Abstract
AIM: To investigate the association between B-mode ultrasound classification of small hepatocellular carcinoma (HCC) and outcome after radiofrequency ablation (RFA).
METHODS: Ninety-seven cases of HCC treated using RFA between April 2001 and March 2006 were reviewed. Ultrasound images were classified as follows: type 1, with halo (n = 29); and type 2, without halo (n = 68). Type 2 was further categorized into three subgroups: type 2a, homogenous hyperechoic (n = 9); type 2b, hypoechoic with smooth margins (n = 43); and type 2c (n = 16), hypoechoic with irregular or unclear margins. Patients with type 2a HCC were excluded from analysis due to the small number of cases.
RESULTS: Two year recurrence rates for type 2b, type 1 and type 2c were 26%, 42% and 69%, respectively, with significant differences between type 2b and type 2c (P < 0.01), and between type 1 and type 2c (P < 0.05). Five year survival rates were 89%, 43% and 65%, respectively. Survival was significantly longer for type 2b than for other types (type 1 vs type 2b, P < 0.01; type 2b vs type 2c, P < 0.05). On univariate analysis, factors contributing to recurrence were number of tumors, tumor stage, serum level of lens culinaris agglutinin-reactive alpha-fetoprotein and ultrasound classification (P < 0.05). Factors contributing to survival were tumor stage and ultrasound classification (P < 0.05). Multivariate analysis identified ultrasound classification as the only factor independently associated with both recurrence and survival (P < 0.05).
CONCLUSION: B-mode ultrasound classification of small HCC is a predictive factor for outcome after RFA.
Keywords: B-mode ultrasound, Hepatocellular carcinoma, Radiofrequency ablation, Recurrence, Prognosis
INTRODUCTION
Treatment strategies for hepatocellular carcinoma (HCC) are decided on the basis of tumor size and number, liver function and performance status[1,2]. Percutaneous local treatments that are less invasive than resection are performed for small HCCs that are unsuitable for resection, with the indications of ≤ 3 lesions, each with diameter ≤ 3 cm, in accordance with the Japanese guidelines[2] and the practice guidelines of the American Association for the Study of Liver Diseases[1].
Percutaneous radiofrequency ablation (RFA) is a well established local treatment for unresectable small HCC[3,4]. RFA is a curative treatment and achieves not only superior local control of the disease, but also better prognosis compared to percutaneous ethanol injection therapy (PEIT)[5,6]. Accordingly, RFA is now recommended over PEIT for the treatment of small HCC. Recently, RFA has also been adopted for patients with resectable early HCC, defined as single tumors > 2 cm in diameter or up to 3 nodules < 3 cm in diameter, with performance status 0 and Child-Pugh class A or B[7].
However, rapid aggressive recurrence with vascular invasion[8-10], intrahepatic dissemination[11,12], seeding or metastasis[13,14] has been reported after RFA. In particular, the risk of seeding is high in patients with poorly differentiated HCC[15]. Furthermore, the prognosis following RFA for poorly differentiated HCC is reportedly unfavorable[16,17]. A large proportion of patients with poorly differentiated HCC show microscopic vascular invasion and intrahepatic metastasis, even when the tumor is small[18]. As a result, curative treatment cannot be achieved using RFA alone and the procedure may thus cause dissemination or metastasis. Clinical diagnosis of poorly differentiated HCC with high-grade malignancy is therefore crucial when determining treatment strategies for small HCC.
Small HCCs show various images on B-mode ultrasound. However, the correlation between B-mode ultrasound image and prognosis has not been elucidated. We have previously reported that classification on B-mode ultrasonography of small hypervascular HCC correlated with histological differentiation and serum level of lens culinaris agglutinin-reactive alpha-feto protein (AFP-L3), an indicator of poor prognosis[19]. In particular, the presence of irregular or unclear margins was very important in screening for small, poorly differentiated HCC. The aim of this study was to determine whether B-mode ultrasound classification is associated with recurrence and survival after RFA.
MATERIALS AND METHODS
Patients
Our prospective database of 97 patients with initial hypervascular HCC (≤ 3 tumors, all ≤ 3 cm in diameter) who had undergone RFA between April 2001 and March 2006 was reviewed. Diagnosis of hypervascular HCC was based on the findings of tumor staining during the arterial phase of contrast-enhanced computed tomography (CT), dynamic magnetic resonance imaging (MRI) or contrast ultrasonography. If any of these diagnostic imaging techniques showed tumor stain in the arterial phase that was washed out in the equilibrium phase, imaging diagnosis was considered definitive. In all patients, tumor stage (tumor-node-metastasis classification as described by the Liver Cancer Study of Japan), etiology of hepatitis, Child-Pugh classification, levels of tumor markers (AFP, AFP-L3 and des-gamma-carboxy prothrombin), fibrosis stage and activity grade of the biopsied liver tissue using the new Inuyama classification[20] were evaluated before RFA. Eligibility criteria for RFA were as follows: (1) no vascular invasion on imaging diagnosis; (2) no severe ascites; (3) platelet count ≥ 5 × 104/mm3; (4) prothrombin time ≥ 50%; (5) total bilirubin < 3 mg/dL; (6) no distant metastases; and (7) in principle, ≤ 3 tumors, all ≤ 3 cm in diameter. No exclusion criteria were set in terms of tumor location (i.e., near main vessels, adjacent organs). Furthermore, all patients with recurrent HCC underwent iterative RFA even when the above criteria for tumor size and number were not met, as long as complete ablation was considered achievable. Written informed consent was obtained from each enrolled patient and the protocol was approved by our institutional review board.
