SUMMARY
Chromatin dynamics play a central role in maintaining genome integrity, but how this is achieved remains largely unknown. Here, we report that microrchidia CW-type zinc finger 2 (MORC2), an uncharacterized protein with a derived PHD finger domain and a conserved GHKL-type ATPase module, is a physiological substrate of p21-activated kinase 1 (PAK1), an important integrator of extracellular signals and nuclear processes. Following DNA damage, MORC2 is phosphorylated on serine 739 in a PAK1 dependent manner, and phosphorylated MORC2 regulates its DNA-dependent ATPase activity to facilitate chromatin remodeling. Moreover, MORC2 associates with chromatin and promotes gamma-H2AX induction in a PAK1 phosphorylation-dependent manner. Consequently, cells expressing MORC2-S739A mutation displayed a reduction in DNA repair efficiency and were hypersensitive to DNA-damaging agent. These findings suggest that the PAK1-MORC2 axis is critical for orchestrating the interplay between chromatin dynamics and the maintenance of genomic integrity through sequentially integrating multiple essential enzymatic processes.
Keywords: Chromatin remodeling, DNA damage response, Genomic stability, Modifier of radiosensitivity, MORC2
INTRODUCTION
Cellular DNA is constantly being damaged by exogenous and endogenous DNA-damaging agents. Cell survival and maintenance of genomic integrity are dependent on the efficient and accurate repair of DNA double-strand breaks (DSBs) in the context of chromatin (Chen et al., 2008). The basic building block of chromatin is the nucleosome, which consists of 146 base pairs of DNA wrapped around an octameric protein complex composed of a histone H3-H4 tetramer and two histone H2A-H2B dimers (Kornberg and Lorch, 1999). By its very nature, the highly condensed structure of chromatin presents a formidable barrier to the DNA repair machinery gaining access to DNA lesion. Accordingly, chromatin must be re-structured to facilitate the access of DNA repair machinery to sites of DNA lesion, thus establishing a mechanism for the function of ATP-dependent chromatin remodeling factors during DNA repair (Bao and Shen, 2007; Morrison and Shen, 2009). These factors can modify chromatin structure through distinct mechanisms including covalent histone modifications, ATP-dependent chromatin remodeling, and histone variant exchange. Once DNA repair is completed, the affected chromatin regions have to be restored to the pre-existing chromatin structure through clearance of post-translational modifications imposed during lesion detection and repair and turning off checkpoint signaling (Groth et al., 2007; Soria et al., 2012). Therefore, mechanistic insights into the dynamics of DSB-containing chromatin are crucial for understanding the regulation of DSB signaling and repair. However, the precise mechanism underlying chromatin dynamics in the process of the signaling, access, and repair of DNA damage is largely unknown.
The microrchidia (MORC) protein family, consisting of five members in humans including MORC1, MORC2, MORC3, MORC4 and the divergent SMCHD1, is characterized by a gyrase, histidine kinase, and MutL (GHKL) domain combined with a C-terminal S5 domain (Inoue et al., 1999; Iyer et al., 2008a; Iyer et al., 2008b). These domains constitute an ATPase module, which is also seen in heat shock protein 90 and several ATP-dependent DNA-manipulating enzymes (such as mismatch repair protein MutL) and the ATPase subunits of different topoisomerases (Iyer et al., 2008a; Iyer et al., 2008b). The presence of a conserved lysine in the S5 domain that is expected to stabilize the hypercharged intermediate during phosphoester hydrolysis predicts that the GHKL domain in MORC family is likely to function as an active ATPase. This conserved basic residue is inferred to function similar to the arginine or lysine finger observed in various phosphohydrolase reactions (Iyer et al., 2008a). In addition, members of the MORC family show fusions to other domains that play a critical role in recognizing different epigenetic marks on chromatin proteins and DNA. Although the exact biochemical roles of eukaryotic MORC proteins remain unknown, the evolutionary contextual and gene neighborhood studies on prokaryotic MORCs and their relatives allowed us to predict that their eukaryotic counterparts could be involved in chromatin remodeling through a yet-to-be defined mechanism.
Here, we discovered that MORC2 (also called ZCWCC1, ZCW3, KIAA0852, or AC004542.C22.1) is a physiological substrate of p21-activated kinase 1 (PAK1), a ubiquitous serine/threonine protein kinase that is widely up-regulated in human cancers and functions as downstream nodes for various oncogenic signaling pathways (Kumar et al., 2006). PAK1 phosphorylation of MORC2 on serine 739 regulates an ATPase-dependent chromatin remodeling following DSB damage and facilitates efficient DSB repair. These findings define a critical role for the PAK1-MORC2 pathway in maintaining chromatin dynamics and genomic stability through sequentially orchestrating multiple essential enzymatic processes.
RESULTS
MORC2 is A Physiologic Substrate of PAK1 Kinase
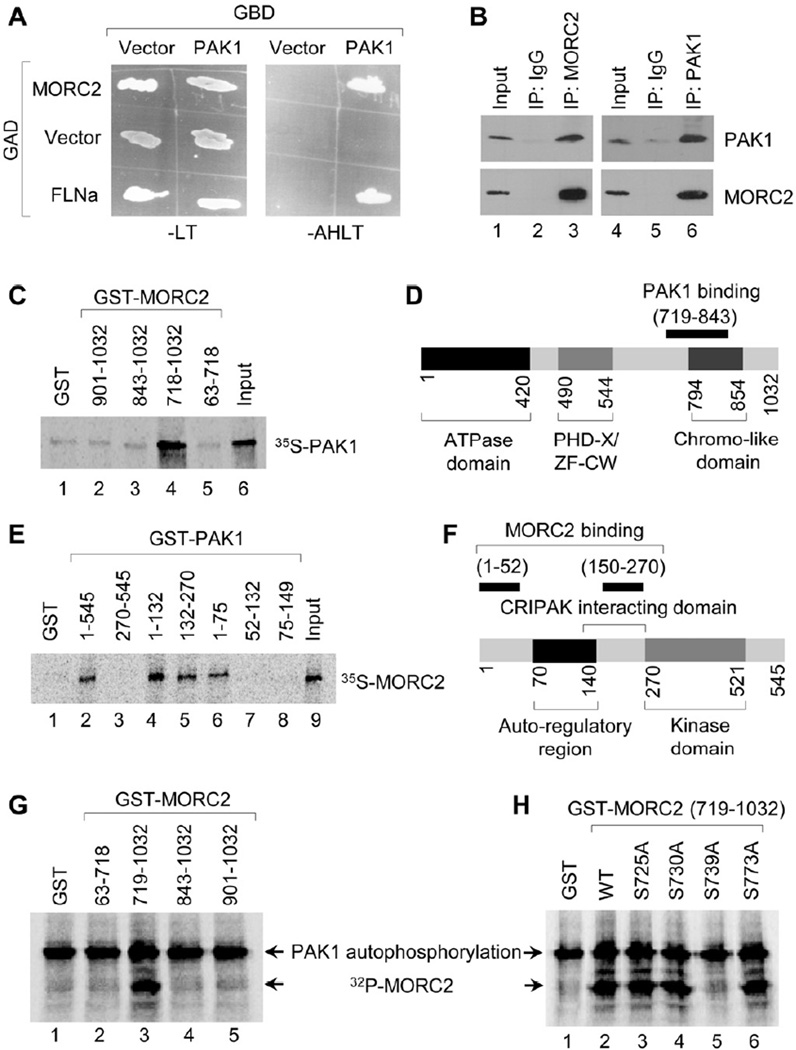
During an earlier study aimed to identify PAK1 interacting proteins, we discovered MORC2 as a novel PAK1 binding partner by a yeast two-hybrid screen using a human mammary gland cDNA library and PAK1 as bait (Figure 1A). We next confirmed that there was an interaction between PAK1 and MORC2 proteins at the endogenous levels by reciprocal immunoprecipitation (IP) assays using protein extracts from HeLa cells (Figure 1B). In vitro glutathione S-transferase (GST) binding assay further demonstrated that in vitro-translated PAK1 interacted with amino acids 719–843 of GST-MORC2 (Figures 1C, 1D and S1A), while MORC2 specifically bound to amino acids 1–52 as well as 150–270 of PAK1 protein (Figures 1E, 1F and S1B).
Figure 1. MORC2 is A Physiologic Substrate of PAK1 Kinase.
(A) Yeast cells were cotransfected with control GAD vector, GAD-MORC2, or GAD-FLNa (filamin-A; positive control) along with GBD vector, or GBD-PAK1. Cotransformants were plated on selection plates lacking leucine and tryptophan (-LT) or adenosine, histidine, leucine, and tryptophan (-AHLT). (B) Lysates from HeLa cells were immunoprecipitated with control IgG, anti-MORC2, or anti-PAK1 antibody, and blotted with the indicated antibodies. (C-D) 35S-labeled, in vitro transcribed-translated PAK1 was incubated with GST-MORC2 deletion constructs and bound proteins were analyzed by SDS-PAGE (C). Schematic representation of the domains of MORC2 for PAK1 binding is shown in D. (E-F) 35S-labeled, in vitro transcribed-translated MORC2 protein was incubated with GST-PAK1 deletion constructs and bound proteins were analyzed by SDS-PAGE (E). Panel F shows the schematic representation of the domains of PAK1 for MORC2 binding. (G) PAK1 phosphorylation of GST-MORC2 deletion constructs by in vitro PAK1 kinase assay. (H) PAK1 phosphorylation of GST-MORC2 (719–1032 amino acids) or its individual mutants.
