Abstract
AIM: To evaluate diagnostic value of α-fetoprotein (AFP)-L3 and prothrombin induced by vitamin K absence-II (PIVKA-II) in hepatocellular carcinoma (HCC).
METHODS: One hundred and sixty-eight patients during routine HCC surveillance were included in this study. Of the 168 patients, 90 (53.6%) had HCC including newly developed HCC (n = 82) or recurrent HCC after treatment (n = 8). Sera were obtained during their first evaluation for HCC development and at the time of HCC diagnosis before commencing HCC treatment. HCC was diagnosed by histological examination, appropriate imaging characteristics-computed tomography or magnetic resonance imaging. Control sera were collected from 78 patients with benign liver disease (BLD), which were obtained during routine surveillance with a suspicion of HCC. AFP, AFP-L3 and PIVKA-II were measured in the same serum by microchip capillary electrophoresis and liquid-phase binding assay on a micro-total analysis system Wako i30 auto analyzer. The performance characteristics of three tests and combined tests for the diagnosis of HCC were obtained using receiver operating characteristic curves in all populations and subgroups with AFP < 20 ng/mL.
RESULTS: Of 90 HCC patients, 38 (42.2%) patients had AFP < 20 ng/mL, 20 (22.2%) patients had AFP 20-200 ng/mL and 32 (35.6%) patients had AFP > 200 ng/mL. Of the 78 BLD patients, 74 (94.9%) patients had AFP < 20 ng/mL. After adjustment for age and HBV infection status, AFP-L3 levels were higher in HCC than in BLD among patients with low AFP levels (< 20 ng/mL) (P < 0.001). In a total of 168 patients, areas under the curve (AUC) for HCC were 0.879, 0.887, 0.801 and 0.939 for AFP, AFP-L3, PIVKA-II and the combined markers, respectively. The combined AUC for three markers showed higher value than the AUCs of individual marker (P < 0.05). AFP-L3 had higher AUC value than PIVKA-II for HCC detection in entire patients (P = 0.043). With combination of AFP-L3 (cut-off > 5%) and PIVKA-II (cut-off > 40 AU/L), the sensitivity were 94.4% and specificity were 75.6% in all patients. In 112 patients with low AFP levels (< 20 ng/mL), AUCs of AFP-L3, PIVKA-II and combine AFP-L3 and PIVKA-II tests were 0.824, 0.774 and 0.939, respectively. AFP-L3 with a cut-off value of 5% showed sensitivity of 71.1% and specificity of 83.8%, and PIVKA-II with a cut-off value of 40 AU/L had sensitivity of 57.9% and specificity of 95.9% in patients with low AFP levels. The combination of AFP-L3 and PIVKA-II increased the sensitivity and specificity up to 92.1% and 79.7%, respectively, in low AFP group. Combined markers detected 81.8% of early stage HCC (Union for International Cancer Control stage I), 86.7% of small sized tumor (< 2 cm) and 91.7% of single tumor of HCC in the low AFP group. In multivariate analysis, AFP-L3 was correlated with AFP and tumor size, and PIVKA-II was correlated with laboratory tests including serum aspartate aminotransferase, total bilirubin, platelets and albumin levels. PIVKA-II had no correlation with AFP, AFP-L3 or tumor characteristics.
CONCLUSION: Combined determination of AFP-L3 and PIVKA-II could improve the diagnostic value for HCC detection in patients with or without increased AFP levels.
Keywords: α-fetoprotein, Prothrombin induced by vitamin K absence-II, Hepatocellular carcinoma, Diagnosis, Tumor marker
INTRODUCTION
Early detection of hepatocellular carcinoma (HCC) is important as the treatment of HCC with surgical resection, liver transplantation or percutaneous ablation can be curative at early stage. Currently, the recommended screening strategy includes measurement of serum α-fetoprotein (AFP) levels and an abdominal ultrasound every 3-6 mo for the detection of HCC at an earlier stage[1-4]. Usually, a serum AFP level of 20 ng/mL is considered as a cut-off value to differentiate HCC from non-HCC[5]. However, serum AFP level has a high false-negative rate for the detection of small or early stage tumors[2,6-9], and it is often markedly elevated in patients with either cirrhosis or those with exacerbated chronic hepatitis without HCC[10,11].
Therefore, additional tumor markers including fucosylated fraction of AFP (AFP-L3) fraction[12,13] and prothrombin induced by vitamin K absence-II (PIVKA-II) or Des-γ-carboxyprothrombin[14-16] have been suggested, and the surveillance program with these markers have been mostly organized by Japanese study groups[1,17]. The lens culinaris agglutinin-reactive, AFP-L3 has been reported to be highly specific for HCC[5,18-20] and in predicting prognosis[21-24]. Recently, a highly sensitive analytical system using the technically advanced microfluidics-based separation system has been introduced[25]. With this micro-total analysis system (μTAS), AFP-L3 can be measured accurately at very low AFP concentrations under 10 ng/mL with high sensitivity[7,19,25,26]. PIVKA-II is an abnormal protein and some reports have indicated the improved specificity of PIVKA-II over AFP in the diagnosis of HCC[2,5,27-29]. The combination of AFP-L3 and PIVKA-II seems to improve the diagnostic value for HCC patients, and these additive assays are especially recommended for patients with low AFP levels according to previous Japanese studies[19,26,27]. However, diagnostic values of these markers are controversial and different cut-off values have been suggested depending on the study design[5,20,26]. In addition, the clinical values of these markers in combination are not conclusive yet, and the clinical and laboratory factors which possibly effect the measured values of AFP-L3 and PIVKA-II have not been sufficiently investigated.
