Abstract
The purpose of this study was to investigate the effect of chronic moderate-intensity training in order to prevent muscle atrophy with a focus on TNF-α and atrogin-1/MAFbx as main proteolytic indicators. Hindlimb unloading model of mice received treadmill running exercise for 1 hr per day during hindlimb unloading period of 6 weeks. The gastrocnemius muscle mass, muscle fiber cross-sectional area, and succinate dehydrogenase (SDH) activity in the muscle fiber were higher in the exercised group, while TNF-α and atrogin-1/MAFbx mRNA expressions were significantly lower. Results in the present study showed that chronic exercise could prevent over expression of TNF-α and atrogin-1/MAFbx in the atrophied skeletal muscle, providing further support to the effects of chronic exercise training on muscle atrophy.
Keywords: TNF-α, atrogin-1/MAFbx, gastrocnemius, muscle atrophy
I. Introduction
Tumor necrosis factor-α (TNF-α) is a polypeptide cytokine that has been associated with muscle wasting and weakness in inflammatory disease [35]. It has been reported that TNF-α stimulates muscle catabolism by activating the ubiquitin/proteasome pathway [23–25], which is the major pathway for selective protein degradation implicated in muscle atrophy, resulting from conditions such as unloading, denervation, and immobilization [34]. In the ubiquitin/proteasome pathway, the targeted protein is labeled by E3 ligases: in skeletal muscle, two E3 proteins: atrogin-1/MAFbx and MuRF1 are up-regulated and appear to be essential for accelerated muscle protein loss in a variety of experimental models of catabolism including hindlimb unloading [6]. In the study by Li et al., they suggest that TNF-α acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin-1/MAFbx in the skeletal muscle [20].
Hindlimb unloading has been used in rodents as a model of disuse to induce muscle atrophy [19, 38]. Previous studies have shown that exercise is an effective countermeasure to attenuate muscle loss induced by hindlimb unloading [1, 8, 10, 13]. In these studies, short-term resistance exercise had been used to attenuate the proteolytic process. However, there haven’t been any studies regarding the effect of chronic exercise training on muscle atrophy induced by hindlimb unloading, focusing on the regulation of muscle proteolytic mediators. Exercise training in moderate intensity can have an influence on skeletal muscles to secrete cytokines in healthy rats [22]. We hypothesized that chronic moderate intensity exercising would down-regulate TNF-α in atrophied skeletal muscle and also down-regulate the atrogin-1/MAFbx level, attenuating the overall ATP dependent Ubiquitin-proteasome muscle proteolysis pathway. In this study, a program of moderate-intensity exercises was used on a hindlimb unloading model of mice, to investigate the effects of chronic exercise on down-regulation of proteolytic mediators like TNF-α, and atrogin-1/MAFbx, which are main atrophy mediators.
II. Materials and Methods
Animal model and experimental design
Twenty-two adult male ICR mice weighing in 32–34 g (Japan SLC, Shizuoka) were used in the present study. The animals were housed in individual cages in a temperature-controlled room at 22±2°C with a light-dark cycle of 12 hr and maintained on mice chow and water ad libitum. The animals were randomly divided into four groups; hindlimb unloading (HU, n=5), hindlimb unloading with exercise (HU+Ex, n=5), control with exercise (Con+Ex, n=7), and age-matched control (Con, n=5) groups. The animals in the HU and HU+Ex groups were applied hindlimb unloading for 6 weeks as described previously [26, 27]. The suspended height was adjusted to allow the forelimbs to maintain contact with the floor and move freely, and to prevent any contact between the hind limbs and any cage surface. Animals in the Con+Ex, and HU+Ex groups ran for 60 min in a day on the treadmill (Osaka Micro System, Osaka, Japan) at a speed of 18 mmin−1. Mice in the HU+Ex group were reloaded on their hindlimbs to run on the treadmill. This exercise was carried out over a period of 6 weeks; 6 times per week, during the 6 weeks period of unloading. The animals’ weights were checked once every week when exercises were not being conducted. This study was approved by the Institution of Animal Care and Use Committee and carried out according to Kobe University Animal Experimentation Regulation. All experiments were conducted in accordance with the principles of laboratory animal care (NIH publication No. 85-23, revised 1985).