RFA technique
Percutaneous RFA using the Cool-tip RF system (Valleylab, Boulder, CO, United States) was performed under ultrasound guidance in all patients. Artificial pleural effusion or artificial ascites was produced using saline when necessary[21]. The impedance control mode was used with a 17-gauge, cooled-tip electrode with a 2 or 3 cm exposed tip. Ablation was started at 40 W for the 2 cm exposed tip and 60 W for the 3 cm exposed tip. Power was increased at a rate of 10 W/min. When a rapid increase in impedance occurred, output was automatically stopped and ablation was restarted after a short time at an output 10 W lower. Duration of a single ablation was 6 min for the 2 cm electrode and 12 min for the 3 cm electrode. After RF exposure, temperature of the needle tip was measured. When the temperature was below 65 °C, additional ablation was performed. The electrode track was not treated by thermo-coagulation in any patients, to prevent seeding or hemorrhage.
Assessment of response and follow-up
Treatment response was assessed by contrast-enhanced CT or MRI at 1-3 d after the final session. Complete response was defined as no enhancement in the entire lesion with a safety margin on imaging. Additional ablation was performed until complete ablation was confirmed in each nodule. All patients were followed up on an outpatient basis every 3-4 mo using contrast-enhanced CT or MRI and measurement of tumor marker levels.
B-mode ultrasound imaging
We used either a SONOLINE Elegra™ Ultrasound Platform (Siemens Medical Systems, Erlangen, Germany) with a 3.5C40 convex probe or a SSA-770A ultrasound system (Toshiba Medical Systems, Tochigi, Japan) with a PVT-674BT ultrasound probe. Tissue harmonic imaging was performed in B-mode.
B-mode ultrasound classification
The B-mode ultrasound classification of small HCC we reported previously was used[19]. Nodules with a halo were regarded as type 1 and halo-free nodules were regarded as type 2. In addition, type 2 nodules were further classified based on the internal echo level and marginal features; uniform hyperechoic nodules were evaluated as type 2a, hypoechoic nodules with regular margins as type 2b and hypoechoic nodules with irregular or unclear margins as type 2c. B-mode ultrasound images were obtained within 1 mo before RFA. All recorded ultrasound images were analyzed by two skilled hepatologists (10 and 19 years of experience in abdominal ultrasonography) who were blinded to patient names. When a discrepancy existed in interpretation between the two hepatologists, a consensus was reached through discussion. If HCCs comprised two or three nodules, the largest nodule was selected and classified using our B-mode classification. B-mode ultrasound classified 29 cases as type 1, 9 as type 2a, 43 as type 2b, and 16 as type 2c. Given the small number of patients with type 2a HCC, these cases were excluded from analysis (Figure 1).
Figure 1.
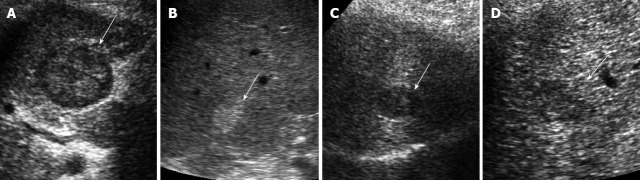
Classification of B-mode ultrasonographic images of small hepatocellular carcinoma. A-D: Hepatocellular carcinoma nodules < 3 cm in diameter were classified into two groups using B-mode ultrasonography: Type 1 with halo (A) and type 2 without halo. Type 2 was then further categorized into three subgroups: Type 2a, homogenous hyperechoic (B); Type 2b, hypoechoic with smooth margins (C); Type 2c, hypoechoic with irregular or unclear margins (D). Hepatocellular carcinoma nodules are indicated by arrows.
Statistical analysis
One factor analysis of variance and the Scheffe test were used to analyze continuous variables. Fisher’s exact test or the χ2 test were used to analyze categorical variables. Cumulative recurrence-free survival rates and cumulative survival rates according to B-mode ultrasound classification were constructed using Kaplan-Meier methods and compared using the log-rank test. Uni- and multivariate analyses using a Cox proportional hazard regression model were performed for factors contributing to tumor recurrence and survival. Results were expressed as hazard ratio with 95%CI. P < 0.05 was considered statistically significant for all analyses using SPSS Statistics Version 19 software (IBM, Tokyo, Japan).
RESULTS
The median follow-up interval was 1018 d. Two year recurrence rates for type 2b, type 1 and type 2c were 26%, 42% and 69%, respectively. Significant differences were seen between type 2b and type 2c (P < 0.01), and between type 1 and type 2c (P < 0.05). Five year survival rates were 89%, 43% and 65%, respectively. Survival was significantly longer for type 2b than for other groups (type 1 vs type 2b, P < 0.01; type 2b vs type 2c, P < 0.05).