As the PAK1 kinase acts on its targets mainly through phosphorylation (Kumar et al., 2006), we next investigated whether PAK1 might phosphorylate MORC2. Interestingly, we found that PAK1 phosphorylates GST-MORC2 containing the C-terminus within amino acids 719–843 (lanes 3–5), but not the N-terminal amino acids 63–718 (lane 2) (Figures 1G and S1C). Sequence analysis of amino acids 719–843 of MORC2 predicted four potential PAK1 phosphosites on serine 725, 730, 739 and 773, respectively. These sites were individually substituted with alanine (termed S725A, S730A, S739A and S773A, respectively) and then subjected to in vitro PAK1 kinase assays. Results showed that the MORC2 mutant containing an alanine substitution on serine 739 (lane 5) but not on other serines (lanes 2–4 and 6) lost PAK1-mediated phosphorylation (Figures 1H and S1D), suggesting that the serine 739 is the PAK1 phosphorylation site in MORC2 protein. Together, these results suggest that MORC2 is a binding partner of PAK1 and is phosphorylated on serine 739 by PAK1 kinase.
MORC2 is a DNA Damage-induced Phosphoprotein via PAK1 Kinase
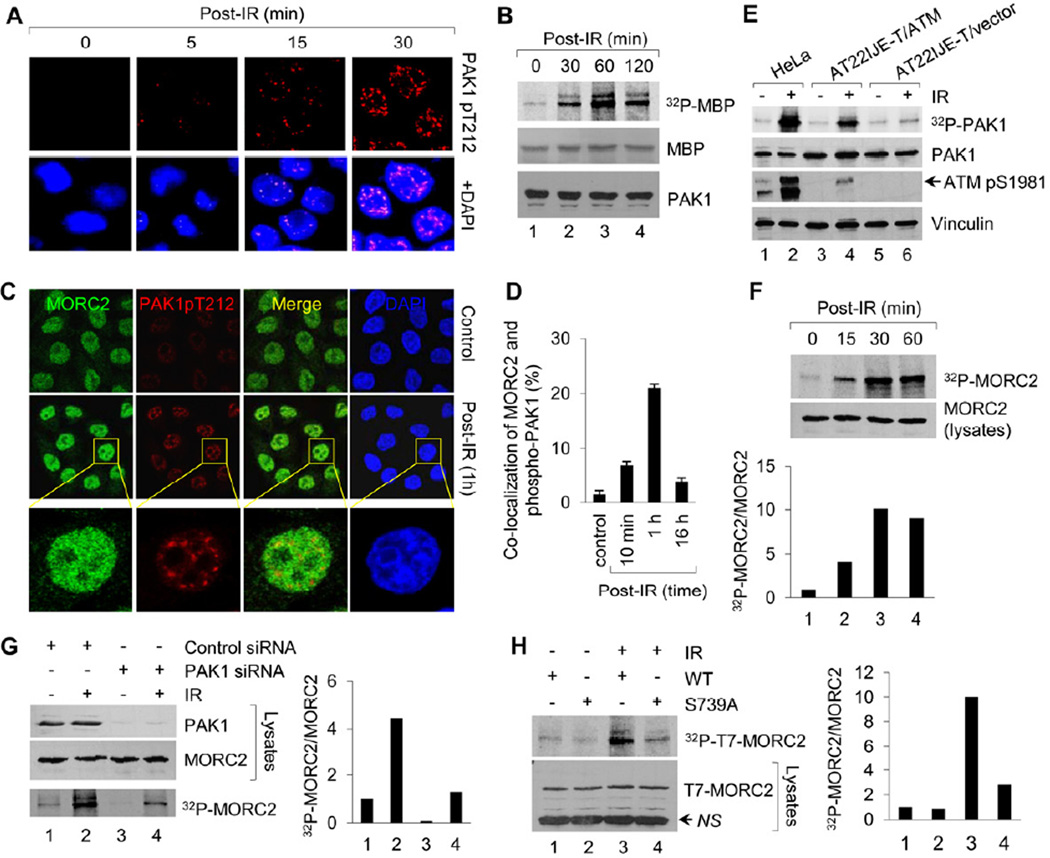
The MORC2 protein has not been well characterized, but it is ubiquitously expressed in human cancer cell lines and tissues and localized predominantly in the nucleus as evaluated by cell fractionation, immunofluorescence and immunohistochemical staining studies (Figures S2A–S2C). Given that MORC2 contains a conserved GHKL-type ATPase domain, related to those found in DNA repair proteins of the MutL family (Iyer et al., 2008a), we speculated that MORC2 might have a previously unrecognized role in the DNA damage response (DDR) pathway. To test this idea, we first questioned whether ionizing radiation (IR), which induces DNA DSBs and is widely being used for cancer radiotherapy by damaging the DNA of cancer cells, could activate PAK1 kinase. Immunofluorescent staining with a specific phospho-PAK1 (Thr212) antibody revealed a marked accumulation of phospho-PAK1 in the nucleus of IR-exposed cells in a time-dependent manner (Figure 2A); phospho-PAK1 was detected as early as 5 min after IR treatment, similar to the kinetics of H2AX phosphorylation in response to IR (Figure S2D). In addition, phospho-PAK1 partially co-localizes with γH2AX foci after DNA damage (Figure S2D). In vitro PAK1 kinase assay using myelin basic protein (MBP) as a substrate also demonstrates that PAK1 kinase activity was dramatically increased in response to IR and reached the maximum level by about 60 min post IR treatment (Figure 2B). Moreover, phosphorylated PAK1 co-localized with MORC2 protein in a time-dependent manner; the maximum level of these co-localizations occurred at 1 h after IR treatment (Figures 2C and 2D). These results suggest that IR promotes phosphorylation and activation of PAK1 kinase, which could be recruited to sites of DNA lesions and involved in early DSB response.
Figure 2. IR Induces MORC2 Phosphorylation in a PAK1-dependent Manner.
(A) HeLa cells were untreated or treated with 10 Gy of IR, harvested at the indicated time points for immunofluorescence staining with the indicated antibodies. Nuclei were visualized by DAPI staining (blue). (B) HeLa cells were untreated or treated with 10 Gy of IR and harvested at the indicating time points. PAK1 immunocomplex was subjected to in vitro kinase assay in the presence of [32P]ATP and MBP as substrates. (C-D) HeLa cells were treated with or without IR and harvested at the indicated time points for immunofluorescence staining of MORC2 and phospho-PAK1 (Thr212). The representative images (C) and quantitation results of co-localization of MORC2 with phospho-PAK1 (D) are shown. (E) Cells were radiolabeled with [32P] orthophosphate overnight. After 1 h of IR treatment, protein extracts were immunoprecipitated with an anti-PAK1antibody, separated by SDS-PAGE, and analyzed autoradiography. Total lysates were immunoblotted with the indicated antibodies for internal controls. (F) HeLa cells were metabolically labeled with [32P] orthophosphoric acid overnight and treated with or without IR. Nuclear extracts were immunoprecipitated with an anti-MORC2 antibody and analyzed by autoradiography. Total lysates were immunoblotted with anti-MORC2 antibody for internal control. (G) HeLa cells were transfected with control siRNA or PAK1 siRNA, metabolically labeled with [32P] orthophosphoric acid overnight. After 1 h of IR treatment, nuclear extracts were subjected to in vivo MORC2 phosphorylation assay. Total lysates were immunoblotted with anti-PAK1 and anti-MORC2 antibodies for internal controls. (H) MCF-7 cells stably expressing wild-type or MORC2-S739A mutant were labeled with [32P]-orthophosphoric acid overnight and treated with or without IR. After 1 h of IR treatment, nuclear extract were immunoprecipitated with anti-T7 agarose beads and subjected to in vivo phosphorylation assay. In F-H, the band densities were quantified using ImageJ software and the results were normalized to the signal for lane 1. NS, non-specific.
The response to IR-induced DSBs occurs primarily through ataxia telangiectasia mutated (ATM) protein kinase, which phosphorylates downstream targets critical for checkpoint activation and DNA repair (Shiloh, 2003). To examine whether ATM is required for PAK1 activation by IR, an ataxia-telangiectasia cell line (AT22IJE-T) stably expressing either wild-type ATM (AT22IJET/ATM) or empty vector (AT22IJE-T/vector)(Ziv et al., 1997) and HeLa cells expressing high levels of endogenous ATM (Gately et al., 1998) were assayed for PAK1 phosphorylation status after IR treatment by in vivo labeling with [32P]-orthophosphoric acid. As shown in Figure 2E, IR induced PAK1 phosphorylation in the AT22IJE-T/ATM and HeLa cells but not in the AT22IJET/vector cells, suggesting that IR activates PAK1 kinase via an ATM-dependent phosphorylation cascade.