Muscle sample preparation
After 24 hr of the last treadmill session, all animals were sacrificed via an overdose of sodium pentobarbital. The gastrocnemius (GAS) muscle was excised and weighed. Thereafter, the lateral portion of GAS muscle was immediately frozen in acetone pre-cooled by dry ice and stored at –80°C until the histological and immunological analyses. For total RNA isolation, the medial portion of GAS muscle was immersed in RNAlater solution (Ambion, TX) and stored.
Histological procedure for fiber type differentiation and SDH activity
The middle part of the muscle belly was attached to the cryostat chuck, and transverse sections (10 µm in thickness) were cut on a cryostat microtome (CM1510S, Leica Instruments, Heidelberg, Germany) at −20°C. The sections were then stained for myofibrillar adenosine triphosphatase (mATPase) at pH 4.55 preincubation to differentiate the muscle fibers as types IID, or IIB on the basis of a previous study by Punkt et al. [33]. For ATPase staining, the sections were preincubated in barbital acetate buffer (pH 4.55) for 5 min at room temperature. Following wash by 0.1 M barbital buffer containing 0.18 M CaCl2 (pH 9.4) for 30 sec, the sections were incubated in 0.1 M barbital buffer containing 0.18 M CaCl2 and 4 mM ATP (pH 9.4) for 45 min at room temperature. The sections were then washed in 1% CaCl2 and 2% CoCl2 for 3 min, washed 0.01 M sodium barbital. Following wash by distilled water, the sections were visualized by 1% ammonium sulfide. The sections stained by ATPase were used to determine the composition of muscle fiber types and to measure cross-sectional areas of each muscle fiber type. The sections were also stained for succinate dehydrogenase (SDH) activity, to determine the level of mitochondrial oxidative capacity. The sections were dried at room temperature for 30 min, incubated in 0.05% nitroblue tetrazolium and 0.05 M sodium succinate in 0.05 M phosphate buffer (pH 7.5) for 45 min at 37°C. Each muscle fiber was matched for ATPase and SDH stains. Images were visualized with a light microscope and photographed by attached CCD camera (BX51, Olympus, Tokyo, Japan). Using the sectional images, the muscle fibers were categorized as types IID (high oxidative), and IIB (low oxidative) fibers. Results were focused on superficial layer of GAS muscle as it has more abundance for fast twitch muscle fibers, which are responsible on IL-6 secretion following exercise [15] and in which TNF-α is more prominent in skeletal muscle [32]. Cross-sectional area and SDH activity of at least 50 muscle fibers were measured using the ImageJ software program (NIH, Bethesda, MD).
ELISA for TNF-α
The right GAS muscle of each animal was homogenized in ice-cooled homogenization buffer (10 mM NaCl and 10 mM Tris-HCL, pH 7.4), containing a protease inhibitor cocktail (1:200, Sigma, MO). The homogenates were centrifuged at 15000 g for 15 min at 4°C. The supernatant was saved, and protein concentration was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA) with bovine serum albumin as a standard. Quantitative assessment of TNF-α protein was carried using a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to manufacturer’s instructions (eBioscience, San Diego, CA).
Real-time quantitative polymerase chain reaction (qPCR) analyses
Total RNA was extracted from ~10 mg of GAS muscle using an extraction kit (QuickGene RNA tissue kit SII, Fujifilm, Tokyo, Japan). Reverse transcription was carried out using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA), and cDNA samples were stored at −20°C. Expression levels of TNF-α (Mn00443258_m1), atrogin-1/MAFbx (Mn00499518_m1), and IL-6 (Mn00446790_m1) mRNA were quantified by TaqMan Gene Expression Assays (Applied Biosystems). Each TaqMan probe and primer set was validated by performing qPCR with a series of cDNA template dilutions to obtain standard curves of threshold cycle time against relative concentration using the normalization gene 18S. The qPCR was performed using PCR Fast Universal Master Mix (Applied Biosystems) in a MicroAmp 96-well reaction plate. Each well contained 1 µL cDNA template, 10 µL PCR Fast Master Mix (Applied Biosystems), 8 µL RNase-free water, and 1 µL TaqMan Gene Expression Assays in a reaction volume of 20 µL. All samples and non-template control reactions were performed in a 7500 Fast Sequence Detection System (Applied Biosystems) at 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min.
