.; Sydney, Australia: 1985. The Rat Nervous System. [Google Scholar]
- 25.Plaitakis A., Caroscio J. T. Abnormal glutamate metabolism in amyotrophic lateral sclerosis. Ann. Neurol. 1987;22:575–579. doi: 10.1002/ana.410220503. [DOI] [PubMed] [Google Scholar]
- 26.Prudovsky I., Tarantini F., Landriscina M., Neivandt D., Soldi R., Kirov A., Small D., Kathir K. M., Rajalingam D., Kumar T. K. Secretion without Golgi. J. Cell. Biochem. 2008;103:1327–1343. doi: 10.1002/jcb.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuss B., von Bohlen und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313:139–157. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Enfedaque A., Bouleau S., Laurent M., Courtois Y., Mignotte B., Vayssiere J. L., Renaud F. FGF1 nuclear translocation is required for both its neurotrophic activity and its p53-dependent apoptosis protection. Biochim. Biophys. Acta. 2009;1793:1719–1727. doi: 10.1016/j.bbamcr.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Rothstein J. D., Kuncl R., Chaudhry V., Clawson L., Cornblath D. R., Coyle J. T., Drachman D. B. Excitatory amino acids in amyotrophic lateral sclerosis: an update. Ann. Neurol. 1991;30:224–225. doi: 10.1002/ana.410300223. [DOI] [PubMed] [Google Scholar]
- 30.Rothstein J. D., Martin L. J., Kuncl R. W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N. Engl. J. Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 31.Rothstein J. D., Jin L., Dykes-Hoberg M., Kuncl R. W. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc. Natl. Acad. Sci. U S A. 1993;901:6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell J. C., Szuflita N., Khatri R., Laterra J., Hossain M. A. Transgenic expression of human FGF-1 protects against hypoxic–ischemic injury in perinatal brain by intervening at caspase-XIAP signaling cascades. Neurobiol. Dis. 2006;22:677–690. doi: 10.1016/j.nbd.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Saito A., Okano H., Bamba H., Hisa Y., Oomura Y., Imamura T., Tooyama I. Low expression of FGF1 (fibroblast growth factor-1) in rat parasympathetic preganglionic neurons. Histol. Histopathol. 2007;22:1327–1335. doi: 10.14670/HH-22.1327. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki K., Tooyama I., Li A. J., Oomura Y., Kimura H. Effects of an acidic fibroblast growth factor fragment analog on learning and memory and on medial septum cholinergic neurons in senescence-accelerated mice. Neuroscience. 1999;92:1287–1294. doi: 10.1016/s0306-4522(99)00087-1. [DOI] [PubMed] [Google Scholar]
- 35.Spreux-Varoquaux O., Bensimon G., Lacomblez L., Salachas F., Pradat P. F., Le Forestier N., Marouan A., Dib M., Meininger V. Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: a reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. J. Neurol. Sci. 2002;193:73–78. doi: 10.1016/s0022-510x(01)00661-x. [DOI] [PubMed] [Google Scholar]
- 36.Stichel C. C., Müller H. W. Experimental strategies to promote axonal regeneration after traumatic central nervous system injury. Prog. Neurobiol. 1998;56:119–148. doi: 10.1016/s0301-0082(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 37.Stock A., Kuzis K., Woodward W. R., Nishi R., Eckenstein F. P. Localization of acidic fibroblast growth factor in specific subcortical neuronal populations. J. Neurosci. 1992;12:4688–4700. doi: 10.1523/JNEUROSCI.12-12-04688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swanson L. W., Hartman B. K. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-β-hydroxylase as a marker. J. Comp. Neurol. 1975;163:467–506. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi S., Masuda C., Maebayashi H., Tooyama I. Immunohistochemical mapping of TRK-fused gene products in the rat brainstem. Acta Histochem. Cytochem. 2012;45:57–64. doi: 10.1267/ahc.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas K. A., Rios-Candelore M., Firzpatrick S. Purification and characterization of acidic fibroblast growth factor from bovine brain. Proc. Natl. Acad. Sci. U S A. 1984;81:357–361. doi: 10.1073/pnas.81.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tooyama I., Sasaki K., Oomura Y., Li A. J., Kimura H. Effect of acidic fibroblast growth factor on basal forebrain cholinergic neurons in senescence-accelerated mice. Exp. Gerontol. 1997;32:171–179. doi: 10.1016/s0531-5565(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 42.Toyoda K., Okano H., Bamba H., Hisa Y., Oomura Y., Imamura T., Tooyama I. Comparison of FGF1 (aFGF) expression between the dorsal motor nucleus of vagus and the hypoglossal nucleus of rat. Acta Histochem. Cytochem. 2006;39:1–7. doi: 10.1267/ahc.05047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Von Lewinski F., Keller B. U. Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS. Trends Neurosci. 2005;28:494–500. doi: 10.1016/j.tins.2005.07.001. [DOI] [PubMed] [Google Scholar]