As IR activates PAK1 (Figures 2A, 2B and S2D), which phosphorylates (Figures 1G and 1H) and co-localizes with MORC2 (Figure 2C), we next determined whether IR treatment could induce MORC2 phosphorylation via PAK1 kinase. Interestingly, we discovered that IR exposure led to a rapid increase in the phosphorylation levels of endogenous MORC2 in HeLa cells as early as 15 min post-IR treatment, and the levels of MORC2 phosphorylation reached maximum at 30–60 min after IR treatment (Figure 2F). Moreover, depletion of endogenous PAK1 by specific siRNAs resulted in a reduction of MORC2 phosphorylation following IR treatment (Figure 2G, compare lane 4 with 2). Furthermore, ATM deficiency did not significantly affect the protein levels of MORC2 in the presence or absence of IR treatment (Figure S2E, compare lanes 5–8 with 1–4), and in vitro ATM kinase assay demonstrated that ATM directly phosphorylated its known substrate p53 (Canman et al., 1998) but not MORC2 (Figure S2F, compare lane 4 with 2 and 3). Thus, these results establish an essential role for PAK1 in MORC2 phosphorylation in response to IR.
To further establish the significance of PAK1 kinase in MORC2 phosphorylation, we next generated the stable pooled clones of MCF-7 cells expressing T7-tagged wild-type MORC2 (T7-MORC2) or the PAK1 phosphosite mutation (T7-MORC2-S739A) using a doxycycline (DOX)-inducible system (Figure S2G). Incubation of cells with a very low concentration of DOX (10 ng/ml) effectively induced T7-MORC2 expression in a time-dependent manner (Figure S2H). As shown in Figure 2H, cells expressing PAK1 phosphosite mutation exhibited a significant decrease in MORC2 phosphorylation in response to IR (compare lane 4 with 3), suggesting that PAK1 may be the primary upstream kinase responsible for increase in MORC2 phosphorylation and that the serine 739 is the primary IR-inducible phosphorylation site in MOCR2 following IR treatment. Together, these findings establish that IR induces MORC2 phosphorylation in a PAK1-dependent manner and the PAK1-MORC2 pathway is involved in the early stage of DDR.
MORC2 Associates with Chromatin in a PAK1 Phosphorylation-dependent Manner
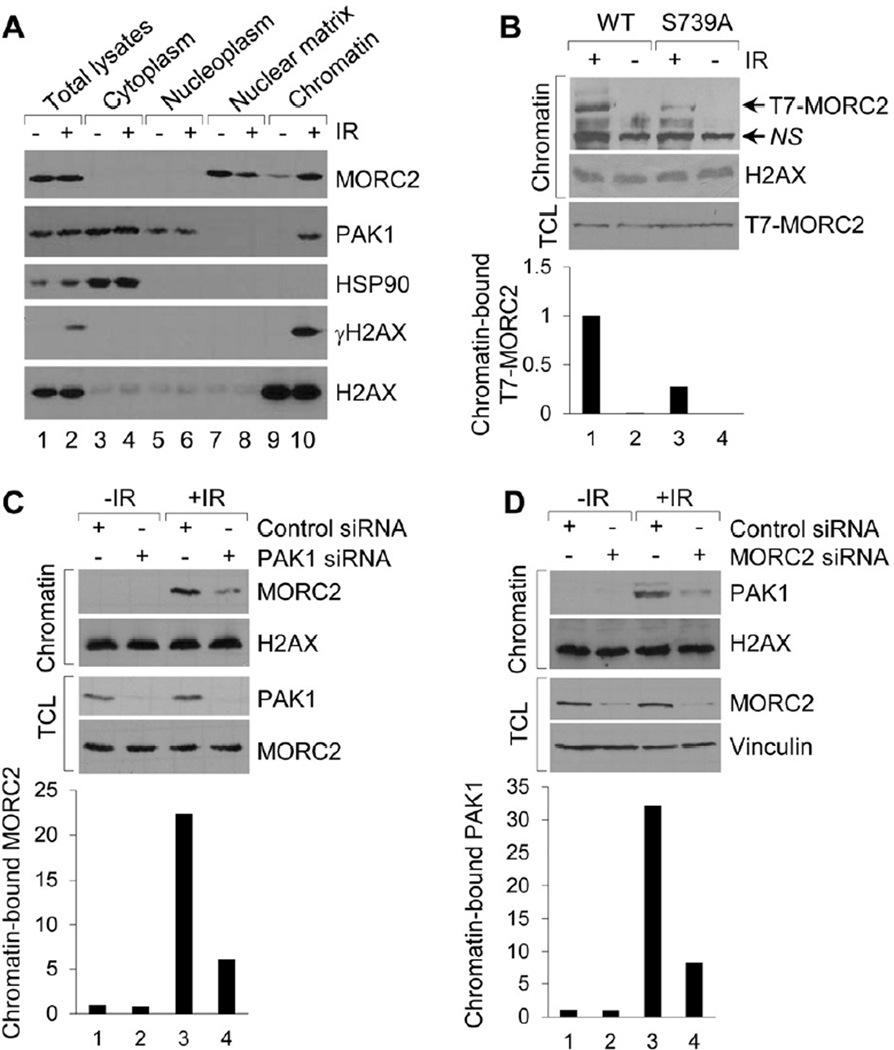
We next examined the sub-cellular localization of MORC2 and PAK1 in HeLa cells treated with or without IR, and found a distinct enrichment of MORC2, as well as of PAK1, to the chromatin fraction in response to IR (Figure 3A, compare lane 10 with 9). Interestingly, MORC2 translocation to the IR-exposed chromatin was accompanied by a simultaneous loss from the nuclear matrix compartment (compare lane 8 with 7), suggesting that MORC2 and PAK1 are dynamically co-recruited to chromatin in a cell state-dependent manner. The nuclear matrix is a dynamic nuclear compartment that plays both direct and indirect role in many nuclear functions including DNA replication and repair (Tsutsui et al., 2005). As the process of association of regulatory molecules with the nuclear matrix is primarily regulated by phosphorylation (Ben-Yehoyada et al., 2003), we next determined whether IR-induced phosphorylation of MORC2 on serine 739 was required for its chromatin association. As shown in Figure 3B, following IR wild-type MORC2 protein became more strongly associated with chromatin as compared with the PAK1 phosphosite mutant (S739A) (compare lane 1 with 3), suggesting that PAK1-mediated MORC2 phosphorylation regulates its association with chromatin following DNA damage. In support of this notion, selective knockdown of endogenous PAK1 significantly reduced the association of MORC2 with chromatin (Figure 3C, compare lane 4 with 3). Similarly, depletion of endogenous MORC2 also compromised the recruitment of PAK1 to damaged chromatin (Figure 3D, compare lane 4 with 3). Collectively, these results suggest that both PAK1 and MORC2 proteins are required for the optimum recruitment of one another to chromatin following IR treatment and that phosphorylation of MORC2 on serine 739 by upstream signaling is essential for this process.
Figure 3. MORC2 Associates with Chromatin following DNA Damage in a PAK1 Phosphorylation-dependent Manner.
(A) Subcellular fractions were prepared from HeLa cells after 1 h of IR treatment and immunoblotted with the indicated antibodies. (B) Chromatin fractions were isolated from MCF-7 cells stably expressing wild-type or MORC2-S739A mutant after 1 h of IR treatment and then immunoblotted with the indicated antibodies. (C-D) HeLa cells were transfected with control siRNA or specific siRNA targeting PAK1 (C) or MORC2 (D). After 48 h of the second transfection, cells were treated with or without IR. Chromatin fractions were isolated after 1 h of IR treatment and then immunoblotted with the indicated antibodies. In B-D, the band densities were quantified using ImageJ software and the results were normalized to the signal for lane 1. TCL, total cellular lysates; NS, non-specific.