Patient background variables at baseline according to B-mode ultrasound classification are compared in Table 1. Significant differences were evident among groups in terms of number of tumors, tumor size, tumor stage and activity grade of hepatitis. Mean tumor size was smaller in type 2b than in other types. Mean number of tumors was smaller in type 2b than in type 2c. High tumor stage was more frequent in type 2c than in other types. Severe activity grade of hepatitis was likewise more frequent in type 2c than in other types. Mean AFP-L3 level was higher in type 2c than in other types.
Table 1.
Comparison of patient characteristics according to B-mode ultrasound-based classification
| Type 1 (n = 29) | Type 2b (n = 43) | Type 2c (n = 16) | P value | |
| Age (yr) | 66.4 ± 9.5 | 66.9 ± 8.7 | 69.3 ± 6.8 | 0.554 |
| Gender (male/female) | 23/6 | 25/18 | 8/8 | 0.085 |
| HCV (positive/negative) | 28/1 | 36/7 | 14/2 | 0.241 |
| Number of tumors | 1.2 ± 0.4 | 1.2 ± 0.5 | 1.6 ± 0.7 | 0.046 |
| Size of tumor (mm) | 22.5 ± 3.8 | 19.0 ± 5.2 | 23.1 ± 5.6 | 0.003 |
| Child-Pugh classification (A/B) | 16/13 | 31/12 | 9/7 | 0.272 |
| Tumor stage (I/II/III) | 10/15/4 | 28/13/2 | 4/7/5 | 0.006 |
| Activity grade (A0, 1/2, 3) | 7/22 | 14/29 | 10/6 | 0.032 |
| Fibrosis stage (F0-2/3, 4) | 8/21 | 10/33 | 7/9 | 0.298 |
| AFP (ng/mL) | 124 ± 246 | 118 ± 274 | 207 ± 406 | 0.564 |
| AFP-L3 (%) | 7.3 ± 17.0 | 5.8 ± 16.5 | 17.5 ± 25.5 | 0.098 |
| DCP (mAU/mL) | 223 ± 489 | 210 ± 482 | 299 ± 486 | 0.817 |
Data are presented as mean ± SD or n/N. HCV: Hepatitis C virus; AFP: Alpha-fetoprotein; AFP-L3: Lens culinaris agglutinin-reactive alpha-fetoprotein; DCP: Des-gamma-carboxy prothrombin.
Recurrence-free survival curves according to B-mode ultrasound classification are compared in Figure 2. Recurrence-free survival was significantly shorter for type 2c HCC than for other types.
Figure 2.
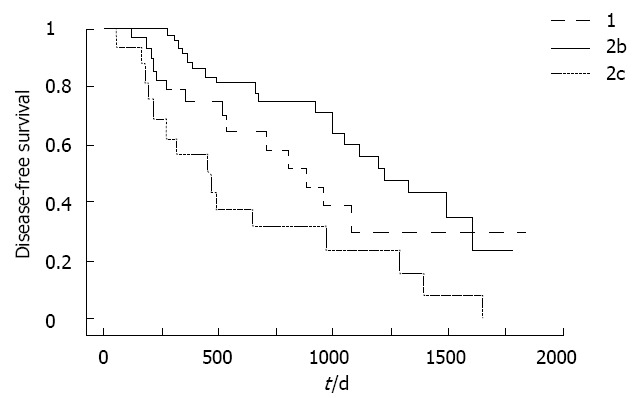
Recurrence-free survival curves according to B-mode ultrasound classification. Recurrence-free survival was significantly shorter for type 2c hepatocellular carcinoma than for other types. P = 0.0454 type 1 vs type 2c; P = 0.0005 type 2b vs type 2c.
Survival curves according to B-mode ultrasound classification are compared in Figure 3. Survival was significantly longer for type 2b than for other groups. No significant difference in survival was evident between types 1 and 2c.
Figure 3.
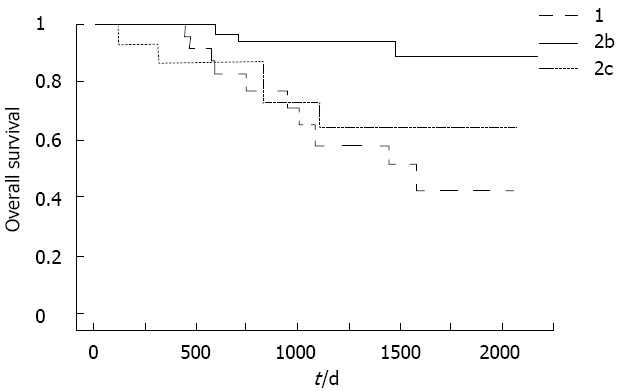
Survival curves according to B-mode ultrasound classification. Survival was significantly longer for type 2b than for other types. P = 0.0006 type 1 vs type 2b; P = 0.0165 type 2b vs type 2c; P = 0.4473 type 1 vs type 2c.