Statistical analyses
The data are expressed as mean±SE. Overall differences were determined using a one-way analysis of variance (ANOVA). When ANOVA was significant, group differences were determined using the Tukey’s post-hoc test. The statistically significant level was set at P<0.05.
III. Results
Body and muscle wet weights
The mean body weights of the animals reflected a continuous weight gain through the six weeks period of the experiment. During the first week after start of unloading, the mean body weights increased in the Con, HU, and HU+Ex groups, i.e. 40.2±0.3, 36.4±0.7, and 35.8±0.3 g, respectively; while the Con+Ex group remained a mean of 32.4±0.6 g in the first week. After 6 weeks of unloading, the mean body weights in the Con, Con+Ex, HU, and HU+Ex groups were 44.6±0.9, 37.7±0.7, 39.7±1.2, and 38.6±1.0 g, respectively. The Con+Ex, HU, and HU+Ex groups mean body weights were significantly less than Con group (Table 1). The mean absolute and relative GAS muscle weights in the HU group were 34% and 26% smaller than those in the Con group, respectively, whereas the values in the HU+Ex group were 24% and 12% smaller than those in the Con group, respectively. The absolute GAS muscle weight in the Hu+Ex group was significantly larger than that in the HU group, and the relative GAS muscle weight in the HU+Ex group showed no significant difference from the Con group. The mean absolute and relative GAS muscle weights in the Con+Ex group were 19% and 2% smaller than those in the Con group, respectively.
Table 1.
Changes in body weight and gastrocnemius muscle weight after six weeks
| Body weight (g) | Muscle wet weight (mg) | Muscle weight/body weight (mg/g) | |
|---|---|---|---|
| Con | 44.6±0.9 | 188±6 | 4.2±0.02 |
| Con+Ex | 37.7±0.7* | 153±4* | 4.1±0.1 |
| HU | 39.7±1.2* | 124±5*† | 3.12±0.2*† |
| HU+Ex | 38.6±1.0* | 143±4*‡ | 3.7±0.1 |
Con, control group; Con+Ex, control with exercise group; HU, hindlimb unloading group; HU+Ex, hindlimb unloading with exercise group. Values are means±SE. *, †, and ‡ are significantly different from the Con, Con+Ex, and HU groups, respectively, at P<0.05.
Muscle fiber cross-sectional area
The fibers in superficial layer of GAS muscle were divided into types IID (high oxidative) and IIB (low oxidative) (Fig. 1). In the mATPase stain, type IID fibers were smaller in size and darker in stain than type IIB fibers, which were bigger in size and lighter in stain. Type IID fibers cross-sectional area mean values were 1257±46, 1313±31, 992±49, and 1215±49 µm2, for the Con, Con+Ex, Hu, and HU+Ex groups, respectively; showing a significant decrease of 21% in the HU group when compared to Con group, also showing a significant increase of 18% in HU+Ex group when compared to the HU group (Fig. 2). There were no significant differences between the Con and Con+Ex groups. Type IIB fibers cross-sectional area mean values were 2639±61, 2249±51, 1959±52, and 2245±56 µm2, for the Con, Con+Ex, HU, and HU+Ex groups, respectively; showing a significant decrease of 26% in the HU group when compared to Con group, also showing a significant increase of 13% in the HU+Ex group when compared to the HU group (Fig. 2). The Con+Ex group showed a significant decrease of 15% when compared to the Con group.
Fig. 1.
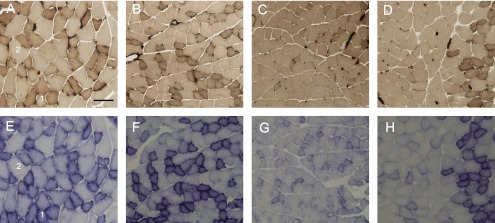
Light microscope images of superficial layer of the gastrocnemius muscle. Transverse sections were assayed for myofibrillar adenosine triphoshphatase (mATPase) at pH 4.55 preincubation (upper), and for succinate dehydrogenase (SDH) activity (lower). 1: type IID (high oxidative); 2: type IIB (low oxidative). A, E: control group; B, F: Con+Ex group; C, G: HU group; D, H: HU+Ex group. Bar=50 µm.