MORC2 Promotes γH2AX Induction but Does not Affect PIKK kinase Expression or Their Activation
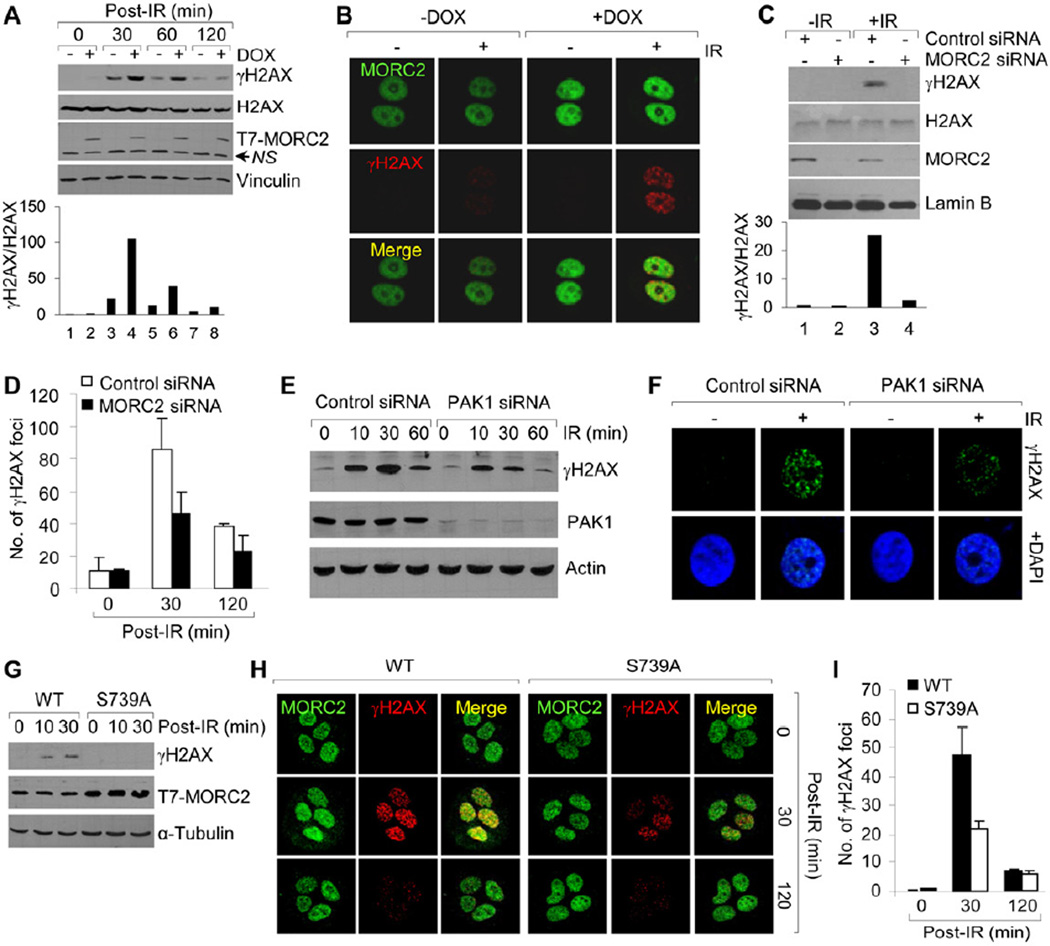
One of the first chromatin-associated events in response to DSBs is the rapid phosphorylation of a serine residue (S129 in S. cerevisiae and S139 in mammals) at the C-terminal region of the histone variant H2AX (Rogakou et al., 1998). This phosphorylation of H2AX (termed γH2AX) is necessary for subsequent recruitment or retention of DNA repair proteins and chromatin remodeling complexes on damaged chromatin and hence the maintenance of genomic integrity (Celeste et al., 2002; Morrison and Shen, 2009; Park et al., 2006; Paull et al., 2000). Given that MORC2 associates with chromatin following DNA damage (Figure 3), we next tested whether MORC2 regulates γH2AX induction in response to IR. Interestingly, we found that induced expression of MORC2 dramatically increased the levels of γH2AX expression (Figure 4A) and γH2AX focus formation (Figure 4B). In contrast, selective knockdown of MORC2 significantly reduced the levels of γH2AX (Figure 4C, compare lane 4 with 3) and the formation of γH2AX repair foci following IR treatment (Figures 4D and S3A). Moreover, knockdown of endogenous PAK1 significantly reduced the levels of γH2AX (Figure 4E) and the formation of γH2AX repair foci (Figure 4F), suggesting that MORC2 facilitates γH2AX induction in a PAK1 phosphorylation dependent manner. In support of this notion, cells expressing MORC2-S739A mutation exhibited a decreased induction of γH2AX after DNA damage (Figures 4G–4I). Given that γH2AX induction is a surrogate marker of DSBs and is an early event in DSB repair (Paull et al., 2000), these results establish a role for MORC2 in the early stage of DDR due to its ability to regulate γH2AX induction. In support of this notion, immunofluorescence staining revealed thatMORC2 partially co-localizes with γH2AX following DNA damage (Figure S3B) and quantitative real-time PCR analysis demonstrated no obvious effect of MORC2 knockdown on the expression of major DSB repair genes (Figure S3C).
Figure 4. MORC2 Promotes γH2AX Induction in a PAK1 Phosphorylation-dependent Manner.
(A-B) MCF-7 cells expressing T7-MORC2 were treated with DMSO or 10 ng/ml of DOX for 12 h and irradiated with or without IR. Cells were harvested at the indicated time points (A) or after 1 h of IR treatment (B), and then subjected to Western blotting (A) or immunofluorescence staining (B) with the indicated antibodies. (C-D) HeLa Cells were transfected with control or MORC2 siRNA. After 48 h of the second transfection, cells were irradiated with or without IR and subjected to immunoblot analysis (C) or immunofluorescence staining (D) with the indicated antibodies. (E-F) HeLa cells were transfected with control or PAK1 siRNA. After 48 h of the second transfection, cells were irradiated with or without IR and then subjected to immunoblot analysis at the indicated time points (E) or immunofluorescence staining after 30 min of IR treatment (F) with the indicated antibodies. (G-I) MCF-7 Cells expressing wild-type or MORC2-S739A mutation were treated with 10 ng/ml DOX for 12 h, irradiated with or without IR, and subjected to immunoblot analysis (G) or immunofluorescence staining (H) with the indicated antibodies. The quantitation results of the immunofluorescence staining of γH2AX foci are shown in panel I.
To further explore how MORC2 regulates γH2AX induction, we next examined the effect of MORC2 on the expression and activation status of members of the phosphoinositide 3-kinase-related kinase (PIKK) family. Of these PIKKs, ATM and DNA-dependent protein kinase (DNA-PK) phosphorylate H2AX in response to DSBs in a partially redundant manner (Stiff et al., 2004), whereas ATM- and Rad3-related (ATR) mediates H2AX phosphorylation in response to replication stress (Ward and Chen, 2001). As shown in Figure S3D, we found that induced expression of MORC2 had no effect on the expression of ATM, DNA-PK and ATR or their activation status in response to IR. Consistently, MORC2 also did not affect the expression and phosphorylation of KRAB-associated protein 1 (KAP1), checkpoint 2 (Chk2) and p53, putative downstream targets of ATM and DNA-PK kinases in response to IR (Matsuoka et al., 1998; Tomimatsu et al., 2009; Ziv et al., 2006). These results suggest that the PAK1-MORC2 pathway may lie downstream of the ATM/DNA-PK-mediated phosphorylation events in response to IR and that MORC2 facilitates γH2AX induction probably by influencing the higher-ordered chromatin structure in such a way as to increase the accessibility of the H2AX-containing nucleosomes to PIKK kinases but not by acting on the expression or activation of these PIKK kinases.
Growth Factor Signaling Modulation of DDR via the PAK1-MORC2 pathway
Experimental evidence from others (Riballo et al., 2004; Stiff et al., 2004) and this work (Figure S2F, compare lanes 3 and 4 with 1) has shown that IR can efficiently induce γH2AX occurrence in human cells lacking ATM. Given that the suggested role of ATM was to activate PAK1 and the fact that PAK1 activity can be effectively stimulated by other extracellular signals such as growth factors (Kumar et al., 2006), we next tested the possibility that growth factor signaling can activate the PAK1-MORC2 pathway independently of ATM, which in turn regulates γH2AX induction following IR treatment. Interestingly, we found that epidermal growth factor (EGF) treatment resulted in a significant increase in γH2AX focus formation in both AT22IJE-T/vector and AT22IJE-T/ATM cells following IR treatment (Figures S4A and S4B), suggesting that EGF-mediated signaling promotes γH2AX induction in response to IR in an ATM-independent manner. Furthermore, knockdown of endogenous MORC2 (Figures S4Cand S4D) or PAK1 (Figures S4E and S4F) compromised the levels of EGF-stimulated γH2AX induction in AT22IJE-T/vector cells following IR treatment, suggesting that growth factor signaling other than ATM also can activate the PAK1-MORC2 pathway, which in turn regulates γH2AX induction in an ATM-independent manner. Since ATM-dependent signaling accounts for no more than 20–25% of overall DSB repair (Goodarzi et al., 2008), these findings may explain the residual DNA repair activity in the ATM-null cells. Thus, we discovered a new pathway to promote γH2AX induction in an ATM-dependent as well as -independent manner following IR treatment, and these findings provide novel insights into the emerging role of the growth factor signaling in the regulation of cellular radiation response and DNA repair in mammalian cells.