In this study, we compared the levels of AFP, AFP-L3 and PIVKA-II in benign liver diseases (BLD) patients and HCC patients, and evaluated the individual and combined diagnostic values of AFP-L3 and PIVKA-II for HCC detection according to the total AFP levels and tumor characteristics. The clinical and laboratory factors affecting AFP-L3 and PIVKA-II were also analyzed.
MATERIALS AND METHODS
Patients
Serum samples were obtained from 168 patients during routine HCC surveillance at the Seoul St. Mary’s Hospital, Seoul, South Korea, during the period between April and November 2011. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital. Of the 168 patients, 90 (53.6%) had HCC including newly developed HCC (n = 82) or recurrent HCC after treatment (n = 8). Sera were obtained during their first evaluation for HCC development and at the time of HCC diagnosis before commencing HCC treatment. HCC was diagnosed by histological examination, appropriate imaging characteristics-computed tomography or magnetic resonance imaging. Tumor stage was assessed by the Union for International Cancer Control (UICC) staging system, which is based on tumor number, size, vascular invasion and metastasis[4,30]. Control sera were collected from 78 patients with BLD, which were obtained during routine surveillance with a suspicion of HCC. All patients were diagnosed by imaging study and were followed up for 7-16 mo after sampling to confirm non-HCC.
Measurement of AFP, AFP-L3 and PIVKA-II
AFP, AFP-L3 and PIVKA-II were measured in the same serum by microchip capillary electrophoresis and liquid-phase binding assay on a μTAS Wako i30 auto analyzer (Wako Pure Chemical Industries, Ltd, Osaka, Japan). The measuring range was 0.3-1000 ng/mL for AFP and 5-50 000 AU/L for PIVKA-II. AFP-L3 levels were calculated in sera with AFP levels over 0.3 ng ⁄mL. The total imprecision for two levels of the three markers were less than 5% CVs in 20 d of evaluation. Serum samples were stored at -80 °C until tested. All processes were preformed automatically and followed the manufacturer’s instructions.
Statistical analysis
Statistical analysis were performed with SPSS version 12.0 (SPSS, Chicago, IL) and Med-Calc version 12. 2. 1. 0 (MedCalc Software, Mariakerke, Belgium). Comparisons were made using t test. The performance characteristics of three tests and combined tests for the diagnosis of HCC were obtained using receiver operating characteristic (ROC) curves in all populations and subgroups with AFP < 20 ng/mL. The sensitivity and specificity of these three markers for the diagnosis of HCC were calculated in all the groups and subgroups according to the total AFP levels. A logistic regression model was performed in SPSS on the three markers and ROC curves analysis was performed for area under the curve (AUC) values for the combined markers. All P values were 2-tailed with P < 0.05 considered statistically significant.
RESULTS
Patient characteristics
Table 1 shows the demographics of the study population. The most common cause of liver disease was hepatitis B virus (HBV) infection in both BLD and HCC patients. HCC patients had a higher frequency of males and HBV infection than that of BLD patients (P < 0.05). Of the 90 HCC patients, 38 (42.2%) patients had AFP < 20 ng/mL, 20 (22.2%) patients had AFP 20-200 ng/mL and 32 (35.6%) patients had AFP > 200 ng/mL. Of the 78 BLD patients, 74 (94.9%) patients had AFP < 20 ng/mL. In four patients with non-HCC and increased AFP levels (45.2-72.8 ng/mL), PIVKA-II levels were less than 40 AU/L, but AFP-L3 levels were above 10%. The levels of AFP, AFP-L3 and PIVKA-II in BLD and HCC patients are plotted with respect to the AFP levels (Figure 1). In patients with AFP < 20 ng/mL, AFP-L3 levels were higher in HCC than that in BLD (P < 0.001). Regarding the patients with total AFP levels of 20-200 ng/mL, not AFP-L3 but PIVKA-II was able to discriminate HCC from BLD (P = 0.010). After adjustment for age (> 50 and < 50 years) and HBV infection status, AFP-L3 levels were still higher in HCC than in BLD among patients with low AFP levels (< 20 ng/mL) (P < 0.001).
Table 1.
Demographics and laboratory test values of patients with benign liver disease and with hepatocellular carcinoma n (%)
| BLD (n = 78) | HCC (n = 90) | P value | |
| Age (mean ± SD), yr | 55.6 ± 12.5 | 59.7 ± 10.1 | 0.020 |
| Male | 51 (65.4) | 64 (71.1) | 0.506 |
| Cause of disease | |||
| HBV | 34 (43.6) | 61 (67.8) | 0.002 |
| HCV | 9 (11.5) | 9 (10.0) | 0.749 |
| Alcohol | 5 (6.4) | 12 (13.3) | 0.139 |
| NANBNC | 1 (1.3) | 8 (8.9) | 0.038 |
| Benign mass | 10 (12.8) | ||
| Others | 19 (24.4) | ||
| AFP < 20 ng/mL | 74 (94.9) | 38 (42.2) | |
| AFP 20-200 ng/mL | 4 (5.1) | 20 (22.2) | |
| AFP > 200 ng/mL | 0 (0) | 32 (35.6) | |
| Tumor stage | |||
| I/II/III/IVa/IVb | 16 (17.8)/31 (34.4)/19 (21.1)/18 (20.0)/6 (6.7) | ||
| Tumor size | |||
| < 2/2-5/> 5 cm | 25 (27.8)/33 (36.7)/32 (35.6) | ||
| Tumor number | |||
| Single/multiple | 40 (44.4)/50 (55.6) | ||
| AFP level | 6.9 ± 13.5 | 473.9 ± 746.0 | < 0.001 |
| (mean ± SD, ng/mL) | |||
| AFP-L3 level | 2.3 ± 4.9 | 40.0 ± 35.7 | < 0.001 |
| (mean ± SD, %) | |||
| PIVKA-II level | 20.0 ± 31.2 | 4469 ± 11 553.8 | < 0.001 |
| (mean ± SD, AU/L) |
HBV: Hepatitis B virus; HCV: Hepatitis C virus; NANBNC: Non-hepatitis A, non-hepatitis B, non-hepatitis C virus; AFP: α-fetoprotein; PIVKA-II: Prothrombin induced by vitamin K absence-II; BLD: Benign liver disease; HCC: Hepatocellular carcinoma.
