Abstract
Background
The treatment options for patients requiring repair of a long segment of the urethra are limited by the availability of autologous tissues. We previously reported that acellular collagen-based tubularized constructs seeded with cells are able to repair small urethral defects in a rabbit model.
Objective
We explored the feasibility of engineering clinically relevant long urethras for surgical reconstruction in a canine preclinical model.
Design, setting, and participants
Autologous bladder epithelial and smooth muscle cells from 15 male dogs were grown and seeded onto preconfigured collagen-based tubular matrices (6 cm in length). The perineal urethral segment was removed in 21 male dogs. Urethroplasties were performed with tubularized collagen scaffolds seeded with cells in 15 animals. Tubularized constructs without cells were implanted in six animals. Serial urethrography and three-dimensional computed tomography (CT) scans were performed pre- and postoperatively at 1, 3, 6, and 12 mo. The animals were euthanized at their predetermined time points (three animals at 1 mo, and four at 3, 6, and 12 mo) for analyses.
Outcome measurements and statistical analysis
Statistical analysis of CT imaging and histology was not needed.
Results and limitations
CT urethrograms showed wide-caliber urethras without strictures in animals implanted with cell-seeded matrices. The urethral segments replaced with acellular scaffolds collapsed. Gross examination of the urethral implants seeded with cells showed normal-appearing tissue without evidence of fibrosis. Histologically, an epithelial cell layer surrounded by muscle fiber bundles was observed on the cell-seeded constructs, and cellular organization increased over time. The epithelial and smooth muscle phenotypes were confirmed using antibodies to pancytokeratins AE1/AE3 and smooth muscle–specific desmin. Formation of an epithelial cell layer occurred in the unseeded constructs, but few muscle fibers formed.
Conclusions
Cell-seeded tubularized collagen scaffolds can be used to repair long urethral defects, whereas scaffolds without cells lead to poor tissue development and strictures. This study demonstrates that long tissue-engineered tubularized urethral segments may be used for urethroplasty in patients.
Keywords: Urethra, Stricture repair, Cell-seeded tubularized urethra reconstruction, Tissue-engineered urethra
1. Introduction
Repair of the anterior urethra is one of the most demanding surgical problems in urology. A multitude of techniques have been used to treat anterior urethral strictures using buccal mucosa or a penile skin flap for reconstruction [1,2]. However, in many cases, an adequate amount of penile skin is not available [3]. In such circumstances, surgeons have been forced to use other externalization techniques such as perineal urethrotomy. In recent years, regenerative medicine and tissue engineering studies have led to the development of novel biomaterials for urethral repair such as decellularized collagen matrices and synthetic polymers [4–8]. These advances have provided the ability to engineer tubularized tissue constructs for the successful management of urethral stricture. Although these materials are biocompatible and facilitate tissue regeneration by bridging tissue defects, studies have shown that the maximum distance of tissue regeneration is limited [9,10]. Also, it has been demonstrated that acellular collagen scaffold materials are unsuitable for tubularized urethral reconstruction because they collapse and lead to further stricture [9,10].
However, recent studies indicate that seeding a tubular urethral scaffold with autologous cells can lead to successful repair of urethral defects [9,11]. This study used a synthetic polymer scaffold for this purpose, and although the construct was able to bridge urethral defects, the stiffness and biocompatibility of synthetic materials are not entirely optimal for urethral applications.
Collagen constructs derived from the bladder submucosa (lamina propria) have been used as a substrate for urethral repair, either with or without cells [8,9,12,13]. These matrices are biocompatible, flexible, and easily prepared in large quantities. We previously demonstrated that collagen-based tubularized constructs seeded with cells are able to repair small urethral defects in a rabbit model. In the present study, we investigated the feasibility of using collagen-based seeded grafts to engineer long urethral segments in canines, a more clinically relevant animal model.
2. Materials and methods
This study was conducted with the approval of the Institutional Animal Care and Use Committee. A total of 21 male beagle dogs were divided into a control group (6 animals) that received unseeded tubularized acellular bladder matrix (ABM) constructs and a group (15 animals) that received cell-seeded tubularized ABM. Constructs were retrieved at 1, 3, 6, and 12 mo after implantation (Fig. 1).
Fig. 1.