Fig. 2.
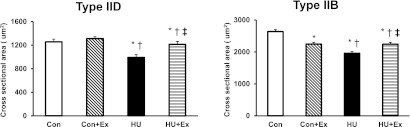
Cross-sectional area of types IID, and IIB fibers in superficial layer of the gastrocnemius muscle. Con, control group; Con+Ex, control with exercise group; HU, hindlimb unloading group; HU+Ex, hindlimb unloading with exercise group. Values are means±SE. *, †, and ‡ are significantly different from the Con, Con+Ex, and HU groups, respectively, at P<0.05.
Succinate dehydrogenase activity
The Con+Ex group showed a significant increase of SDH activity in both types of fibers compared to other groups (Fig. 3). The SDH activity of types IID, and IIB fibers showed a significant decrease in the HU group when compared to the other groups, also the HU+Ex group showed a significant increase in SDH activity of types IID and IIB fibers when compared to HU group (Fig. 3).
Fig. 3.
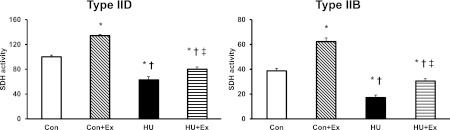
SDH activity of types IID (high oxidative), and IIB (low oxidative) fibers in superficial layer of the gastrocnemius muscle. Con, control group; Con+Ex, control with exercise group; HU, hindlimb unloading group; HU+Ex, hindlimb unloading with exercise group. Values are means±SE. *, †, and ‡ are significantly different from the Con, Con+Ex, and HU groups, respectively, at P<0.05.
TNF-α expression
The expression level of TNF-α protein concentration in the HU group significantly increased by 4-fold from the Con group (Fig. 4A). In contrast, there were no significant differences between the Con, Con+Ex and HU+Ex groups. The expression level of TNF-α mRNA in the HU group showed a significant increase by 3-fold from the Con group (Fig. 4B). The value in the HU+Ex group was significantly less than that in the HU group, maintained at control level.
Fig. 4.
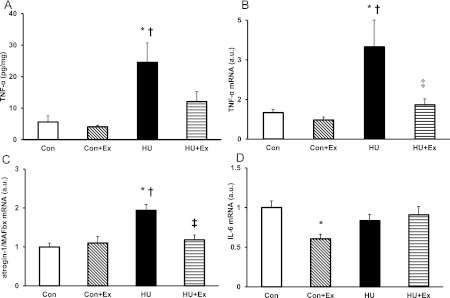
TNF-α protein concentration level (A), and mRNA expression levels of TNF-α (B), atrogin-1/MAFbx (C), and IL-6 (D) in the gastrocnemius muscle. Con, control group; Con+Ex, control with exercise group; HU, hindlimb unloading group; HU+Ex, hindlimb unloading with exercise group. Values are means±SE. *, †, and ‡ are significantly different from the Con, Con+Ex, and HU groups, respectively, at P<0.05.
Atrogin-1/MAFbx expression
The expression level of atrogin-1/MAFbx mRNA in the HU group showed a significant increase by 2-fold from the Con group, while the HU+Ex group expression level was significantly lower than the HU group, being maintained at the Con group level (Fig. 4C). There were no significant differences between the Con and Con+Ex groups.
IL-6 expression
The expression level of IL-6 mRNA showed a significant decrease in the Con+Ex group from the Con group only, while there were no significant differences between the other groups (Fig. 4D).