Figure 1.
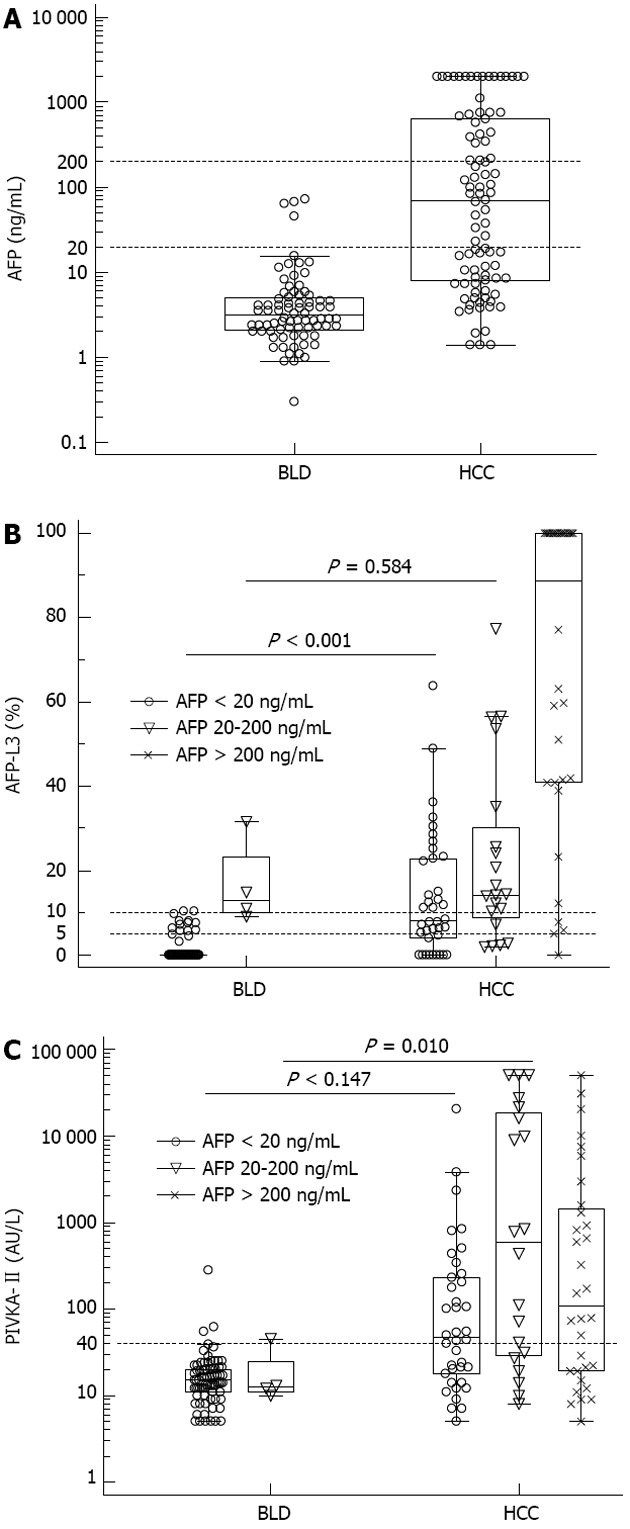
α-fetoprotein (A), α-fetoprotein-L3 (B) and prothrombin induced by vitamin K absence-II (C) values in benign liver disease and hepatocellular carcinoma patients. α-fetoprotein (AFP)-L3 and prothrombin induced by vitamin K absence (PIVKA)-II levels are plotted by AFP concentrations (AFP < 20 ng/mL, AFP 20-200 ng/mL and AFP > 200 ng/mL). In patients with AFP < 20 ng/mL, AFP-L3 levels were higher in hepatocellular carcinoma (HCC) (P < 0.001) (B). In patients with AFP 20-200 ng/mL, PIVKA-II showed higher levels in HCC (P = 0.010) (C). The horizontal dotted lines are cut-off values used for performance analysis in this study. BLD: Benign liver disease.
ROC curve analysis of three markers for the detection of HCC
The ROC curves of AFP, AFP-L3 and PIVKA-II for the diagnosis of HCC in all of 168 patients are shown in Figure 2. The maximum AUCs for distinguishing between HCC and BLD were 0.879, 0.887 and 0.801 for AFP, AFP-L3 and PIVKA-II, respectively. AFP-L3 had higher AUC than PIVKA-II for HCC (P = 0.043). Using logistic regression model, the combined AUC for three markers was 0.939 and showed higher AUC values than the individual markers (P < 0.05).
Figure 2.