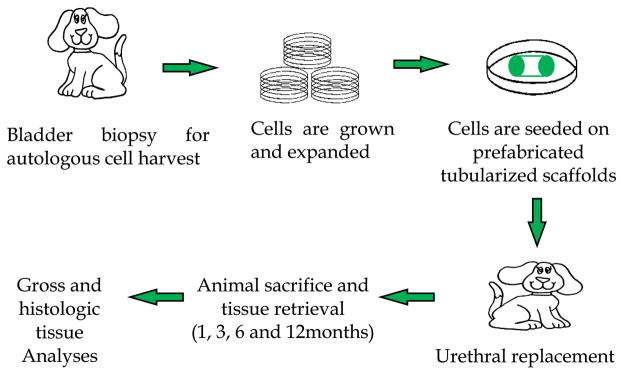
Study overview. Autologous urothelial and smooth muscle cells, isolated from the bladder, are seeded on tubularized acellular collagen scaffolds for urethral replacement. The animals were followed for up to 12 mo for analyses.
2.1. Preparation of the acellular bladder matrix
Porcine acellular bladder matrix was processed as described previously [14]. Briefly, the mucosa (urothelium/suburothelium) of the bladder was removed by surgical delamination. The bladder matrix was rinsed with water in a stirring flask (200 rpm) for 2 d at 4°C, followed by treatment with 0.03% trypsin for 1 h. The matrix was then treated with a solution of Triton X-100 (0.5%) and ammonium hydroxide (0.05%) in distilled water for 72 h at 4°C. The solution was changed every 24 h. Histology and DNA quantification were performed to confirm the removal of all cells. The tissue was washed, frozen, lyophilized, and sterilized using gamma irradiation (800 rads) [14].
2.2. Bladder biopsy
A 2 × 2 cm bladder biopsy was obtained from 15 study group animals. The animals were anesthetized using 10 mg/kg ketamine (Fort Dodge, Fort Dodge, IA, USA) and 1.5 mg/kg xylazine (VEDCO, Inc., St. Joseph, MO, USA) and maintained using 1–3% isoflurane (Abbott Laboratories, North Chicago, IL, USA). After surgical prep, a small (5 cm) paramedian incision was made and the bladder was exposed. A 2 × 2 cm piece of the bladder wall was sampled. The bladder, muscle, and skin were then closed in layers. An intramuscular injection of Baytril (enrofloxacin, 5 mg/kg, Bayer Corp., Shawnee Mission, KS, USA) was given to each animal prior to the procedure and daily for 3 d postsurgery.
2.3. Cell isolation and expansion
Cells were isolated and expanded according to previously published protocols [15]. The mucosal and muscle layer were mechanically separated. Muscle fragments were placed on culture dishes, and muscle cells were expanded from these explants in Dulbecco’s Modified Eagle Medium (DMEM, Sigma Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA). The urothelial layer was mechanically separated from the bladder tissue using a scalpel, and the cells were expanded in a similar manner in Keratinocyte Serum-Free Medium (KSFM, Gibco). Each cell culture required an average of five to six passages to reach appropriate numbers for seeding and implantation. Prior to seeding, the urothelial cells were labeled with PKH 26 (red dye) and the muscle cells were labeled with PKH 67 (green dye). Cell labeling was confirmed by fluorescent microscopy prior to the seeding process.
2.4. Scaffold preparation and cell seeding
Each scaffold was sterilized and tubularized by suturing a 7-cm-long segment of the decellularized bladder matrix around a 14F sterile urethral catheter under aseptic conditions. For the control group, the scaffold was incubated for 24 h in DMEM supplemented with 10% fetal bovine serum (FBS) prior to implantation. For seeded scaffolds, muscle cells were seeded onto the outside of the scaffold at a concentration of 10 × 106 cells per square centimeter in a spinner flask filled with DMEM/10% FBS. The catheter was left in place to prevent internal seeding. During culture, the medium was stirred at 75 rpm to allow for uniform distribution of cells over a 48-h period. At the end of this period, spinning was stopped and the cells were allowed to settle for another 48 h. Urothelial cells were statically seeded on the internal surface of the scaffold using a glass pipette at a concentration of 10 × 105 cells per square centimeter. The cells were allowed to settle and attach to the scaffold in the incubator for 48 h. The final seeding was performed with muscle cells. These were statically seeded onto the outside of the scaffold and left again to settle for 48 h prior to implantation. After the initial 3 d of dynamic cell seeding, the construct was place in a 50/50 medium of DMEM and KSFM (Sigma Aldrich).