IV. Discussion
The present study demonstrated the preventive effects of chronic exercise training on muscle atrophy, induced by hindlimb unloading for 6 weeks in mouse gastrocnemius muscle. In the present study, the gastrocnemius muscle showed a significant atrophy after 6 weeks of unloading; this is consistent with previous studies [21, 27, 29]. The exercise protocol used in the present study attenuated a decrease in muscle weight and cross-sectional area in types IID and IIB muscle fibers during unloading. Running exercise in the present study did not cause muscle hypertrophy in the Con+Ex group as shown in Table 1. But it was capable of attenuating muscle atrophy in the HU+Ex group through decreasing protein degeneration process, which continued in the non-exercised HU group, resulting in a significant decrease in HU group’s muscle weight, and muscle weight/body weight ratio. This suggests that moderate intensity treadmill training is capable of preventing muscle atrophy. Although most of the studies on exercise countermeasures for hindlimb unloading induced atrophy used resistance exercise protocols [5, 11, 14], our results in the present study are consistent with a study done by Gwag et al. [12] in the ability of endurance exercises in attenuating muscle atrophy induced by hindlimb unloading. Endurance exercise training is known to affect oxidative muscle fiber types, and transformation of glycolytic fibers into oxidative types [7]. This can explain why type IIB fibers cross-sectional area in Con+Ex group was smaller than Con group, also in HU+Ex group, type IIB fibers cross-sectional area was significantly bigger than HU group, but also significantly smaller than Con, and Con+Ex groups, meaning that exercise protocol did not affect type IIB fibers cross-sectional area in a hypertrophic manner. The chronic exercise training resulted in the inhibition of the over expressions of TNF-α and atrogin-1/MAFbx mRNA in the atrophied muscle, suggesting evidence for the role of chronic exercise training in regulating proteolytic pathway mediators (mainly TNF-α) which is considered as one of the triggering cytokines of many proteolytic pathways.
It has been reported that reactive oxygen species (ROS) and elevated proinflammatory cytokines, in particular TNF-α, mediate muscle atrophy; ROS can also initiate the mitochondrial-driven apoptosis pathway [4]. In the present study, the SDH activity of type IID (high oxidative) and type IIB (low oxidative) fibers in the HU group were significantly lower than the other groups. Mukhina et al. [28] stated that unloading inhibits SDH activity in muscles. The exercised groups (i.e. the Con+Ex and HU+Ex groups) showed significant increases in the SDH activity in both types of fibers, this is consistent with a previous study in which running exercises increased SDH activity in both types of fibers of the mouse tibialis anterior [17]. In our study, chronic exercise program prevented the atrophic changes on the oxidative level, due to the ability of endurance exercises to increase oxidative capacity and decrease the effects of ROS, as stated by Wenz et al. [39].
TNF-α is a polypeptide cytokine that has been associated with muscle wasting and weakness in inflammatory disease [35], it acts via p38 to increase atrogin-1/MAFbx gene expression in the skeletal muscle. atrogin-1/MAFbx gene product is up-regulated in response to TNF-α [20]. Furthermore, TNF-α is considered essential for accelerated muscle protein loss in a variety of experimental models of catabolism, including hindlimb suspension [20]. It has been reported that the decrease in the muscle fiber’s cross-sectional area with atrophy was attenuated in atrogin-1/MAFbx-deficient mice [6]; the functional importance of atrogin-1/MAFbx is evident. In both ELISA and qPCR results for TNF-α in our study, the HU group’s expression levels were significantly elevated compared to control groups; while exercises maintained a low level of TNF-α concentration, and a significantly low expression of TNF-α mRNA compared to the HU group. Exercise training is able to decrease the level of TNF-α through muscle contraction and release of epinephrine, which in turn decreases elevated TNF-α [36]. Additionally, atrogin-1/MAFbx mRNA level was significantly elevated in the HU group, while exercises maintained a significant decrease of atrogin-1/MAFbx mRNA expression in the HU+Ex group. According to Li et al. [20], down-regulation of TNF-α through exercise training resulted in down-regulation of atrogin-1/MAFbx production, reflecting the preventive effect of chronic exercise training on attenuation of muscle atrophy. IL-6 is an immunomodulatory cytokine, and increases 100-fold in skeletal muscle during acute exercise, remaining elevated in the circulation after prolonged exercise session (~1-h post-exercise, [30]). Additionally, it has been reported that IL-6 has a role in inhibiting TNF-α production [31]. IL-6 mRNA expression levels in the present study showed a significant difference in the Con+Ex group only, being decreased from the Con group; but not showing any differences among the other groups. This low level of IL-6 mRNA expression in mouse muscle tissue is consistent with a study on human muscle tissue done by Fischer et al. [9] as they stated that IL-6 derived from contracting skeletal muscle is required less in the trained state. A study by Lira et al. [22] showed the ability of chronic exercise in decreasing IL-6 in healthy rat skeletal muscle, and same result was obtained in our study in skeletal muscle of healthy mouse represented by Con+Ex group. Many studies on muscle derived IL-6 focused on immediate responses of resistive or eccentric bouts of exercises [18, 37]. It was speculated that the difference in exercise protocol between present (endurance exercise) and previous studies (resistance exercise) caused different results for IL-6. Jonsdottir et al. [18] concluded that IL-6 is locally produced in the skeletal muscle after muscle contractions, with no significant differences between the various muscle fiber types. While it was stated by Plomgraad et al. [32] that the cytokine expression is fiber-type specific, the protein expression of IL-6 is higher in the fast fiber than in the slow fiber.