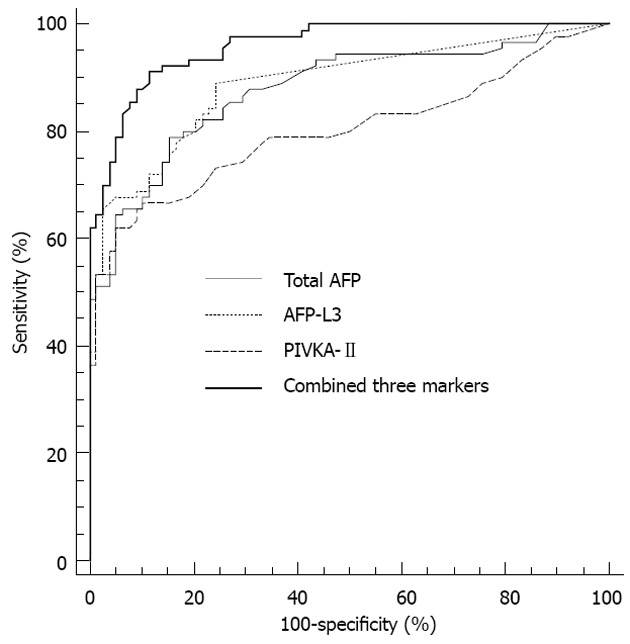
Receiver operating characteristic curves of total α-fetoprotein, α-fetoprotein-L3, prothrombin induced by vitamin K absence-II and three combined markers for the diagnosis of hepatocellular carcinoma in all patients. The area under the curve values were 0.879 for total α-fetoprotein (AFP), 0.887 for AFP-L3, 0.801 for prothrombin induced by vitamin K absence (PIVKA)-II and 0.959 for the three combined markers.
In 112 patients with AFP < 20 ng/mL (HCC, n = 38; BLD, n = 74), the AUC for HCC detection was 0.824 for AFP-L3, 0.774 for PIVKA-II and 0.939 for the two combined markers. Combined AUC was significantly higher than each AUC (P < 0.05), and the AUC value was not different between AFP-L3 and PIVKA-II (P = 0.516) (Figure 3).
Figure 3.
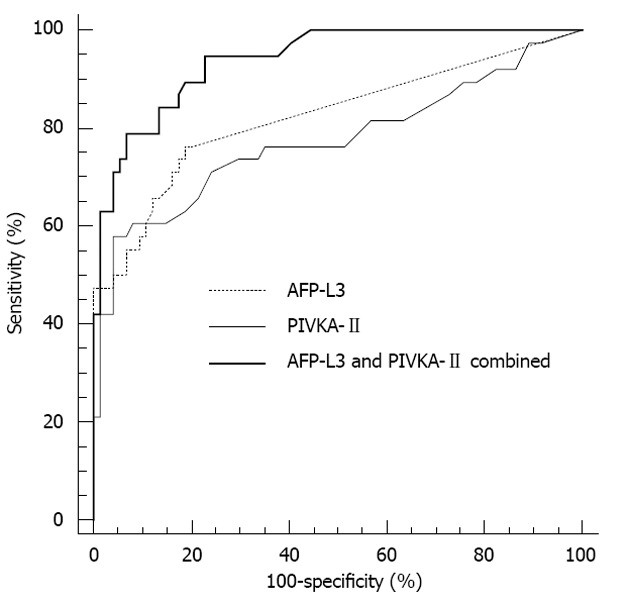
Receiver operating characteristic curves of α-fetoprotein-L3 and prothrombin induced by vitamin K absence-II for the diagnosis of hepatocellular carcinoma in patients with total α-fetoprotein < 20 ng/mL. The area under the curve values were 0.824 for α-fetoprotein (AFP)-L3, 0.774 for prothrombin induced by vitamin K absence (PIVKA)-II and 0.939 for the two combined markers.
Sensitivity and specificity of three markers for HCC diagnosis
The sensitivities and specificities of the three markers for all patients and subgroups are shown in Table 2. In all patients, the total AFP showed a sensitivity of 78.9% and a specificity of 84.6% with a cut-off of 10 ng/mL. AFP-L3 with a cut-off value of 5% increased the sensitivity up to 82.2%. When the cut-off value for AFP-L3 and PIVKA-II were set at 10% and 40 AU/L, the specificities were improved up to 93.6% and 94.9%, respectively.
Table 2.
Performance of α-fetoprotein, α-fetoprotein-L3 and prothrombin induced by vitamin K absence-II in detecting hepatocellular carcinoma in all patients and in subgroups according to total α-fetoprotein levels: Total α-fetoprotein
| Cut-off value | Sensitivity | Specificity | ||
| Entire group (n = 168, 90 HCC) | AFP | 10 ng/mL | 78.9% | 84.6% |
| AFP-L3 | 5% | 82.2% | 79.5% | |
| 10% | 67.8% | 93.6% | ||
| PIVKA-II | 40 AU/L | 62.2% | 94.9% | |
| AFP-L3 or PIVKA-II | 5% or 40 AU/L | 94.4% | 75.6% | |
| 10% or 40 AU/L | 84.4% | 89.7% | ||
| AFP < 20 ng/mL (n = 112, 38 HCC) | AFP-L3 | 5% | 71.1% | 83.8% |
| 10% | 47.4% | 97.3% | ||
| PIVKA-II | 40 AU/L | 57.9% | 95.9% | |
| AFP-L3 or PIVKA-II | 5% or 40 AU/L | 92.1% | 79.7% | |
| 10% or 40 AU/L | 78.9% | 93.2% | ||
| AFP < 200 ng/mL (n = 136, 58 HCC) | AFP-L3 | 5% | 74.1% | 79.5% |
| 10% | 56.9% | 93.6% | ||
| PIVKA-II | 40 AU/L | 62.1% | 94.9% | |
| AFP-L3 or PIVKA-II | 5% or 40 AU/L | 93.1% | 75.6% | |
| 10% or 40 AU/L | 82.8% | 89.7% |
AFP: α-fetoprotein; PIVKA-II: Prothrombin induced by vitamin K absence-II; HCC: Hepatocellular carcinoma.
In the subgroups according to the total AFP levels (AFP < 20 ng/mL, n = 112; AFP < 200 ng/mL, n = 136), the sensitivity and specificity of AFP-L3 were close to 70% and 80% respectively with a cut-off of 5%, and there was no significant difference compared to that of the entire group. The sensitivity and specificity of PIVKA-II was approximately 60% and 95% respectively with a cut-off of 40 AU/L in all patients and subgroups.