2.5. Surgical procedure
Under anesthesia, a suprapubic bladder catheter was surgically inserted into the bladder and left in place for 4 wk postoperatively to ensure proper drainage of urine during healing. Subsequently, the bulbar and proximal penile urethra was exposed and dissected through an inverted Y-shaped perineal incision. A 6-cm-long segment of urethra starting distal to the membranous urethra was transected and removed. The cell-seeded urethral scaffold was interposed and its ends were anastomosed with the native ends of urethral tissues using 6/0 Vicryl sutures. The wound was closed in layers in a routine fashion. Gentle compression was applied to the surgical wound. The bandage was kept in place for 3 d before reapplication whenever possible. Analgesia (buprenorphine, 0.01 mg/kg) was administered intramuscularly at the end of the procedure followed by another administration every 8 h for the first 2 d and as needed thereafter.
2.6. Follow-up
The urethral tubes were left in place for 3–4 wk whenever possible. Upon removal, retrograde and voiding computed tomography (CT) urethrocystography (Toshiba 32-slice Aquilion scanner) was performed. The CT study was repeated at 1, 3, 6, and 12 mo after surgery. The suprapubic catheter was closed at the time of urethral stent removal and was removed 1 wk later in all experimental animals. In the control group, removal of the suprapubic catheter depended on the degree of patency of the urethra.
The experimental animals that received cell-seeded urethral constructs (n = 15) were divided over four time points. Three animals were humanely killed at 1 mo postoperatively, and four animals were killed at 3, 6, and 12 mo. Animals in the control group (n = 6) were killed when urethral stricture or fistulae developed and caused severe voiding difficulty or urinary leakage. At death, the bladder, prostate, urethra, and corporal bodies were removed for gross assessment and histologic sectioning. The retrieved tissues were divided into normal urethra, proximal and distal anastomotic lines, and engineered urethral tissue. All tissues were formalin fixed, paraffin embedded, and sectioned (8 μm). Hematoxylin and eosin and Mason’s trichrome staining were performed. Immunohistochemical staining was performed using monoclonal antibodies against both the urothelial cell layer (pancytokeratins AE1/AE3) and smooth muscle cell layer (desmin). Unstained sections were also examined under immunofluorescence microscopy to identify the PKH-labeled implanted cells.
3. Results
3.1. In vitro analysis
The processed collagen-based scaffolds showed the presence of extracellular matrices with absence of cells, as evidenced by histomorphologic analysis (Fig. 2). The decellularized scaffolds were assessed for acellularity using DNA quantification. DNA quantification showed an average of 0.206–0.244 ng/mg DNA in the acellular bladder matrix, which is within acceptable limits (<0.3 ng/mg) for decellularized materials. Successful cell cultures were obtained from every bladder biopsy used in the study. Bladder epithelial and smooth muscle cells were grown and expanded separately until sufficient numbers of cells were obtained. Both the urothelial and smooth muscle cells showed expression of AE1/AE3 and desmin, respectively (Fig. 3). Prior to implantation, a 1-cm piece of every seeded scaffold was removed for testing to ensure proper seeding of the cells. All scaffolds showed attachment of multilayered urothelial cells in the inner layer and muscle cells on the outer surface of the construct (Fig. 4).
Fig. 2.
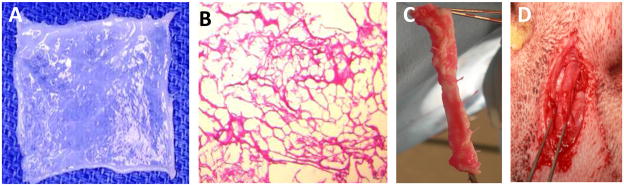
(a) Collagen-based scaffolds derived from bladder were chemically treated to remove cells. (b) The processed collagen-based scaffolds show absence of cells. Hematoxylin and eosin (H&E) staining showed no presence of cells after the decellularization process. (c) Tubularized urethral graft seeded with cells prior to surgery. (d) Implanted tubularized urethral graft at the time of surgery.
Fig. 3.
(a) Bladder epithelial and (b) smooth muscle cells are grown and expanded in culture. Both the urothelial and smooth muscle cells show expression of (c) AE1/AE3 and (D) desmin, respectively.
Fig. 4.