Previous studies have been reported that resistance exercise protocols contribute to induce hypertrophy of skeletal muscle and decrease the loss of muscle mass induced by hindlimb unloading [2, 3, 16]. In the study by Lira et al., a program of chronic exercises has managed to decrease cytokine production including TNF-α in healthy rat skeletal muscles [22]. Our results in the present study showed that chronic exercise could prevent overexpression of TNF-α and atrogin-1/MAFbx in the atrophied skeletal muscle. This provides further evidence for the effect of exercise on muscle atrophy. It was found that not only resistance exercise protocols, but also chronic endurance exercises could attenuate skeletal muscle atrophy induced by hindlimb unloading through down-regulation of TNF-α levels and indirect down-regulation of atrogin-1/MAFbx gene expression.
V. Acknowledgment
This study was supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
VI. References
- 1.Adams G. R., Haddad F., Bodell P. W., Tran P. D., Baldwin K. M. Combined isometric, concentric, and eccentric resistance exercise prevents unloading-induced muscle atrophy in rats. J. Appl. Physiol. 2007;103:1644–1654. doi: 10.1152/japplphysiol.00669.2007. [DOI] [PubMed] [Google Scholar]
- 2.Allen D. L., Linderman J. K., Roy R. R., Bigbee A. J., Grindeland R. E., Mukku V., Edgerton V. R. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am. J. Physiol. 1997;273:C579–C587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- 3.Allen D. L., Linderman J. K., Roy R. R., Grindeland R. E., Mukku V., Edgerton V. R. Growth hormone/IGF-I and/or resistive exercise maintains myonuclear number in hindlimb unweighted muscles. J. Appl. Physiol. 1997;83:1857–1861. doi: 10.1152/jappl.1997.83.6.1857. [DOI] [PubMed] [Google Scholar]
- 4.Andrianjafiniony T., Dupré-Aucouturier S., Letexier D., Couchoux H., Desplanches D. Oxidative stress, apoptosis, and proteolysis in skeletal muscle repair after unloading. Am. J. Physiol. Cell Physiol. 2010;299:C307–C315. doi: 10.1152/ajpcell.00069.2010. [DOI] [PubMed] [Google Scholar]
- 5.Bajotto G., Shimomura Y. Determinants of disuse-induced skeletal muscle atrophy: exercise and nutrition countermeasures to prevent protein loss. J. Nutr. Sci. Vitaminol. (Tokyo) 2006;52:233–247. doi: 10.3177/jnsv.52.233. [DOI] [PubMed] [Google Scholar]
- 6.Bodine S. C., Latres E., Baumhueter S., Lai V. K., Nunez L., Clarke B. A., Poueymirou W. T., Panaro F. J., Na E., Dharmarajan K. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 7.Díaz-Herrera P., Torres A., Morcuende J., García-Castellano J., Calbet A., Sarrat R. Effect of endurance running on cardiac and skeletal muscle in rats. Histol. Histopathol. 2001;16:29–35. doi: 10.14670/HH-16.29. [DOI] [PubMed] [Google Scholar]
- 8.Dupont-Versteegden E. E., Fluckey J. D., Knox M., Gaddy D., Peterson C. A. Effect of flywheel-based resistance exercise on processes contributing to muscle atrophy during unloading in adult rats. J. Appl. Physiol. 2005;101:202–212. doi: 10.1152/japplphysiol.01540.2005. [DOI] [PubMed] [Google Scholar]
- 9.Fischer C. P., Plomgaard P., Hansen A. K., Pilegaard H., Saltin B., Pedersen B. K. Endurance training reduces the contraction-induced interleukin-6 mRNA expression in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2004;287:E1189–1194. doi: 10.1152/ajpendo.00206.2004. [DOI] [PubMed] [Google Scholar]