Results of univariate analysis of background variables associated with tumor recurrence are shown in Table 2. Number of tumors, tumor stage, AFP-L3 levels and B-mode ultrasound classification were identified as significant contributing factors for recurrence after RFA. These significant variables were then entered into multivariate analysis. The results of multivariate analysis are shown in Table 3, with type 2c of B-mode ultrasound classification identified as the only independent factor contributing to tumor recurrence.
Table 2.
Univariate analysis of factors contributing to recurrence
| Variables | HR | 95%CI | P value |
| Age (yr) | 1.007 | 0.972-1.043 | 0.717 |
| Gender | |||
| Female | 1 | ||
| Male | 1.036 | 0.577-1.859 | 0.906 |
| HCV | |||
| Negative | 1 | ||
| Positive | 1.176 | 0.527-2.625 | 0.693 |
| Number of tumors | 1.557 | 1.009-2.404 | 0.045 |
| Size of tumor (mm) | 1.035 | 0.980-1.092 | 0.214 |
| Child-Pugh classification | |||
| A | 1 | ||
| B | 1.481 | 0.838-2.617 | 0.177 |
| Tumor stage | |||
| I | 1 | ||
| II | 1.214 | 0.657-2.244 | 0.535 |
| III | 5.055 | 2.123-12.034 | 0.000 |
| Activity grade | |||
| A0, 1 | 1 | ||
| A2, 3 | 1.346 | 0.745-2.430 | 0.325 |
| Fibrosis stage | |||
| F0-2 | 1 | ||
| F3, 4 | 1.323 | 0.689-2.540 | 0.401 |
| AFP (ng/mL) | 1.000 | 1.000-1.001 | 0.318 |
| AFP-L3 (%) | 1.017 | 1.004-1.030 | 0.023 |
| DCP (mAU/mL) | 1.000 | 1.000-1.001 | 0.332 |
| B-mode classification | |||
| Type 2b | 1 | ||
| Type 1 | 1.531 | 0.767-3.053 | 0.227 |
| Type 2c | 3.179 | 1.623-6.227 | 0.001 |
HR: Hazard ratio; HCV: Hepatitis C virus; AFP: Alpha-fetoprotein; AFP-L3: Lens culinaris agglutinin-reactive alpha-fetoprotein; DCP: Des-gamma-carboxy prothrombin.
Table 3.
Multivariate analysis of factors contributing to recurrence
| Variables | HR | 95%CI | P value |
| n | 1.411 | 0.719-2.770 | 0.317 |
| Tumor stage | |||
| I | 1 | ||
| II | 0.645 | 0.283-1.467 | 0.296 |
| III | 2.540 | 0.784-8.224 | 0.120 |
| AFP-L3 | |||
| Negative | 1 | ||
| Positive | 1.531 | 0.642-3.649 | 0.337 |
| B-mode classification | |||
| Type 2b | 1 | ||
| Type 1 | 1.850 | 0.878-3.899 | 0.106 |
| Type 2c | 2.438 | 1.107-5.373 | 0.027 |
HR: Hazard ratio; AFP-L3: Lens culinaris agglutinin-reactive alpha-fetoprotein.
The results of univariate analysis of background variables associated with survival are shown in Table 4. Tumor stage and B-mode ultrasound classification were identified as significant contributing factors for survival. All significant variables on univariate analysis were then entered into multivariate analysis. The results of multivariate analysis are shown in Table 5, with type 1 of B-mode ultrasound classification identified as the only independent factor contributing to survival.
Table 4.
Univariate analysis of factors contributing to survival
| Variables | HR | 95%CI | P value |
| Age (yr) | 0.979 | 0.929-1.032 | 0.440 |
| Gender | |||
| Female | 1 | ||
| Male | 1.161 | 0.435-3.096 | 0.766 |
| HCV | |||
| Negative | 1 | ||
| Positive | 0.947 | 0.218-4.124 | 0.943 |
| Number of tumors | 1.700 | 0.840-3.440 | 0.140 |
| Size of tumor (mm) | 1.030 | 0.945-1.123 | 0.504 |
| Child-Pugh classification | |||
| A | 1 | ||
| B | 1.789 | 0.710-4.512 | 0.218 |
| Tumor stage | |||
| I | 1 | ||
| II | 1.588 | 0.551-4.579 | 0.392 |
| III | 3.847 | 1.081-13.690 | 0.037 |
| Activity grade | |||
| A0, 1 | 1 | ||
| A2, 3 | 1.978 | 0.698-5.606 | 0.200 |
| Fibrosis stage | |||
| F0-2 | 1 | ||
| F3, 4 | 1.292 | 0.425-3.930 | 0.652 |
| AFP (ng/mL) | 1.001 | 1.000-1.002 | 0.087 |
| AFP-L3 (%) | 1.012 | 0.995-1.031 | 0.212 |
| DCP (mAU/mL) | 1.000 | 0.999-1.001 | 0.919 |
| B-mode classification | |||
| Type 2b | 1 | ||
| Type 1 | 6.911 | 1.897-25.176 | 0.003 |
| Type 2c | 4.466 | 1.066-18.709 | 0.041 |
HR: Hazard ratio; HCV: Hepatitis C virus; AFP: Alpha-fetoprotein; AFP-L3: Lens culinaris agglutinin-reactive alpha-fetoprotein; DCP: Des-gamma-carboxy prothrombin.