MORC2 Exerts PAK1 Phosphorylation- and DNA-dependent ATPase Activity
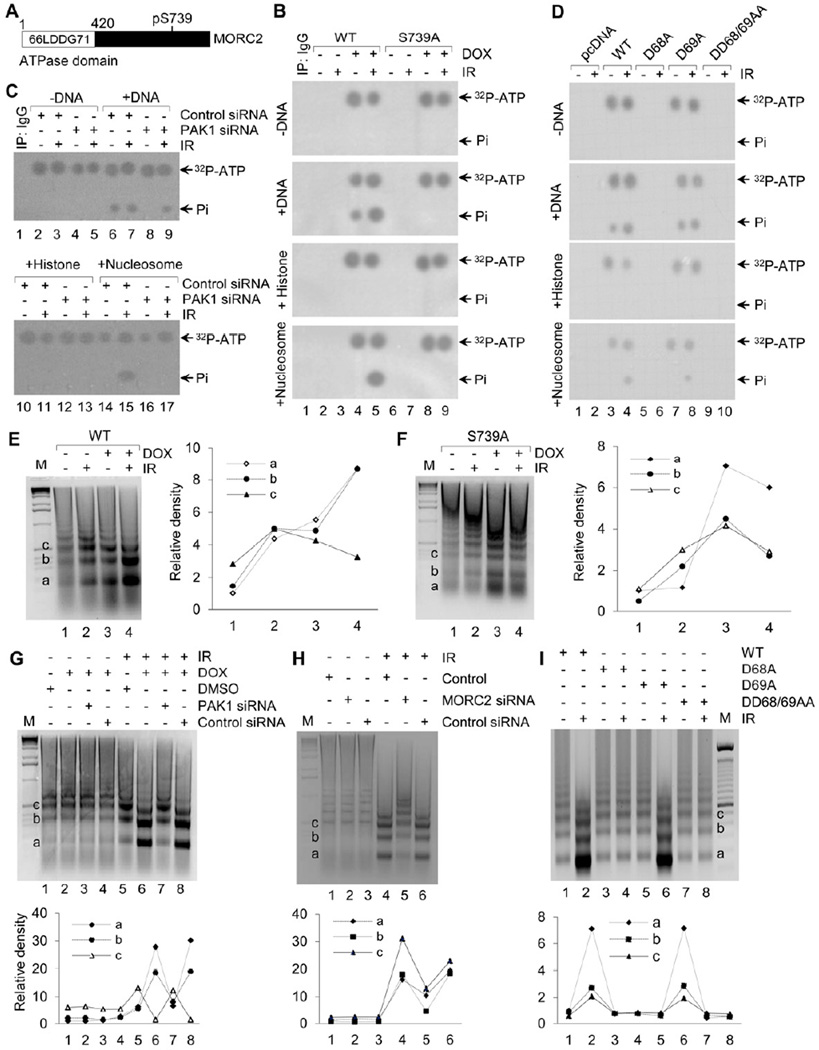
As MORC2 contains a conserved GHKL-type ATPase domain at its N-terminus (Inoue et al., 1999; Iyer et al., 2008a) (Figure 5A), we speculated that MORC2 possesses an intrinsic ATPase activity, which might be required for ATP-dependent chromatin remodeling following DNA damage. To test this notion, we carried out the ATPase assay to analyze the ability of MORC2 to hydrolyze ATP in the presence or absence of equal amounts of naked DNA, core histones, or nucleosomes purified from HeLa cells (Bochar et al., 2000). We discovered that MORC2 possessed the ATPase activity in the presence of DNA or nucleosome, whereas did not observe any ATP hydrolysis in presence of core-histones alone or absence of DNA (Figure 5B). Furthermore, the noted ATPase activity of MORC2 was stimulated following IR treatment in the presence of DNA or nucleosome (compare lane 5 with 4), suggesting that IR-induced DDR signaling cascade is essential for activation of the ATPase activity of MORC2. In support of this notion, mutation of PAK1 phosphosite (S739A) in MORC2 resulted in a loss of the ATPase activity (Figure 5B, lanes 8 and 9). Consistently, depletion of endogenous PAK1 (Figure S5A) compromised the ATPase activity in the presence of DNA (Figure 5C, compare lane 8 with 6 and compare lane 9 with 7, respectively) or nucleosomes (Figure 5C, compare lane 17 with 15). These findings suggest that MORC2 is a DNA-dependent ATPase that requires PAK1 phosphorylation and IR-dependent upstream signals.
Figure 5. MORC2 Possesses An Intrinsic ATPase Activity and Facilitates Chromatin Relaxation in Response to DNA Damage.
(A) Schematic diagram shows the conserved ATPase domain in the N-terminus of MORC2. (B) MCF-7 cells expressing wild-type MORC2 or S739A mutant were incubated with DMSO or 10 ng/ml of DOX for 12 h. After 1 h of IR treatment, nuclear extracts were immunoprecipitated with T7-tagged agarose beads and subjected to ATPase assays using 100 ng of double-stranded plasmid DNA, histone, or mononucleosomes from HeLa cells. (C) MCF-7 cells expressing wild-type MORC2 were transfected with control or PAK1 siRNA, and treated with or without IR after 48 h of the second transfection. Nuclear extracts were subjected to ATPase assays. (D) HEK293T cells were transfected with the indicated expression vectors. After 48 h of transfection, cells were treated with or without 10 Gy of IR and harvested after 1 h of IR treatment for ATPase assays. (E–F) MCF-7 cells expressing wild-type (E) or S739A mutant (F) were treated with DMSO or 10 ng/ml of DOX for 12 h. After 1 h of IR treatment, nuclear extracts were incubated with 5 units of MNase for 10 min, and DNA was visualized by ethidium bromide staining. (G-H) HeLa cells were transfected with control or specific siRNA targeting PAK1 (G) or MORC2 (H). After 48 h of the second transfection, cells were treated with or without IR and nuclear extracts were subjected to MNase assays. (I) HEK 293T cells were transfected with the indicated expression vectors. After 48 h of transfection, nuclei were prepared after 1 h of IR treatment and subjected to MNase assay. In E-I, the band densities were quantified using ImageJ software and the results were normalized to the signal for a1.
To further map the amino acids essential for the ATP-hydrolysis and ATP-binding activities of MORC2, we mutated conserved, predicted catalytic aspartic acid residue at (D68) and its neighboring residue D69 (Iyer et al., 2008a) to alanine individually or in both (termed D68A, D69A, or DD68/69AA) and determined the ATP binding and hydrolysis activity (Figure 5D and S5B) We found that mutation of D68 (lanes 5 and 6) but not D69 (lanes 7 and 8) abolished the ATP binding and ATPase activities of MORC2 as compared with its wild-type counterpart (lanes 3 and 4). Moreover, the double mutant, DD68/69AA, did not show any ATPase activity (lanes 9 and 10), suggesting that the amino acid D68, but not D69, is indeed a key catalytic residue for the ATPase activity of MORC2.
Phosphorylated MORC2 Facilitates ATPase-dependent Chromatin Remodeling
As MORC2 associates with chromatin (Figure 3) and possesses an intrinsic DNA-dependent ATPase activity (Figures 5B–5D) following DNA damage, we next examined whether MORC2 alters chromatin structure by analyzing the sensitivity of chromatin to micrococcal nuclease (MNase), which preferentially digests the linker region of DNA between nucleosomes (Ziv et al., 2006). Therefore, increased MNase sensitivity is generally interpreted to reflect relaxed chromatin, which is more rapidly digested with MNase and is frequently associated with DNA repair (Ziv et al., 2006). We found that induced expression of wild-type MORC2 (Figure S5C) resulted in a moderate increase in MNase accessibility in chromatin (Figure 5E, compare lanes 2 with 1) and the effect was significantly enhanced following IR treatment (compare lanes 4 with 3). In contrast, chromatin from cells expressing mutant MORC2 S739A did not display the significantly altered sensitivity to MNase (Figure 5F, compare lanes 8 with 7). These findings demonstrate that MORC2 facilitates chromatin relaxation in response to DSBs in a PAK1-phosphorylation dependent manner.
We next depleted endogenous PAK1 and examined the extent of chromatin relaxation following DNA damage (Figure S5D), and found that the magnitude of chromatin relaxation was markedly reduced in PAK1-knocked down cells as compared with control siRNA transfected cells (Figure 5G, compare lanes 8 with 7). Furthermore, selective knockdown of endogenous MORC2 (Figure S5E) also reduced chromatin accessibility to MNase (Figure 5H, compare lanes 6 with 5). More interestingly, the observed MORC2-mediated chromatin relaxation in response to IR was compromised in cells expressing ATPase mutants (D68A and DD68/69AA) (Figure 5I, lanes 3 and 4, and lanes 7 and 8, respectively), indicating that ATPase activity of MORC2 is required for chromatin relaxation in response to IR-induced DSBs. Together, these findings suggest that MORC2 regulates chromatin remodeling through a sequential set of events including PAK1 phosphorylation and ATP hydrolysis.