- 10.Fluckey J. D., Dupont-Versteegden E. E., Montague D. C., Knox M., Tesch P., Peterson C. A., Gaddy-Kurten D. A rat resistance exercise regimen attenuates losses of musculoskeletal mass during hindlimb suspension. Acta Physiol. Scand. 2002;176:293–300. doi: 10.1046/j.1365-201X.2002.01040.x. [DOI] [PubMed] [Google Scholar]
- 11.Graham S. C., Roy R. R., West S. P., Thomason D., Baldwin K. M. Exercise effects on the size and metabolic properties of soleus fibers in hindlimb-suspended rats. Aviat. Space Environ. Med. 1989;60:226–234. [PubMed] [Google Scholar]
- 12.Gwag T., Lee K., Ju H., Shin H. J., Lee W., Choi I. Stress and signaling responses of rat skeletal muscle to brief endurance exercise during hindlimb unloading: a catch-up process for atrophied muscle. Cell. Physiol. Biochem. 2009;24:537–546. doi: 10.1159/000257510. [DOI] [PubMed] [Google Scholar]
- 13.Haddad F., Adams G. R., Bodell P. W., Baldwin K. M. Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J. Appl. Physiol. 2006;100:433–441. doi: 10.1152/japplphysiol.01203.2005. [DOI] [PubMed] [Google Scholar]
- 14.Herbert M. E., Roy R. R., Edgerton V. R. Influence of one-week hindlimb suspension and intermittent high load exercise on rat muscles. Exp. Neurol. 1988;102:190–198. doi: 10.1016/0014-4886(88)90093-3. [DOI] [PubMed] [Google Scholar]
- 15.Hiscock N., Chan M. H., Bisucci T., Darb I. A., Febbraio M. A. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J. 2004;18:992–994. doi: 10.1096/fj.03-1259fje. [DOI] [PubMed] [Google Scholar]
- 16.Hurst J. E., Fitts R. H. Hindlimb unloading-induced muscle atrophy and loss of function: protective effect of isometric exercise. J. Appl. Physiol. 2003;95:1405–1417. doi: 10.1152/japplphysiol.00516.2002. [DOI] [PubMed] [Google Scholar]
- 17.Ishihara A., Hirofuji C., Nakatani T., Itoh K., Itoh M., Katsuta S. Effects of running exercise with increasing loads on tibialis anterior muscle fibers in mice. Exp. Physiol. 2002;87:113–116. doi: 10.1113/eph8702340. [DOI] [PubMed] [Google Scholar]
- 18.Jonsdottir I. H., Schjerling P., Ostrowski K., Asp S., Richter E. A., Pedersen B. K. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J. Physiol. 2000;528:157–163. doi: 10.1111/j.1469-7793.2000.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasper C. E., McNulty A. L., Otto A. J., Thomas D. P. Alterations in skeletal muscle related to impaired physical mobility: an empirical model. Res. Nurs Health. 1993;16:265–273. doi: 10.1002/nur.4770160405. [DOI] [PubMed] [Google Scholar]
- 20.Li Y. P., Chen Y., John J., Moylan J., Jin B., Mann D. L., Reid M. B. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liantonio A., Pierno S., Digennaro C., Giannuzzi V., Gramegna G., Desaphy J. F., Camerino D. C. The hindlimb unloading model of microgravity differently affects skeletal muscle properties of young and adult mice. Basic Applied Myology. 2009;19:107–112. [Google Scholar]
- 22.Lira F. S., Koyama C. H., Yamashita A. S., Rosa J. C., Zanchi N. E., Batista M. L., Jr., Seelaender M. C. Chronic exercise decreases cytokine production in healthy rat skeletal muscle. Cell Biochem. Funct. 2009;27:458–461. doi: 10.1002/cbf.1594. [DOI] [PubMed] [Google Scholar]
- 23.Llovera M., Martinez C. G., Agell N., Lopez-Soriano F. J., Argilés J. M. TNF can directly induce the expression of ubiquitin-dependent proteolytic system in rat soleus muscles. Biochem. Biophys. Res. Commun. 1997;230:238–241. doi: 10.1006/bbrc.1996.5827. [DOI] [PubMed] [Google Scholar]