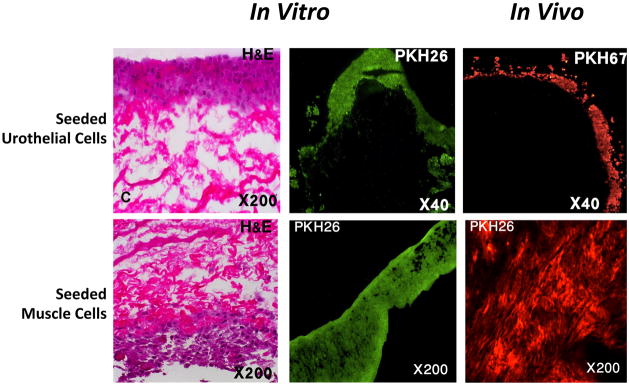
All scaffolds seeded with cells showed attachment of multilayered urothelial cells in the inner layer and muscle cells on the outer surface of the construct. The labeled cells survived after implantation in vivo. H&E = hematoxylin and eosin.
3.2. Surgical outcomes
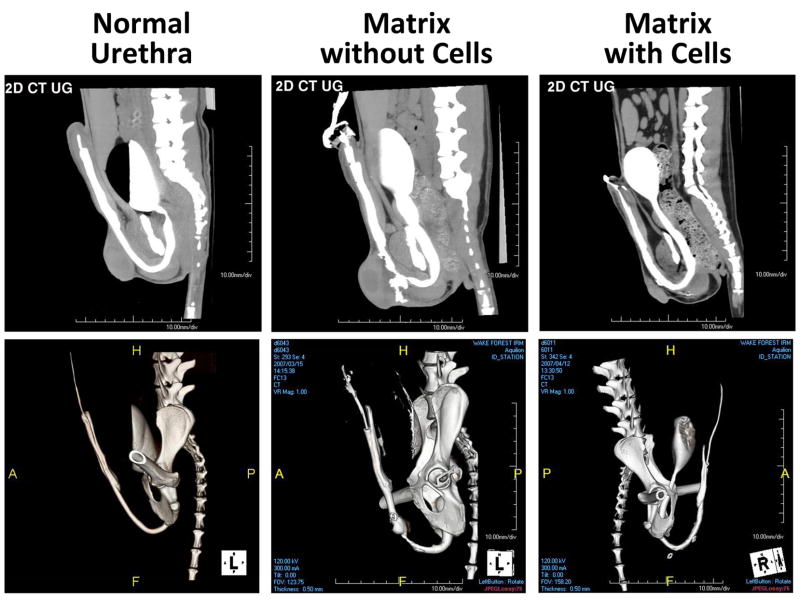
All six control animals implanted with acellular matrices resulted in early urine leakage after removal of the urethral stent, which was attributed to fistulae formation at the anastomotic site. All animals in this group developed urethral strictures within 3 mo of implantation as shown via CT urethrocystography, and they were euthanized when voiding became difficult and their urethras were severely obstructed (Fig. 5). Gross anatomic and histologic assessment postmortem showed evidence of stricture formation and fibrosis throughout the entire implanted construct.
Fig. 5.
(Top panel) Two-dimensional (2D) computed tomography (CT) urethrocystography; (bottom panel) three-dimensional reconstructed images. Animals that were treated with tubularized matrix without cells showed strictured urethra with fistula. Animals treated with cell-seeded tubularized matrix showed wide patent urethras at 12 mo after surgery.
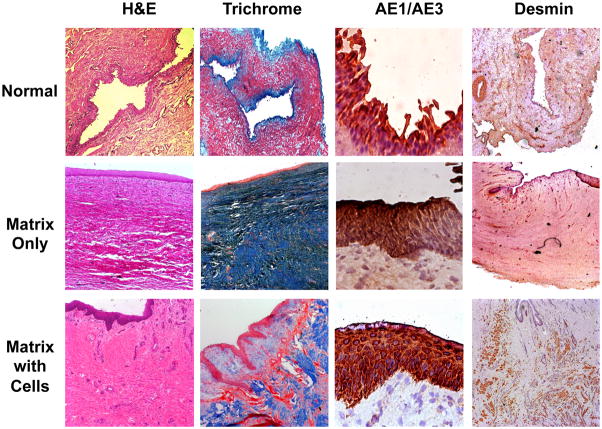
All animals in the experimental group (n = 15) showed a patent urethra with normal caliber up to 12 mo after surgery (Fig. 5). Four of the 15 animals experienced early accidental removal of the urethral stents, which were replaced under fluoroscopic guidance. Histologic analysis of the cell-seeded constructs at 1 mo confirmed the presence of multilayered urothelium and muscle. The levels of tissue formation developed over time with a progressive increase in muscle content. In contrast, extensive fibrosis and scarce smooth muscle were seen in animals treated with matrix without cells (Fig. 6). One animal in the experimental group developed acute urinary retention 3 wk after stent removal, and a stent was inserted for an additional 2 wk. Urethrocystography did not show any narrowing of the urethral lumen in this animal, and the retention was attributed to bladder irritation.