PAK1-MORC2 Axis as A New Arm of the ATM-mediated DDR
Given that ATM also phosphorylates its downstream substrate KAP1, which in turn regulates chromatin relaxation following DNA damage (Goodarzi et al., 2011a; Ziv et al., 2006), we next investigated whether the emerging role of the ATM-PAK1-MORC2 axis in chromatin relaxation following DNA damage depends on the ATM-KAP1 pathway. As shown in Figure S3C, induced expression of MORC2 did not affect the expression and activation of KAP1, indicating that both pathways may function independently in the context of DDR. In support of this notion, we found that depletion of endogenous MORC2 and PAK1 using specific siRNAs did not affect the levels of KAP1 and phosphorylated KAP1 on serine 824 (Figures S6A and S6B). In contrast, the levels of γH2AX were compromised in response to DNA damage following knockdown of endogenous PAK1 or MORC2. Moreover, siRNA-mediated knockdown of KAP1 did not affect MORC2 phosphorylation, whereas knockdown of PAK1 decreased the levels of MORC2 phosphorylation following IR treatment (Figure S6C). These results suggest that the ATM-PAK1-MORC2 axis and the ATM-KAP1 pathway function independently in response to IR-induced DNA damage. Moreover, although MORC2 interacted with KAP1 but this interaction was IR-independent as well as PAK1 phosphorylation-independent (Figures S6D and S6E). However, whether both KAP1 and MORC2 proteins could be part of the same complex but independently regulated following DNA damage remains to be investigated.
MORC2 Disrupts Nucleosomal Structure in a Phosphorylation-dependent Manner
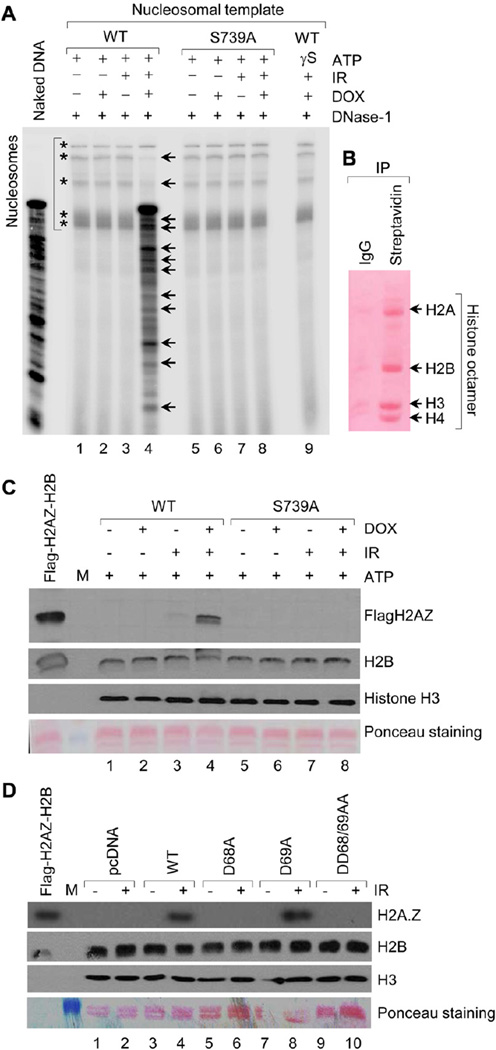
To further elucidate whether MORC2 can alter the nucleosomal structure in an ATP-dependent manner, we performed mono-nucleosome disruption assay (Hamiche et al., 1999) in MCF-7 cells expressing wild-type and S739A-mutant MORC2 in the presence or absence of IR. Results showed that wild-type MORC2 can mobilize nucleosome (increased sensitivity to DNase Ι digestion) in response to IR (Figure 6A, lane 4). In contrast, nucleosome remodeling activity was completely compromised when PAK1 phosphosite mutant MORC2-S739A was substituted in the assay (Figure 6A, lane 8). Further, we demonstrate that nucleosome remodeling function of phosphorylated MORC2 was dependent on ATP binding as this activity was completely lost when non-hydrolysable form ATP-γ-S was used in the assay (Figure 6A, lane 9).
Figure 6. MORC2 Exhibits a Histone-exchange Activity and Promotes Signaling-dependent Nucleosome Mobilization.
(A) Nucleosome remodeling assay was carried out using 30 µl of T7-MORC2 or its mutant (S739A) using salt gradient exchange. Total 100 ng of end labeled nucleosomal template was incubated with T7 immunoprecipitates and further digested with DNase1 (0.5U) for 1 min at room temperature. Reaction mix was precipitated, electrophoresed on 10% urea-TBE gel, and exposed to phosphor screen. (B) Schematic for the preparation of native biotinylated chromatin on streptavidin-magnetic beads. (C) Histone exchange assay was carried out using 30 µl of T7-MORC2 with 100 ng of Flag-H2A.Z-H2B dimer and 50 ng of streptavidin bound native chromatin in total of 50 µl exchange reaction at 37 °C for 2 h. Beads were washed with 400 mM KCl and bound proteins were eluted using SDS-Laemmli buffer and analyzed on 16% SDS-PAGE using indicated antibodies. Presence of Flag-H2AZ in elutes demonstrates the exchange of H2AZ for H2A due to chromatin remodeling. (D) HEK293 cells were transfected with the indicated expression vectors. After 48 h of transfection, cells were harvested after 1 h of IR treatment for histone exchange assay as describe above.
We next utilized an in vitro histone exchange assay to demonstrate the nucleosome remodeling functions of MORC2. Consistent with the above results, we observed that wild-type MORC2 could use ATP hydrolysis to perform dimer exchange within a nucleosome, thereby replacing canonical H2A-H2B dimers with a mixture of H2AZ-H2B dimers (Figures 6B and 6C, lane 4). Interestingly, PAK1 phosphosite mutant MORC2-S739A (Figure 6C, lane 8) and ATPase mutant MORC2-D68A (Figure 6D, lanes 6 and 10) did not display any dimer exchange, thus establishing a role for MORC2 in chromatin remodeling events following DNA damage in a PAK1 phosphorylation- and ATPase-dependent manner.
MORC2 Facilitates DSB Repair in a PAK1 Phosphorylation-dependent Manner
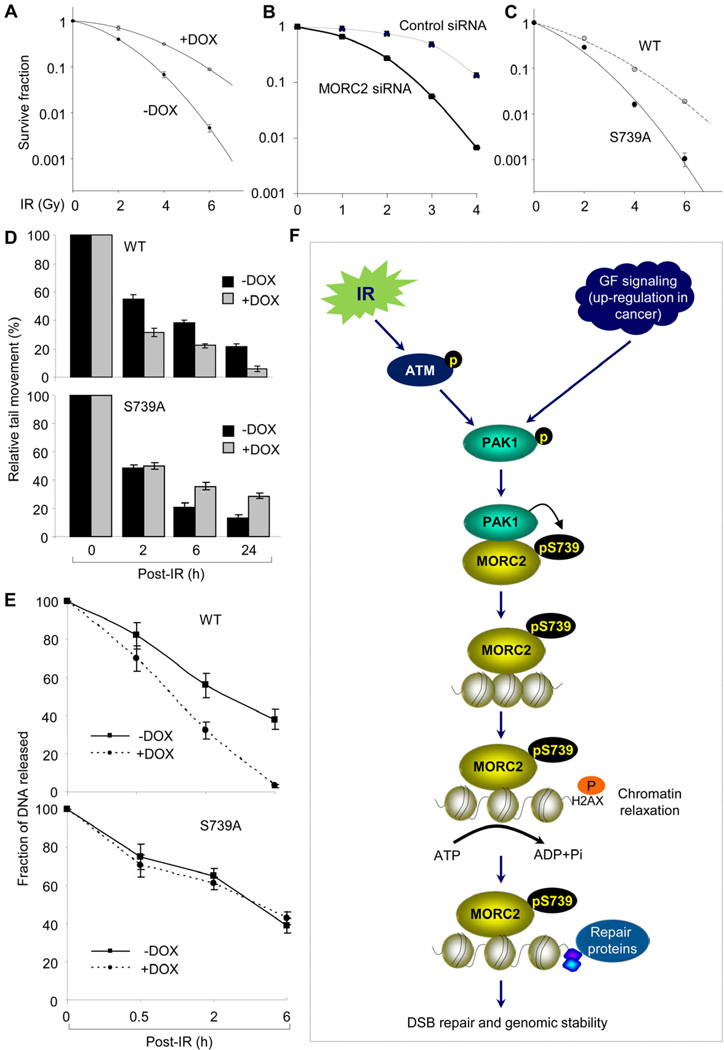
To assess the functional role of MORC2 in DDR, we next examined the effect of induced expression of MORC2 on cell survival in response to IR using a clonogenic survival assay. We found that induced expression of MORC2 markedly enhanced the clonogenic survival following IR treatment as compared with control cells (Figures 7A and S7A). In contrast, knockdown of endogenous MORC2 using specific siRNAs led to an increased sensitivity to IR as compared with control siRNA-treated cells (Figures 7B and S7B). Interestingly, cells expressing S739A mutation were highly sensitive to IR as compared with its wild-type counterparts (Figure 7C). These results suggest an important role for MORC2 in DSB repair pathways and cell survival after DNA damage in a PAK1 phosphorylation-dependent manner.
Figure 7. MORC2 Promotes DSB Repair and Enhances Radioresistance Following DNA Damage.