- 24.Martinez C. G., Agell N., Llovera M., Lopez-Soriano F. J., Argilés J. M. Tumor necrosis factor-α increases the ubiquitinization of rat skeletal muscle proteins. FEBS Lett. 1993;323:211–214. doi: 10.1016/0014-5793(93)81341-v. [DOI] [PubMed] [Google Scholar]
- 25.Martinez C. G., Llovera M., Agell N., Lopez-Soriano F. J., Argilés J. M. Ubiquitin gene expression in skeletal muscle is increased during sepsis: involvement of TNF-alpha but not IL-1. Biochem. Biophys. Res. Commun. 1995;217:839–844. doi: 10.1006/bbrc.1995.2848. [DOI] [PubMed] [Google Scholar]
- 26.Morey E. R., Sabelman E. E., Turner R. T., Baylink D. J. A new rat model simulating some aspects of space flight. Physiologist. 1979;22:S23–S24. [PubMed] [Google Scholar]
- 27.Morey-Holton E. R., Globus R. K. Hindlimb unloading rodent model: technical aspects. J. Appl. Physiol. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- 28.Mukhina A. M., Nemirovskaia T. L., Larina I. M., Pastushkova L. Kh., Vasil’eva GIu., Istomina V. E., Veselova O. M., Turtikova O. V., Shenkman B. S. Effects of creatine phosphokinase competitive inhibitor on system and tissue energy metabolism in rats in the norm and during unloading. Aviakosm. Ekolog. Med. 2008;42:35–39. [PubMed] [Google Scholar]
- 29.Parco M. S., Emidio E. P., Stephen E. A. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading. J. Appl. Physiol. 2008;105:1695–1705. doi: 10.1152/japplphysiol.90800.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen B. K., Akerström T. C., Nielsen A. R., Fischer C. P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007;103:1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 31.Petersen A. M., Pedersen B. K. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J. Physiol. Pharmacol. 2006;57:43–51. [PubMed] [Google Scholar]
- 32.Plomgaard P., Penkowa M., Pedersen B. K. Fiber type specific expression of TNF-alpha, IL-6 and IL-18 in human skeletal muscles. Exerc. Immunol. Rev. 2005;11:53–63. [PubMed] [Google Scholar]
- 33.Punkt K., Naupert A., Asmussen G. Differentiation of rat skeletal muscle fibres during development and ageing. Acta Histochem. 2004;106:145–154. doi: 10.1016/j.acthis.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Reid M. B. Response of the ubiquitin-proteasome pathway to changes in muscle activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1423–R1431. doi: 10.1152/ajpregu.00545.2004. [DOI] [PubMed] [Google Scholar]
- 35.Reid M. B., Li Y. P. Tumor necrosis factor-α and muscle wasting: a cellular perspective. Respir. Res. 2001;2:269–272. doi: 10.1186/rr67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starkie R., Ostrowski S., Jauffred S., Febbraio M., Pedersen B. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-α production in humans. FASEB J. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 37.Steensberg A., Keller C., Starkie R. L., Osada T., Febbraio M. A., Pedersen B. K. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2002;283:E1272–E1278. doi: 10.1152/ajpendo.00255.2002. [DOI] [PubMed] [Google Scholar]
- 38.Thomason D. B., Booth F. W. Atrophy of the soleus muscle by hindlimb unweighting. J. Appl. Physiol. 1990;68:1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Wenz T., Diaz F., Hernandez D., Moraes C. T. Endurance exercise is protective for mice with mitochondrial myopathy. J. Appl. Physiol. 2009;106:1712–1719. doi: 10.1152/japplphysiol.91571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