Table 5.
Multivariate analysis of factors contributing to survival
| Variables | HR | 95%CI | P value |
| Tumor stage | |||
| I | 1 | ||
| II | 1.189 | 0.406-3.481 | 0.752 |
| III | 3.763 | 0.896-15.796 | 0.070 |
| B-mode classification | |||
| Type 2b | 1 | ||
| Type 1 | 7.146 | 1.924-26.538 | 0.003 |
| Type 2c | 3.055 | 0.668-13.970 | 0.150 |
HR: Hazard ratio.
DISCUSSION
This review of a prospective database for patients who had undergone RFA for primary HCC found B-mode ultrasound image classification as an independent factor strongly influencing post-RFA recurrence and disease outcomes. This result supports the earlier finding that the B-mode ultrasound image classification we devised is capable of evaluating the malignant potential of HCC[19].
The most common B-mode ultrasound image classification for primary HCCs ≤ 3 cm in diameter that had undergone RFA was type 2b, followed by types 1b, 2c and 2a, in that order. Substantial bias in the distribution of ultrasound types was thus evident. We have previously reported that type 2a HCC shows the smallest mean diameter in B-mode ultrasound classification and represents well-differentiated HCC with fat deposition[19]. Type 2a can therefore be considered as the ultrasound classification associated with the lowest malignant potential. Our present database of patients with hypervascular HCC included only 9 type 2a patients and data for these cases were excluded from analyses due to insufficient numbers. However, type 2a HCC seems likely to represent the patient group associated with the best prognosis.
Type 2b showed a longer interval until recurrence and better outcomes compared with types 1 and 2c. Of course, mean tumor diameter is smaller in type 2b than in types 1 and 2c and tumor stage also shows a higher proportion of early-stage cases. Outcomes for type 2b could thus be due to these reasons. However, multivariate analysis identified ultrasound categorization as an independent factor exerting greater influence on recurrence and survival than either tumor diameter or stage. For that reason, the malignant potential of type 2b HCC can be considered lower than that of types 1 and 2c.
Type 1 in the B-mode ultrasound image classification represents HCC with a clear margin accompanied by a halo. The halo represents a fibrous capsule, a finding commonly seen in dedifferentiated and moderately differentiated HCCs during the course of multistep carcinogenesis. In contrast, a type 2c ultrasound image is commonly seen in poorly differentiated HCCs, showing no halo and irregular or unclear margins[19]. In addition, a type 2c ultrasound image is frequently seen in HCCs that are positive for AFP-L3 and type 2c HCCs thus have higher malignant potential than type 1 HCCs[19]. When the recurrence-free curve following RFA was stratified for types 2b, 1 and 2c, type 2c was seen to include the most cases of early recurrence. This difference can be thought to reflect the malignant potential of HCC. Conversely, we found no difference in survival rates between types 1 and 2c. HCC does not just show a high recurrence rate; it is a cancer that also undergoes repeated recurrence, as a result of which the level of compliance with the follow-up schedule and administration of treatment for recurrent lesions also greatly influence the outcome. The patient database used in the present study was prospective, in accordance with the predetermined follow-up schedule. For that reason we think that compliance with the follow-up schedule exerted little effect on outcomes in this study. However, the possibility that treatment at the time of recurrence influenced the results cannot be ignored. We actively performed RFA for our patients, not only in cases where they satisfied the indications for RFA at the time of recurrence, but even when criteria of tumor size and number were not met, as long as the imaging findings were evaluated as suggesting that the lesions could be controlled. Radical re-treatment rates, including iterative RFA or resection for type 1, type 2b and type 2c, were 93%, 95% and 80%, respectively. No significant differences were seen among radical re-treatment rates according to ultrasound classification. Our analysis of prognostic factors in this study did not include the treatment method at the time of recurrence. However, early detection of recurrence due to the prudent follow-up schedule and the effectiveness of our active treatment of recurrent lesions may have been reasons for the lack of significant differences in survival rate between types 1 and 2c.
Studies to date have identified various risk factors for recurrence following RFA for HCC, including tumor diameter[22-27], tumor number[28-30], tumor stage[24], histological poor differentiation[24], insufficient safety margin[25,29,31], a tumor location[25,27] that is problematic for RFA, such as adjacent to a large blood vessel or the liver surface, hepatitis C virus[28] and/or hepatitis B virus infection[23], AFP level[22,25,28], liver fibrosis and platelet count[23,30]. Santambrogio et al[32] recently reported that intraoperative ultrasound score can predict recurrent HCC after radical treatment. However, no previous reports have identified B-mode ultrasonogram for small HCC using external ultrasound as a risk factor for recurrence. The present study identified that risk factors for recurrence included, not only the previously reported tumor number, tumor stage and tumor markers, but also ultrasound image type. Moreover, multivariate analysis identified ultrasound image type 2c as the only significant independent risk factor. Ultrasound image type thus seems more closely associated with recurrence of HCC after RFA than previously reported risk factors.