To increase the diagnostic value for HCC detection, we combined the AFP-L3 and PIVKA-II results. If either AFP-L3 or PIVKA-II level was above the cut-off value, the combined result was defined as positive. With combination of AFP-L3 (cut-off > 5%) and PIVKA-II (cut-off > 40 AU/L), the sensitivities were 92.1%-94.4% and specificities were 75.6%-79.7% in all patients and subgroups. When a cut-off value was set at 10% for AFP-L3 and combined with PIVKA-II, the sensitivities were approximately 80% (78.9%-84.4%) and the specificities were maintained close to 90%.
Sensitivity of AFP-L3 and PIVKA-II according to the tumor characteristics in patients with AFP < 20 ng/mL
To establish the sensitivity of AFP-L3 (cut-off 5%) and PIVKA-II (cut-off 40 AU/L) for the detection of small tumors and early stage HCC especially in 38 HCC patients with AFP < 20 ng/mL, we subgrouped the HCC patients according to the UICC stage (I, II, III, IVa, IVb), tumor size (< 2, 2-5, > 5 cm) and tumor number (single and multiple tumor) (Table 3). The sensitivity of AFP-L3 and PIVKA-II in UICC stage I -II (n = 27) was 63.6%-75.0% and 45.5%-56.3%. The combination of AFP-L3 and PIVKA-II increased the sensitivity up to 81.8%-100% (Table 3). In HCC patients with tumor size < 2 (n = 15) and 2-5 cm (n = 17), the sensitivity of AFP-L3 was 46.7% and 47.1%, respectively. The sensitivity of PIVKA-II for the detection of HCC with tumor size < 2 and 2-5 cm was 40.0% and 64.7%, and combination of AFP-L3 and PIVKA-II resulted in an enhancement of sensitivity up to 86.7% and 94.1%, respectively. With a combined test (AFP-L3 and PIVKA-II), 22 (91.7%) of 24 HCCs with a single tumor were detected.
Table 3.
Sensitivity of α-fetoprotein-L3 and prothrombin induced by vitamin K absence-II according to the tumor characteristics in 38 hepatocellular carcinoma patients with α-fetoprotein < 20 ng/mL n (%)
| n | AFP-L3 | PIVKA-II | AFP-L3 and PIVKA-II combined | |
| (> 5%) | (> 40 AU/L) | (> 5%, > 40 AU/L) | ||
| Tumor stage (UICC) | ||||
| I | 11 | 7 (63.6) | 5 (45.5) | 9 (81.8) |
| II | 16 | 12 (75.0) | 9 (56.3) | 16 (100) |
| III | 9 | 6 (66.7) | 6 (66.7) | 8 (88.9) |
| IVa | 2 | 2 (100) | 2 (100) | 2 (100) |
| Tumor size | ||||
| < 2 cm | 15 | 7 (46.7) | 6 (40.0) | 13 (86.7) |
| 2-5 cm | 17 | 8 (47.1) | 11 (64.7) | 16 (94.1) |
| > 5 cm | 6 | 3 (50.0) | 5 (83.3) | 6 (100) |
| Tumor number | ||||
| Single | 24 | 16 (66.7) | 12 (50.0) | 22 (91.7) |
| Multiple | 14 | 11 (78.6) | 10 (71.4) | 13 (92.7) |
AFP: α-fetoprotein; PIVKA-II: Prothrombin induced by vitamin K absence-II; UICC: International Union Against Cancer; HCC: Hepatocellular carcinoma.
Correlation analysis
To investigate the clinical and laboratory factors which may affect the measured values of AFP-L3 and PIVKA-II, we performed univariate and multivariate analyses. In univariate correlation analysis, there was a correlation between AFP-L3 and PIVKA-II. AFP-L3 and PIVKA-II levels were correlated with tumor stage (UICC), tumor size, aspartate aminotransferase (AST) and albumin. Tumor number and AFP levels were correlated with only AFP-L3 levels.
In multivariate analysis, only AFP and tumor size were significant correlates of AFP-L3 (Table 4). In terms of PIVKA-II, laboratory tests including serum AST, total bilirubin, platelets and albumin levels were correlated with the PIVKA-II results. However, PIVKA-II had no correlation with AFP, AFP-L3 or tumor characteristics.
Table 4.
Stepwise multiple linear regression analysis using dependent variables α-fetoprotein-L3 and prothrombin induced by vitamin K absence-II
| Variable | AFP-L3 | PIVKA-II | ||
| β coefficient | P value | β coefficient | P value | |
| AFP | 0.839 | < 0.001 | NS | |
| Tumor size | 0.190 | 0.002 | NS | |
| AST | NS | 0.550 | < 0.001 | |
| Total bilirubin | NS | 0.336 | < 0.001 | |
| Platelet | NS | 0.566 | 0.001 | |
| Albumin | NS | -0.674 | < 0.001 | |
AFP: α-fetoprotein; PIVKA-II: Prothrombin induced by vitamin K absence-II; AST: Aspartate aminotransferase; NS: Not significant.
DISCUSSION
In patients at risk for developing HCC, systematic screening is required for detection of small tumors at an early stage. Although the diagnostic strategy for early detection of HCC has been changed with the technical development in imaging diagnosis[3], measurement of tumor markers for HCC at regular intervals is still a common practice. AFP is the most widely used tumor marker for HCC. However, recent studies reported that AFP lacks adequate sensitivity and specificity for effective surveillance[6,9-11]. Our data showed that 38 (42.2%) of 90 HCC patients had AFP < 20 ng/mL, and 4 (5.1%) of 78 non-HCC patients had AFP > 20 ng/mL, which support the inadequacy of AFP as a tumor marker shown by previous reports. Therefore, we examined the individual and combined diagnostic values of AFP-L3 and PIVKA-II for HCC according to the AFP levels (< 20 ng/mL and < 200 ng/mL), and investigated the clinical and laboratory factors that influence the measured values of AFP-L3 and PIVKA-II.