(A) MCF-7 cells expressing wild-type MORC2 were treated with DMSO or 10 ng/ml of DOX for 12 h, and then subjected to clonogenic survival assays. (B) HeLa cells were transfected with control or MORC2 siRNA. After 48 h of the second transfection, cells were treated with or without IR at the indicated doses and then subjected to clonogenic survival assays. (C) MCF-7 cells expressing wild-type MORC2 or its S739A mutant were treated with DMSO or 10 ng/ml of DOX for 12 h and then subjected to clonogenic survival assays. (D-E) MCF-7 cells expressing wild-type MORC2 or its S739A mutant were incubated with DMSO or 10 ng/ml of DOX for 12 h, and treated with or without IR. Cells were harvested at the indicated time points for comet assays (D) or PFGE assays (E). (F) The proposed working model for MORC2 functions in the DDR. Extracellular signals, such as IR and growth factors (GFs), activate the PAK1 kinase, which interacts with and phosphorylates MORC2 on serine 739. Phosphorylated MORC2 associates with chromatin and facilitates an ATPase-dependent chromatin relaxation in response to DNA damage, which, in turn, might regulate DSB signaling.
We next used the neutral comet assay that specifically measures DNA DSBs in individual eukaryotic cells (Olive and Banath, 2006) to examine DNA-repair efficiency in MORC2-inducible cells. Results showed that immediately after irradiation (0 h), approximately the same amount of DNA fragments were generated in cells expressing MORC2 or control cells. However, when residual unrepaired DNA fragments were monitored at various time points post IR treatment, induced expression of wild-type MORC2 led to a decrease in the levels of damaged DNA as compared with un-induced control cells (Figure 7D, upper panel). In contrast, cells expressing MORC2-S739A mutant exhibited increased levels of damaged DNA compared to its control cells (Figure 7D, lower panel), suggesting that MORC2 is required for efficient DSB repair in a PAK1 phosphorylation-dependent manner.
Next, we directly determined the rate of DNA repair after IR treatment in wild-type or MORC2-S739A mutant expressing cells by pulsed-field gel electrophoresis (PFGE), a functional assay to measure DSB repair (Joshi and Grant, 2005). The PFGE assay can measure pre-existing damage as well as induction of DSBs by chemical, physical, or biological agents, and has been used extensively to study DNA repair by observing the reduction in migrating DNA when cells are allowed a period of repair following genotoxic insult (Joshi and Grant, 2005). Consistently, we found that induced expression of wild-type MORC2 resulted in a significant decrease in the levels of un-repaired DNA damage (Figures 7E, upper panel, and S7C). In contrast, no significant change in DSBs was observed in MORC2 S739A mutant expressing cells (Figures 7E, lower panel, and S7C). Collectively, these results suggest that MORC2 is required for efficient DNA repair and MORC2 phosphorylation on serine 739 plays a pivotal role in conferring improved clonogenic survival following IR-induced DSBs.
DISCUSSION
In this study, we focused on addressing the role of MORC2 signaling in the DDR. We demonstrate for the first time that MORC2 exerts an intrinsic DNA-dependent ATPase activity and regulates chromatin remodeling in a PAK1 phosphorylation-dependent manner, thus contributing to the fine-tuning of damage signaling and DSB repair (Figure 7F). In this context, MORC2 ATPase mutant (D68A) cannot bind and hydrolyze ATP (Figure 5D) and failed to alter nucleosomal structure following DNA damage (Figures 5I and 6D). Moreover, PAK1 phosphosite mutant (S739A) exhibited intact ATP binding activity but lost its ATPase activity (Figure 5B), resulting in impaired chromatin remodeling activity, decreased DSB repair and enhanced sensitivity to DNA-damaging agents. Thus, our findings highlight a sequential set of events that drives MORC2-mediated chromatin remodeling involving signaling dependent phosphorylation and its ATPase activity. Given the same experimental conditions used in these analyses, the observed profound differences in ATPase activity between wild-type and PAK1 phosphosite-mutant MORC2 would be accounted from the signaling-dependent modification of MORC2. However, future work will be required to rule out the possibility that MORC2-interacting proteins are involved in MORC2-mediated chromatin remodeling. Moreover, although the role of other ATP-dependent chromatin remodeling factors in chromatin remodeling has been documented after DNA damage (Bao and Shen, 2007), the mechanistic cooperation between signaling-dependent phosphorylation and chromatin remodeling activity has not been hitherto observed. Thus, to our knowledge, MORC2 may represent the first example of a chromatin remodeling factor that can disrupt nucleosomal structure in a phosphorylation-dependent manner.
It is generally accepted that unperturbed heterochromatin is a barrier to the DDR and repair processes and thus, the nature of the heterochromatic activity must be alleviated to enable DSB repair (Goodarzi et al., 2011b; Goodarzi et al., 2008). Recent studies have demonstrated a role for MORC proteins in heterochromatin condensation and gene silencing (Moissiard et al., 2012; Shao et al., 2010). If MORC2 is a heterochromatic/repressive factor, one question is how MORC2 contributes to the DDR? Our data showed that MORC2 plays a role in reorganizing higher order chromatin structure following DNA damage through inducing chromatin relaxation in a PAK1 phosphorylation- and ATPase-dependent manner (Figure 5). Given that phospho-PAK1 and MORC2 only partially co-localized with γH2AX (Figures S2D and S3B), a surrogate marker of DDR triggered by IR, we propose a model wherein the PAK1-MORC2 axis contributes to global chromatin relaxation in response to DNA damage, which, in turn, regulates DSB signaling. Conceivably, MORC2 would enable faster access of DNA repair proteins to the DSBs, allowing efficient DSB repair to occur. As the PAK1-MORC2 pathway may lie downstream of ATM (Figure S3D), our findings support the notion that ATM is required for the efficient repair of DNA lesions in heterochromatin by affecting the transcriptional silencing complex formed by the heterochromatin-building factors (Goodarzi et al., 2008). As chromatin affects DDR signaling as well as gene transcription (Sulli et al., 2012), it remains to be addressed whether MORC2 also potentially participates in the DDR through transcriptional regulation of yet unidentified DDR gene expression. Another mechanism for altering chromatin compaction following DNA damage is the ATM-dependent KAP1 phosphorylation, which triggers heterochromatin de-condensation and facilitates efficient DSB repair in heterochromatin (Goodarzi et al., 2008; Sulli et al., 2012; Ziv et al., 2006). Interestingly, several lines of evidence suggest that the ATM-PAK1-MORC2 axis might function independently of the ATM-KAP1 pathway in the context of DDR (Figures S3D and S5). Thus, our study proposes a new model that ATM kinase regulates chromatin remodeling through a previously unrecognized ATM-PAK1-MORC2 pathway.
In support of the established role of MORC2 in chromatin remodeling, we discovered that MORC2 regulates γH2AX induction that is regardless with the expression and activation of ATM and DNA-PK kinases (Figure S3C). One possible mechanism is that, following DNA damage, MORC2 promotes chromatin relaxation in a phosphorylation- and ATPase-dependent manner, resulting in an increase of the accessibility of the H2AX-containing nucleosomes to PIKK kinases and enhanced γH2AX induction. Given the crucial role for γH2AX in efficient DSB repair (Celeste et al., 2002; Morrison and Shen, 2009; Paull et al., 2000), these findings suggest that the PAK1-MORC2 pathway facilitates DSB repair, at least in part, by stimulating γH2AX formation by acting on chromatin (Park et al., 2006). Interestingly, the emerging role of MORC2 in the regulation of H2AX phosphorylation is similar to that of the SWI/SNF chromatin remodeling complex, whose inactivation compromises γH2AX induction but does not affect the expression of ATM, DNA-PK and ATR, or their activation and/or recruitment to DSBs following DNA damage (Park et al., 2006). Thus, it will be particularly interesting to determine whether there is a crosstalk between MORC2 and the SWI/SNF complex in chromatin organization in the future study.
In summary, we report a previously unknown function of the PAK1-MORC2 axis in the DDR through facilitating phosphorylation-dependent, ATPase-coupled chromatin remodeling. The MORC2 protein, in particular, shows a conserved domain architecture that is only preserved in deuterostomes (chordates and echinoderms), suggesting that its role in DNA repair-associated chromatin remodeling is likely to be of particular significance in vertebrates that lacks direct cognates in model systems from fungi (Saccharomyces), nematodes (Caenorhabditis) or arthropods (Drosophila). Given that PAK1 is up-regulated in a wide variety of human cancers and linked to cancer progression and therapeutic resistance (Kumar et al., 2006), targeting the PAK1-MORC2 DNA repair pathway might sensitize cancer cells to DNA-damaging based radio- and chemo-therapy by the accumulation of unrepaired DNA breaks.
EXPERIMENTAL PROCEDURES
Cell Culture and Ionizing Radiation
MCF-7, HEK293T, and HeLa cells were obtained from American Type Culture Collection. AT22IJE-T/vector and AT22IJE-T/ATM cell lines were provided by Dr. Yosef Shiloh. For ionizing radiation, cells were irradiated with a Nasatron 137Cs irradiator (Li et al., 2009).
Immunofluorescence Staining
Immunofluorescence staining was performed as described previously (Vadlamudi et al., 2002). Cells were incubated with primary antibodies, washed in PBS, and then incubated with secondary antibody conjugated with 555-Alexa (red) or 488-Alexa (green), respectively. The blue DNA dye 4', 6-diamidino-2-phenylindole (DAPI) was used as a nuclear staining. Microscopic analyses were performed using a Zeiss LSM 710 or an Olympus FV1000 confocal microscope.