On the other hand, the prognostic factors for RFA that have been reported to date are similar to the risk factors for recurrence; in addition to factors such as tumor diameter, tumor number, tumor stage, tumor differentiation grade and tumor markers, patient age and hepatic function (Child’s classification) have also been cited[33-37]. The present study found that tumor stage and B-mode ultrasound image type all represented significant prognostic factors and multivariate analysis identified ultrasound image type as the only significant independent risk factor. Accordingly, B-mode ultrasound image type appears more closely associated with the outcome of RFA than the previously reported risk factors for both recurrence and prognosis. When deciding therapeutic strategies for small HCC in the future, the greatest attention and importance should be placed on the B-mode ultrasound image type.
Ultrasound achieves superior spatial resolution compared with CT and MRI and is the most capable modality for depicting the morphological details of tumors. For that reason, ultrasound is considered to closely reflect the gross morphology of tumors. The gross morphology of HCC is a prognostic factor. With regard to the nodular type, the contiguous multi-nodular type and the single nodular type with extra-nodular growth are reportedly less histologically differentiated than the single nodular type and, because they show higher incidences of vascular invasion and intrahepatic metastasis, recurrence following resection is an early indicator of poor prognosis[38-40]. We think that the reason ultrasound image type is strongly associated with recurrence and outcomes following RFA is that the B-mode ultrasound classification we devised closely reflects the macroscopic type of HCC and enables identification of small HCC with poorer differentiation and higher malignant potential.
Some limitations of the present study were the design as a small-scale retrospective study, with a large degree of bias in the distribution of B-mode ultrasound image types. This prevented us from analyzing post-RFA recurrence and prognosis in relation to type 2a small HCC. We were also unable to investigate the effects of therapy on hepatitis, which presumably influences the recurrence and outcomes of small HCC, or the effects of treatments administered at the time of recurrence. However, even with those limitations, we were able to generate very interesting results indicating that the B-mode ultrasound image type is strongly associated with outcomes following RFA.
In conclusion, this study demonstrated B-mode ultrasound image classification type as a factor that strongly influences outcomes following RFA of small HCC. This new knowledge is likely to influence the therapeutic strategies of physicians treating patients with small HCC. That is, even if a patient satisfies the indications for RFA, the malignant potential of lesions should be evaluated on the basis of B-mode ultrasound image classification and tumor marker levels. Since type 1 and type 2c small HCC have high malignant potential, potentially more effective therapeutic strategies that include other treatment methods, such as resection or concurrent transcatheter arterial chemoembolization etc., should be carefully devised rather than simply selecting RFA by default.
COMMENTS
Background
Percutaneous radiofrequency ablation (RFA) is a minor invasive and radical treatment for small hepatocellular carcinomas (HCCs). However, rapid aggressive recurrence with vascular invasion, intrahepatic dissemination, seeding or metastasis has been reported after RFA. The risk of seeding is high in patients with poorly differentiated HCC. Furthermore, the prognosis following RFA for poorly differentiated HCC is reportedly unfavorable. Clinical diagnosis of poorly differentiated HCC with high-grade malignancy is therefore very important when selecting treatment for small HCC.
Research frontiers
Small HCCs show various images on B-mode ultrasound. However, the correlation between B-mode ultrasound image and prognosis has not been elucidated. In the present study, the authors investigated the association between B-mode ultrasound image of small hypervascular HCC and outcome after RFA. The authors have previously reported that classification on B-mode ultrasonography of small hypervascular HCC correlated with histological differentiation.
Innovations and breakthroughs
This is the first study to report that B-mode ultrasound image of small hypervascular HCC is a predictive factor for outcome after RFA. Nodules with a halo and halo-free hypoechoic nodules with irregular or unclear margins have higher malignant potential and treatment for such lesions should be selected with care.
Applications
B-mode ultrasound image of small HCC is likely to influence the therapeutic strategies of physicians treating patients with small HCC.
Terminology
The halo sign is a hypoechoic band around the tumor, corresponding to a thin fibrous capsule around the HCC.
Peer review
The authors report a series of 97 patients affected by HCC undergoing RFA. They demonstrated that the ultrasonographic pattern of HCC predicts the risk of recurrence after RFA. It is an interesting and innovative issue.