The AFP-L3 fraction has been reported to be more sensitive than AFP for small sized or early stage HCC[20,31]. AFP-L3 is also known to be highly specific for HCC and reflect tumor characteristics including poor differentiation or malignant invasion[32-35]. The present data of all patients showed that the AUC value of AFP-L3 for HCC detection was 0.887, which was not different with the AUC value of AFP (0.879), but was higher than the AUC of PIVKA-II. In focusing on the patients with AFP < 20 ng/mL, AFP-L3 levels were significantly higher in HCC than in non-HCC, and the sensitivity of AFP-L3 for HCC was 71.1% and 47.4% with cut-offs of 5% and 10%, respectively. These values are higher than those reported in previous studies showing sensitivity of 25%-50% with cut-offs between 5% and 10%[19,20]. The high specificity for HCC is an important advantage of AFP-L3 measurement and higher sensitivity without a concomitant reduction in specificity is useful in clinical settings. In the present study, the specificities of AFP-L3 for HCC were 79.5%-97.3% in all patients or in patients with low AFP levels. The values are similar to those in previous reports showing the specificities of AFP-L3 for HCC as over 85% among patients with low total AFP-levels[19,20]. Overall, in patients with low AFP levels, AFP-L3 (cut-off 5%) had reasonable AUC value, sensitivity and specificity (0.824, 71.1% and 83.8%, respectively) which are similar or improved results compare to previously reported data[19,36].
In the present study, PIVKA-II alone had sensitivities and specificities close to 60% and 95% in all patients and patients with low AFP levels. PIVKA-II was shown to have a higher specificity in previous studies[2,5,27], and all four patients with non-HCC and increased AFP/AFP-L3 levels showed lower values of PIVKA-II in the present study. This suggests that the PIVKA-II test may be useful in detecting false-positive AFP results.
The levels of AFP-L3 and PIVKA-II had no correlation between them and these two may be compensative markers reflecting a different developmental form of HCC[37]. In multivariate analysis, AFP-L3 correlated with total AFP and tumor size. In contrast, PIVKA-II had no correlation with AFP or tumor characteristics, but was correlated with some liver function tests including serum AST, tuberculosis, platelet and albumin.
In our study, the combination of PIVKA-II and AFP-L3 improved the sensitivity close to 90% and showed significantly increased AUC (0.939) even in patients with low AFP. In addition, the combined tests showed approximately 90% sensitivity for the detection of early stage, small sized or single HCC tumors in patients with low AFP levels. These results suggest that these two biomarkers should be measured simultaneously and in combination with imaging tests to improve the diagnostic accuracy.
Potential limitations of our study include the small number of newly diagnosed HCC patients, lack of randomization and relative short-term follow-up. Our results should be validated with a larger number of patients with age- and sex-matched control subjects. Since AFP-L3 has been suggested as a prognostic marker in patients with HCC[22,23,26], future studies using longitudinal AFP-L3 data analysis or studies regarding the effect of AFP-L3 effect on the survival rate of HCC patients after treatment are needed. Despite these limitations, the present study indicates that AFP-L3 and PIVKA-II are useful additive tumor markers for the diagnosis of HCC.
In conclusion, combined determination of AFP-L3 and PIVKA-II improved the diagnostic value for HCC detection in patients with or without increased AFP levels. The utility of improved surveillance protocol based on these tumor markers needs to be investigated.
COMMENTS
Background
In patients at risk for developing hepatocellular carcinoma (HCC), systematic screening is required for detection of small tumors at an early stage. Although, α-fetoprotein (AFP) is the most widely used tumor marker for HCC, AFP lacks adequate sensitivity and specificity for effective surveillance. Therefore, additional tumor markers including fucosylated fraction of AFP (AFP-L3) fraction and prothrombin induced by vitamin K absence-II (PIVKA-II) have been suggested.
Research frontiers
The clinical values of AFP, AFP-L3 and PIVKA-II in combination are not conclusive yet, and the clinical and laboratory factors which possibly affect the measured values of AFP-L3 and PIVKA-II have not been sufficiently investigated. In this study, the authors evaluated the individual and combined diagnostic values of AFP-L3 and PIVKA-II for HCC detection according to the total AFP levels and tumor characteristics. The clinical and laboratory factors affecting AFP-L3 and PIVKA-II were also analyzed.
Innovations and breakthroughs
In the study, the combination of PIVKA-II and AFP-L3 improved the sensitivity close to 90% and showed significantly increased areas under the curve (0.939) even in patients with low AFP. In addition, the combined tests showed approximately 90% sensitivity for the detection of early stage, small sized or single HCC tumors in patients with low AFP levels. In multivariate analysis, AFP-L3 correlated with total AFP and tumor size. In contrast, PIVKA-II had no correlation with AFP or tumor characteristics, but was correlated with some liver function tests including serum aspartate aminotransferase, tuberculosis, platelet and albumin. The present study indicates that AFP-L3 and PIVKA-II are useful additive tumor markers for the diagnosis of HCC.
Applications
Combined determination of AFP-L3 and PIVKA-II could improve the diagnostic value for HCC detection in patients with or without increased AFP levels. The utility of improved surveillance protocol based on these tumor markers needs to be investigated.
Peer review
This study confirmed that the combined determination of AFP-L3 and PIVKA-II improved the diagnostic value for HCC detection in patients with or without increased AFP levels. The authors performed the multivariate analysis and revealed that AFP-L3 and PIVKA-II had no correlation between them and might be useful additive and compensate tumor markers for the diagnosis of HCC. The results are interesting and can be used to elucidate the different development form of HCC.