PAK1 Kinase Assay
In vitro PAK1 kinase assays were performed in HEPES buffer (50 mM HEPES, 10 mM MgCl2, 2 mM MnCl2, and 1 mM DTT) containing 20 µg of MBP, 10 µCi of [γ-32P] ATP, and 25 µM cold ATP (Vadlamudi et al., 2002). Samples were resolved by SDS-PAGE and auto-radiographed using Storm™ 865 scanner.
In Vivo [32P] Orthophosphate Labeling
Cells were incubated with freshly prepared phosphate-free DMEM medium supplemented with 20 µCi/ml [32P] orthophosphate overnight and then subjected to IR treatment. Protein extracts were subjected to IP analysis with the indicated antibodies, followed by isolation by SDS-PAGE and autography using Storm™ 865 scanner.
Clonogenic Survival Assay
The clonogenic survival assay has been described previously (Franken et al., 2006). Colonies were counted and the plating efficiency and surviving fraction for given treatments were calculated on the basis of the survival rates of non-irradiated cells.
Micrococcal Nuclease (MNase) Assay
MNase assay was carried out as previously described (Ziv et al., 2006). Freshly isolated nuclei were digested at 25°C with MNase at a concentration of 5 U per 250 µl of digestion buffer (15 mM Tris–HCl at pH 7.4, 60 mM KCl, 15 mM NaCl, 0.25 M sucrose, 1 mM CaCl2 and 0.5 mM DTT). Genomic DNA was purified and separated by electrophoresis in 1.2% agarose gel.
ATPase Assays
ATPase assays were performed as described previously (Bochar et al., 2000). Briefly, 25 µl of reaction mixture containing 20 mM Tris-HCl, pH 7.4, 8 mM MgCl2, 0.1 mM DTT, 50 mM KCl, 2% glycerol, 50 µg/ml of BSA, 0.5 µCi [γ-32P]ATP and where indicated 100 ng of double-stranded plasmid DNA, core histones, or nucleosomes purified from HeLa cells. Samples were incubated for 30 min at 30°C, and resolved on a 12% polyacrylamide gel containing 7 M urea for 1.0 h at 150 V. Wet gels were auto-radiographed for 10 min at −80°C.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ratna K. Vadlamudi, Feng Li, and Zhibo Yang for their technical assistance in the early phase of this study; Xifeng Wu and Uma Raju (M.D. Anderson Cancer Center, Houston, TX) for their helps with the comet and clonogenic survival assays, respectively. We are grateful to Ronald C. Conaway (Stowers Institute for Medical Research, Kansas City, MO), Yosef Shiloh (Tel Aviv University, Ramat Aviv, Israel) and Weidong Wang (National Institutes of Health, Baltimore, MD) for providing essential expression vectors and cell lines. The MORC2 structural and proteomic work was supported by the McCormick Genomic and Proteomic Center. LA is supported by the intramural funds of the National Library of Medicine, National Institutes of Health (NIH). This study was supported by the NIH grant CA139573 (to RK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes extended Experimental Procedures, seven figures, and two tables.
REFERENCES
- Bao Y, Shen X. Chromatin remodeling in DNA double-strand break repair. Curr Opin Genet Dev. 2007;17:126–131. doi: 10.1016/j.gde.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Ben-Yehoyada M, Ben-Dor I, Shaul Y. c-Abl tyrosine kinase selectively regulates p73 nuclear matrix association. J Biol Chem. 2003;278:34475–34482. doi: 10.1074/jbc.M301051200. [DOI] [PubMed] [Google Scholar]
- Bochar DA, Savard J, Wang W, Lafleur DW, Moore P, Cote J, Shiekhattar R. A family of chromatin remodeling factors related to Williams syndrome transcription factor. Proc Natl Acad Sci U S A. 2000;97:1038–1043. doi: 10.1073/pnas.97.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San- Martin B, Coppola V, Meffre E, Difilippantonio MJ, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Carson JJ, Feser J, Tamburini B, Zabaronick S, Linger J, Tyler JK. Acetylated lysine 56 on histone H3 drives chromatin assembly after repair and signals for the completion of repair. Cell. 2008;134:231–243. doi: 10.1016/j.cell.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- Gately DP, Hittle JC, Chan GK, Yen TJ. Characterization of ATM expression, localization, and associated DNA-dependent protein kinase activity. Mol Biol Cell. 1998;9:2361–2374. doi: 10.1091/mbc.9.9.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi AA, Kurka T, Jeggo PA. KAP-1 phosphorylation regulates CHD3 nucleosome remodeling during the DNA double-strand break response. Nat Struct Mol Biol. 2011a;18:831–839. doi: 10.1038/nsmb.2077. [DOI] [PubMed] [Google Scholar]
- Goodarzi AA, Kurka T, Jeggo PA. KAP-1 phosphorylation regulates CHD3 nucleosome remodeling during the DNA double-strand break response. Nat Struct Mol Biol. 2011b;18:831–839. doi: 10.1038/nsmb.2077. [DOI] [PubMed] [Google Scholar]
- Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M, Jeggo PA. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31:167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128:721–733. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Hamiche A, Sandaltzopoulos R, Gdula DA, Wu C. ATP-dependent histone octamer sliding mediated by the chromatin remodeling complex NURF. Cell. 1999;97:833–842. doi: 10.1016/s0092-8674(00)80796-5. [DOI] [PubMed] [Google Scholar]
- Inoue N, Hess KD, Moreadith RW, Richardson LL, Handel MA, Watson ML, Zinn AR. New gene family defined by MORC, a nuclear protein required for mouse spermatogenesis. Hum Mol Genet. 1999;8:1201–1207. doi: 10.1093/hmg/8.7.1201. [DOI] [PubMed] [Google Scholar]
- Iyer LM, Abhiman S, Aravind L. MutL homologs in restriction-modification systems and the origin of eukaryotic MORC ATPases. Biol Direct. 2008a;3:8. doi: 10.1186/1745-6150-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer LM, Anantharaman V, Wolf MY, Aravind L. Comparative genomics of transcription factors and chromatin proteins in parasitic protists and other eukaryotes. Int J Parasitol. 2008b;38:1–31. doi: 10.1016/j.ijpara.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Joshi N, Grant SG. DNA double-strand break damage and repair assessed by pulsed-field gel electrophoresis. Methods Mol Biol. 2005;291:121–129. doi: 10.1385/1-59259-840-4:121. [DOI] [PubMed] [Google Scholar]
- Kornberg RD, Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98:285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rew Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- Li DQ, Ohshiro K, Reddy SD, Pakala SB, Lee MH, Zhang Y, Rayala SK, Kumar R. E3 ubiquitin ligase COP1 regulates the stability and functions of MTA1. Proc Natl Acad Sci U S A. 2009;106:17493–17498. doi: 10.1073/pnas.0908027106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- Morrison AJ, Shen X. Chromatin remodelling beyond transcription: the INO80 and SWR1 complexes. Nat Rew Mol Cell Biol. 2009;10:373–384. doi: 10.1038/nrm2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive PL, Banath JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1:23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- Park JH, Park EJ, Lee HS, Kim SJ, Hur SK, Imbalzano AN, Kwon J. Mammalian SWI/SNF complexes facilitate DNA double-strand break repair by promoting gamma-H2AX induction. EMBO J. 2006;25:3986–3997. doi: 10.1038/sj.emboj.7601291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- Riballo E, Kuhne M, Rief N, Doherty A, Smith GC, Recio MJ, Reis C, Dahm K, Fricke A, Krempler A, et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol Cell. 2004;16:715–724. doi: 10.1016/j.molcel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rew Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- Soria G, Polo SE, Almouzni G. Prime, repair, restore: the active role of chromatin in the DNA damage response. Mol Cell. 2012;46:722–734. doi: 10.1016/j.molcel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- Sulli G, Di Micco R, di Fagagna FD. Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat Rew Cancer. 2012;12:709–720. doi: 10.1038/nrc3344. [DOI] [PubMed] [Google Scholar]
- Tomimatsu N, Mukherjee B, Burma S. Distinct roles of ATR and DNA-PKcs in triggering DNA damage responses in ATM-deficient cells. EMBO Rep. 2009;10:629–635. doi: 10.1038/embor.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui KM, Sano K, Tsutsui K. Dynamic view of the nuclear matrix. Acta Med Okayama. 2005;59:113–120. doi: 10.18926/AMO/31953. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nature Cell Biol. 2002;4:681–690. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Bar-Shira A, Pecker I, Russell P, Jorgensen TJ, Tsarfati I, Shiloh Y. Recombinant ATM protein complements the cellular A-T phenotype. Oncogene. 1997;15:159–167. doi: 10.1038/sj.onc.1201319. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Bielopolski D, Galanty Y, Lukas C, Taya Y, Schultz DC, Lukas J, Bekker-Jensen S, Bartek J, Shiloh Y. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat Cell Biol. 2006;8:870–876. doi: 10.1038/ncb1446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.