Footnotes
Peer reviewer: Luca Vigano, MD, Department of HPB and Digestive Surgery, Ospedale Mauriziano Umberto I, Largo Turati 62, 10128 Torino, Italy
S- Editor Xiong L L- Editor Roemmele A E- Editor Xiong L
References
- 1.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 2.Clinical Practice Guidelines for Hepatocellular Carcinoma - The Japan Society of Hepatology 2009 update. Hepatol Res. 2010;40 Suppl 1:2–144. doi: 10.1111/j.1872-034X.2010.00650.x. [DOI] [PubMed] [Google Scholar]
- 3.Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759–768. doi: 10.2214/ajr.167.3.8751696. [DOI] [PubMed] [Google Scholar]
- 4.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 5.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Seki T, Tamai T, Ikeda K, Imamura M, Nishimura A, Yamashiki N, Nakagawa T, Inoue K. Rapid progression of hepatocellular carcinoma after transcatheter arterial chemoembolization and percutaneous radiofrequency ablation in the primary tumour region. Eur J Gastroenterol Hepatol. 2001;13:291–294. doi: 10.1097/00042737-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Takada Y, Kurata M, Ohkohchi N. Rapid and aggressive recurrence accompanied by portal tumor thrombus after radiofrequency ablation for hepatocellular carcinoma. Int J Clin Oncol. 2003;8:332–335. doi: 10.1007/s10147-003-0328-6. [DOI] [PubMed] [Google Scholar]
- 10.Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, Guglielmi A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10:1137–1140. doi: 10.3748/wjg.v10.i8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicoli N, Casaril A, Abu Hilal M, Mangiante G, Marchiori L, Ciola M, Invernizzi L, Campagnaro T, Mansueto G. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am J Surg. 2004;188:165–167. doi: 10.1016/j.amjsurg.2003.12.061. [DOI] [PubMed] [Google Scholar]
- 12.Mori Y, Tamai H, Shingaki N, Moribata K, Shiraki T, Deguchi H, Ueda K, Enomoto S, Magari H, Inoue I, et al. Diffuse intrahepatic recurrence after percutaneous radiofrequency ablation for solitary and small hepatocellular carcinoma. Hepatol Int. 2009;3:509–515. doi: 10.1007/s12072-009-9131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 14.Portolani N, Tiberio GA, Ronconi M, Coniglio A, Ghidoni S, Gaverini G, Giulini SM. Aggressive recurrence after radiofrequency ablation of liver neoplasms. Hepatogastroenterology. 2003;50:2179–2184. [PubMed] [Google Scholar]
- 15.Imamura J, Tateishi R, Shiina S, Goto E, Sato T, Ohki T, Masuzaki R, Goto T, Yoshida H, Kanai F, et al. Neoplastic seeding after radiofrequency ablation for hepatocellular carcinoma. Am J Gastroenterol. 2008;103:3057–3062. doi: 10.1111/j.1572-0241.2008.02153.x. [DOI] [PubMed] [Google Scholar]
- 16.Akamatsu M, Ishikawa T, Shiratori Y, Koike Y, Shiina S, Teratani T, Hamamura K, Obi S, Sato S, Tateishi R, et al. Factors predisposing to poorly differentiated hepatocellular carcinoma and its recurrence. Hepatogastroenterology. 2005;52:391–397. [PubMed] [Google Scholar]
- 17.Kim SH, Lim HK, Choi D, Lee WJ, Kim SH, Kim MJ, Kim CK, Jeon YH, Lee JM, Rhim H. Percutaneous radiofrequency ablation of hepatocellular carcinoma: effect of histologic grade on therapeutic results. AJR Am J Roentgenol. 2006;186:S327–S333. doi: 10.2214/AJR.05.0350. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda S, Itamoto T, Nakahara H, Kohashi T, Ohdan H, Hino H, Ochi M, Tashiro H, Asahara T. Clinicopathologic features and prognostic factors of resected solitary small-sized hepatocellular carcinoma. Hepatogastroenterology. 2005;52:1163–1167. [PubMed] [Google Scholar]
- 19.Moribata K, Tamai H, Shingaki N, Mori Y, Enomoto S, Shiraki T, Deguchi H, Ueda K, Inoue I, Maekita T, et al. Assessment of malignant potential of small hypervascular hepatocellular carcinoma using B-mode ultrasonography. Hepatol Res. 2011;41:233–239. doi: 10.1111/j.1872-034X.2010.00763.x. [DOI] [PubMed] [Google Scholar]
- 20.Adachi E, Maeda T, Kajiyama K, Kinukawa N, Matsumata T, Sugimachi K, Tsuneyoshi M. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer. 1996;77:2022–2031. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2022::AID-CNCR9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78 Suppl 1:113–124. doi: 10.1159/000315239. [DOI] [PubMed] [Google Scholar]
- 22.Harrison LE, Koneru B, Baramipour P, Fisher A, Barone A, Wilson D, Dela Torre A, Cho KC, Contractor D, Korogodsky M. Locoregional recurrences are frequent after radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2003;197:759–764. doi: 10.1016/S1072-7515(03)00750-6. [DOI] [PubMed] [Google Scholar]