Footnotes
Supported by The Industrial Core Technology Development Program funded by the Ministry of Knowledge Economy, No. 10033183
P- Reviewers Aspinall RJ, Yang SF, Kataoka H S- Editor Wen LL L- Editor A E- Editor Li JY
References
- 1.Izumi N. Diagnostic and treatment algorithm of the Japanese society of hepatology: a consensus-based practice guideline. Oncology. 2010;78 Suppl 1:78–86. doi: 10.1159/000315234. [DOI] [PubMed] [Google Scholar]
- 2.Saffroy R, Pham P, Reffas M, Takka M, Lemoine A, Debuire B. New perspectives and strategy research biomarkers for hepatocellular carcinoma. Clin Chem Lab Med. 2007;45:1169–1179. doi: 10.1515/CCLM.2007.262. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 5.Durazo FA, Blatt LM, Corey WG, Lin JH, Han S, Saab S, Busuttil RW, Tong MJ. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1541–1548. doi: 10.1111/j.1440-1746.2008.05395.x. [DOI] [PubMed] [Google Scholar]
- 6.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–438. [PubMed] [Google Scholar]
- 7.Toyoda H, Kumada T, Tada T. Highly sensitive Lens culinaris agglutinin-reactive α-fetoprotein: a new tool for the management of hepatocellular carcinoma. Oncology. 2011;81 Suppl 1:61–65. doi: 10.1159/000333263. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda M, Asakawa M, Amemiya H, Fujii H. Lens culinaris agglutinin-reactive fraction of AFP is a useful prognostic biomarker for survival after repeat hepatic resection for HCC. J Gastroenterol Hepatol. 2011;26:731–738. doi: 10.1111/j.1440-1746.2010.06532.x. [DOI] [PubMed] [Google Scholar]
- 9.Cui R, He J, Zhang F, Wang B, Ding H, Shen H, Li Y, Chen X. Diagnostic value of protein induced by vitamin K absence (PIVKAII) and hepatoma-specific band of serum gamma-glutamyl transferase (GGTII) as hepatocellular carcinoma markers complementary to alpha-fetoprotein. Br J Cancer. 2003;88:1878–1882. doi: 10.1038/sj.bjc.6601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae JS, Park SJ, Park KB, Paik SY, Ryu JK, Choi CK, Hwang TJ. Acute exacerbation of hepatitis in liver cirrhosis with very high levels of alpha-fetoprotein but no occurrence of hepatocellular carcinoma. Korean J Intern Med. 2005;20:80–85. doi: 10.3904/kjim.2005.20.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu CW, Hwang SJ, Luo JC, Lai CR, Tsay SH, Li CP, Wu JC, Chang FY, Lee SD. Clinical, virologic, and pathologic significance of elevated serum alpha-fetoprotein levels in patients with chronic hepatitis C. J Clin Gastroenterol. 2001;32:240–244. doi: 10.1097/00004836-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Taketa K, Sekiya C, Namiki M, Akamatsu K, Ohta Y, Endo Y, Kosaka K. Lectin-reactive profiles of alpha-fetoprotein characterizing hepatocellular carcinoma and related conditions. Gastroenterology. 1990;99:508–518. doi: 10.1016/0016-5085(90)91034-4. [DOI] [PubMed] [Google Scholar]
- 13.Taketa K, Endo Y, Sekiya C, Tanikawa K, Koji T, Taga H, Satomura S, Matsuura S, Kawai T, Hirai H. A collaborative study for the evaluation of lectin-reactive alpha-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res. 1993;53:5419–5423. [PubMed] [Google Scholar]
- 14.Liebman HA. Isolation and characterization of a hepatoma-associated abnormal (des-gamma-carboxy)prothrombin. Cancer Res. 1989;49:6493–6497. [PubMed] [Google Scholar]
- 15.Okuda H, Obata H, Nakanishi T, Furukawa R, Hashimoto E. Production of abnormal prothrombin (des-gamma-carboxy prothrombin) by hepatocellular carcinoma. A clinical and experimental study. J Hepatol. 1987;4:357–363. doi: 10.1016/s0168-8278(87)80546-9. [DOI] [PubMed] [Google Scholar]
- 16.Yano Y, Yamashita F, Kuwaki K, Fukumori K, Kato O, Yamamoto H, Ando E, Tanaka M, Sata M. Clinical features of hepatitis C virus-related hepatocellular carcinoma and their association with alpha-fetoprotein and protein induced by vitamin K absence or antagonist-II. Liver Int. 2006;26:789–795. doi: 10.1111/j.1478-3231.2006.01310.x. [DOI] [PubMed] [Google Scholar]
- 17.Kudo M. Early detection and curative treatment of early-stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2005;3:S144–S148. doi: 10.1016/s1542-3565(05)00712-3. [DOI] [PubMed] [Google Scholar]
- 18.Hirai Y, Waki I, Momose A, Fukazawa T, Aida T, Takagi K, Hirano T. Increase of O 2p unoccupied electronic states within the ab plane of YBa2Cu3O6.8 due to a superconducting transition. Phys Rev B Condens Matter. 1992;45:2573–2576. doi: 10.1103/physrevb.45.2573. [DOI] [PubMed] [Google Scholar]
- 19.Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A, Kanke F, Satomura S. Clinical utility of highly sensitive Lens culinaris agglutinin-reactive alpha-fetoprotein in hepatocellular carcinoma patients with alpha-fetoprotein & lt; 20 ng/mL. Cancer Sci. 2011;102:1025–1031. doi: 10.1111/j.1349-7006.2011.01875.x. [DOI] [PubMed] [Google Scholar]