- 23.Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J, Tung H, Tso WK, Fan ST, Poon RT. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg. 2008;207:20–29. doi: 10.1016/j.jamcollsurg.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Yu HC, Cheng JS, Lai KH, Lin CP, Lo GH, Lin CK, Hsu PI, Chan HH, Lo CC, Tsai WL, et al. Factors for early tumor recurrence of single small hepatocellular carcinoma after percutaneous radiofrequency ablation therapy. World J Gastroenterol. 2005;11:1439–1444. doi: 10.3748/wjg.v11.i10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006;59:432–441. doi: 10.1016/j.ejrad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Waki K, Aikata H, Katamura Y, Kawaoka T, Takaki S, Hiramatsu A, Takahashi S, Toyota N, Ito K, Chayama K. Percutaneous radiofrequency ablation as first-line treatment for small hepatocellular carcinoma: results and prognostic factors on long-term follow up. J Gastroenterol Hepatol. 2010;25:597–604. doi: 10.1111/j.1440-1746.2009.06125.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang B, Zou J, Xia J, Ren Z, Gan Y, Wang Y, Zhang B, Ge N, Wang D, Chen Y, et al. Risk factors for recurrence of small hepatocellular carcinoma after long-term follow-up of percutaneous radiofrequency ablation. Eur J Radiol. 2011;79:196–200. doi: 10.1016/j.ejrad.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Izumi N, Asahina Y, Noguchi O, Uchihara M, Kanazawa N, Itakura J, Himeno Y, Miyake S, Sakai T, Enomoto N. Risk factors for distant recurrence of hepatocellular carcinoma in the liver after complete coagulation by microwave or radiofrequency ablation. Cancer. 2001;91:949–956. [PubMed] [Google Scholar]
- 29.Horiike N, Iuchi H, Ninomiya T, Kawai K, Kumagi T, Michitaka K, Masumoto T, Onji M. Influencing factors for recurrence of hepatocellular carcinoma treated with radiofrequency ablation. Oncol Rep. 2002;9:1059–1062. [PubMed] [Google Scholar]
- 30.Yamanaka Y, Shiraki K, Miyashita K, Inoue T, Kawakita T, Yamaguchi Y, Saitou Y, Yamamoto N, Nakano T, Nakatsuka A, et al. Risk factors for the recurrence of hepatocellular carcinoma after radiofrequency ablation of hepatocellular carcinoma in patients with hepatitis C. World J Gastroenterol. 2005;11:2174–2178. doi: 10.3748/wjg.v11.i14.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zytoon AA, Ishii H, Murakami K, El-Kholy MR, Furuse J, El-Dorry A, El-Malah A. Recurrence-free survival after radiofrequency ablation of hepatocellular carcinoma. A registry report of the impact of risk factors on outcome. Jpn J Clin Oncol. 2007;37:658–672. doi: 10.1093/jjco/hym086. [DOI] [PubMed] [Google Scholar]
- 32.Santambrogio R, Costa M, Strada D, Bertolini E, Zuin M, Barabino M, Opocher E. Intraoperative ultrasound score to predict recurrent hepatocellular carcinoma after radical treatments. Ultrasound Med Biol. 2011;37:7–15. doi: 10.1016/j.ultrasmedbio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Machi J, Bueno RS, Wong LL. Long-term follow-up outcome of patients undergoing radiofrequency ablation for unresectable hepatocellular carcinoma. World J Surg. 2005;29:1364–1373. doi: 10.1007/s00268-005-7829-6. [DOI] [PubMed] [Google Scholar]
- 34.Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684–692. doi: 10.1007/s00330-006-0461-5. [DOI] [PubMed] [Google Scholar]
- 35.Guglielmi A, Ruzzenente A, Sandri M, Pachera S, Pedrazzani C, Tasselli S, Iacono C. Radio frequency ablation for hepatocellular carcinoma in cirrhotic patients: prognostic factors for survival. J Gastrointest Surg. 2007;11:143–149. doi: 10.1007/s11605-006-0082-y. [DOI] [PubMed] [Google Scholar]
- 36.Yan K, Chen MH, Yang W, Wang YB, Gao W, Hao CY, Xing BC, Huang XF. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336–347. doi: 10.1016/j.ejrad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi S, Kudo M, Chung H, Inoue T, Ishikawa E, Kitai S, Tatsumi C, Ueda T, Nagai T, Minami Y, et al. PIVKA-II is the best prognostic predictor in patients with hepatocellular carcinoma after radiofrequency ablation therapy. Oncology. 2008;75 Suppl 1:91–98. doi: 10.1159/000173429. [DOI] [PubMed] [Google Scholar]
- 38.Hui AM, Takayama T, Sano K, Kubota K, Akahane M, Ohtomo K, Makuuchi M. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol. 2000;33:975–979. doi: 10.1016/s0168-8278(00)80131-2. [DOI] [PubMed] [Google Scholar]
- 39.Shimada M, Rikimaru T, Hamatsu T, Yamashita Y, Terashi T, Taguchi K, Tanaka S, Shirabe K, Sugimachi K. The role of macroscopic classification in nodular-type hepatocellular carcinoma. Am J Surg. 2001;182:177–182. doi: 10.1016/s0002-9610(01)00682-1. [DOI] [PubMed] [Google Scholar]
- 40.Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res. 2003;26:142–147. doi: 10.1016/s1386-6346(03)00007-x. [DOI] [PubMed] [Google Scholar]