- 20.Tamura Y, Igarashi M, Kawai H, Suda T, Satomura S, Aoyagi Y. Clinical advantage of highly sensitive on-chip immunoassay for fucosylated fraction of alpha-fetoprotein in patients with hepatocellular carcinoma. Dig Dis Sci. 2010;55:3576–3583. doi: 10.1007/s10620-010-1222-5. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita F, Tanaka M, Satomura S, Tanikawa K. Prognostic significance of Lens culinaris agglutinin A-reactive alpha-fetoprotein in small hepatocellular carcinomas. Gastroenterology. 1996;111:996–1001. doi: 10.1016/s0016-5085(96)70067-7. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi M, Kuroiwa T, Suda T, Tamura Y, Kawai H, Igarashi M, Fukuhara Y, Aoyagi Y. Fucosylated fraction of alpha-fetoprotein, L3, as a useful prognostic factor in patients with hepatocellular carcinoma with special reference to low concentrations of serum alpha-fetoprotein. Hepatol Res. 2007;37:914–922. doi: 10.1111/j.1872-034X.2007.00147.x. [DOI] [PubMed] [Google Scholar]
- 23.Toyoda H, Kumada T, Kaneoka Y, Osaki Y, Kimura T, Arimoto A, Oka H, Yamazaki O, Manabe T, Urano F, et al. Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. J Hepatol. 2008;49:223–232. doi: 10.1016/j.jhep.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Saito Y, Shimada M, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Yamada S, Asanoma M. Prediction of recurrence of hepatocellular carcinoma after curative hepatectomy using preoperative Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein. Hepatol Res. 2012;42:887–894. doi: 10.1111/j.1872-034X.2012.01004.x. [DOI] [PubMed] [Google Scholar]
- 25.Kagebayashi C, Yamaguchi I, Akinaga A, Kitano H, Yokoyama K, Satomura M, Kurosawa T, Watanabe M, Kawabata T, Chang W, et al. Automated immunoassay system for AFP-L3% using on-chip electrokinetic reaction and separation by affinity electrophoresis. Anal Biochem. 2009;388:306–311. doi: 10.1016/j.ab.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 26.Hanaoka T, Sato S, Tobita H, Miyake T, Ishihara S, Akagi S, Amano Y, Kinoshita Y. Clinical significance of the highly sensitive fucosylated fraction of α-fetoprotein in patients with chronic liver disease. J Gastroenterol Hepatol. 2011;26:739–744. doi: 10.1111/j.1440-1746.2010.06585.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang CS, Lin CL, Lee HC, Chen KY, Chiang MF, Chen HS, Lin TJ, Liao LY. Usefulness of serum des-gamma-carboxy prothrombin in detection of hepatocellular carcinoma. World J Gastroenterol. 2005;11:6115–6119. doi: 10.3748/wjg.v11.i39.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, Toyota T, Takahashi T, Kasukawa R. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol. 2000;95:1036–1040. doi: 10.1111/j.1572-0241.2000.01978.x. [DOI] [PubMed] [Google Scholar]
- 29.Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, Lok AS. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology. 2003;37:1114–1121. doi: 10.1053/jhep.2003.50195. [DOI] [PubMed] [Google Scholar]
- 30.Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, Hokotate H, Inoue H, Baba Y, Imamura Y, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res. 2002;24:395–403. doi: 10.1016/s1386-6346(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 31.Shiraki K, Takase K, Tameda Y, Hamada M, Kosaka Y, Nakano T. A clinical study of lectin-reactive alpha-fetoprotein as an early indicator of hepatocellular carcinoma in the follow-up of cirrhotic patients. Hepatology. 1995;22:802–807. [PubMed] [Google Scholar]
- 32.Khien VV, Mao HV, Chinh TT, Ha PT, Bang MH, Lac BV, Hop TV, Tuan NA, Don LV, Taketa K, et al. Clinical evaluation of lentil lectin-reactive alpha-fetoprotein-L3 in histology-proven hepatocellular carcinoma. Int J Biol Markers. 2001;16:105–111. doi: 10.1177/172460080101600204. [DOI] [PubMed] [Google Scholar]
- 33.Hagiwara S, Kudo M, Kawasaki T, Nagashima M, Minami Y, Chung H, Fukunaga T, Kitano M, Nakatani T. Prognostic factors for portal venous invasion in patients with hepatocellular carcinoma. J Gastroenterol. 2006;41:1214–1219. doi: 10.1007/s00535-006-1950-7. [DOI] [PubMed] [Google Scholar]
- 34.Tada T, Kumada T, Toyoda H, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Kitabatake S, Kuzuya T, Nonogaki K, et al. Relationship between Lens culinaris agglutinin-reactive alpha-fetoprotein and pathologic features of hepatocellular carcinoma. Liver Int. 2005;25:848–853. doi: 10.1111/j.1478-3231.2005.01111.x. [DOI] [PubMed] [Google Scholar]
- 35.Oka H, Saito A, Ito K, Kumada T, Satomura S, Kasugai H, Osaki Y, Seki T, Kudo M, Tanaka M. Multicenter prospective analysis of newly diagnosed hepatocellular carcinoma with respect to the percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein. J Gastroenterol Hepatol. 2001;16:1378–1383. doi: 10.1046/j.1440-1746.2001.02643.x. [DOI] [PubMed] [Google Scholar]
- 36.Choi J, Park Y, Kim JH, Kim HS. Evaluation of revisited fucosylated alpha-fetoprotein (AFP-L3) with an autoanalyzer μTAS in a clinical laboratory. Clin Chim Acta. 2012;413:170–174. doi: 10.1016/j.cca.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi K, Kumada T, Nakano S, Takeda I, Sugiyama K, Kiriyama S, Sone Y, Miyata A, Shimizu H, Satomura S. Usefulness of measurement of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein as a marker of prognosis and recurrence of small hepatocellular carcinoma. Am J Gastroenterol. 1999;94:3028–3033. doi: 10.1111/j.1572-0241.1999.01378.x. [DOI] [PubMed] [Google Scholar]