Fig. 6.
Histologic analysis of reconstructed urethras. Extensive fibrosis and scarce smooth muscle were seen in animals treated with matrix without cells. Animals treated with cell-seeded matrix show formation of complete layers of transitional epithelium and well-developed smooth muscle. H&E = hematoxylin and eosin.
4. Discussion
Various urethral conditions often require extensive urethral reconstruction for which onlay grafts would not be suitable [16]. A multistage urethroplasty is generally recommended [17]. Many urethral tissue substitutes have been used for tubularized urethroplasties, but they have not been satisfactory and often lead to complications [18,19]. Buccal mucosa remains the most widely used source of tissue for urethral replacement, especially in cases of complex urethral reconstruction. However, anastomotic strictures and fistulas are commonly reported [17]. In addition, when used in a staged procedure, healing of buccal mucosa grafts is not similar in all patients, and multiple revisions of the graft bed may be necessary to obtain a satisfactory urethral bed before closure [17]. Significant donor site morbidity has also been reported after harvesting buccal mucosa [20,21]. Another commonly used graft material is bladder mucosa, which has also been associated with a number of complications including meatal stenosis, prolapse, and a granulomatous reaction at the urethral meatus [22].
For these reasons, many attempts have been made to identify alternative tissues that would serve as adequate urethral substitutes. Tissue engineering techniques may be a suitable option for the creation of biocompatible urethral tissues [23]. Acellular collagen matrices derived from the bladder have already been successfully applied both experimentally and clinically for urethral repair as an onlay graft [12,24]. However, experimental data have shown that in cases of tubularized urethral replacement, cell seeding is necessary to prevent stricture formation when matrices are used [16]. In this study, tissue-engineered tubularized grafts seeded with autologous cells were successfully used for the replacement of longer urethral segments and are thus applicable to a number of clinical situations. From a bladder biopsy, we were able to isolate both urothelial and smooth muscle cells and expand them until sufficient numbers for scaffold seeding were obtained. In this study we demonstrate that the urothelial cells formed a multilayered epithelial lining on the luminal side of the scaffold while the muscle cells formed a corresponding layer on the outer surface.
To demonstrate that the seeded cells are able to contribute to tissue formation in vivo, we labeled the cells with a fluorescent marker and followed them in vivo. We showed that both urothelial and smooth muscle cells that were seeded on the implanted scaffolds are able to survive and proliferate in the newly formed tissue until retrieval. Both cell types were shown to contribute significantly to the multilayered tissue structure formed 3 mo after implantation.
Importantly, the animals receiving the cell-seeded tubularized urethral grafts showed improvement over the control group in all histologic and functional aspects. Urothelial coverage and a complete muscle layer were seen in all experimental animals beginning at 1 mo postoperatively. These layers continued to develop and approximate normal tissues throughout the duration of the study, with no abnormal findings. In comparison, the control animals developed at most a single layer of stratified epithelium within 1–3 mo postoperatively. All of these animals developed urethral strictures of either a portion of or the entire implanted scaffold within the first 3 mo. Fistula formation, urine extravasation, and hematoma formation were also noted in all of the control animals; only four of the experimental animals experienced minor complications. These results confirm and supplement our previous study using shorter urethral defects in the rabbit model [16]. We hypothesize that when cell-seeded scaffolds are used, the rapid development of a urothelial barrier along the luminal surface of the urethra may prevent urine leakage into the suburothelial tissue and associated fibrosis. In the control animals, which do not develop a sufficient urothelial layer, urinary irritation may be one of the major factors implicated in the inflammatory infiltration and fibrous tissue deposition that led to stricture formation. Another contributing factor to the success of cell-seeded tubular urethral replacement could be the support provided by the rapidly developing muscle layer that keeps the urethra from collapsing and prevents wall adhesions. This mechanism would not be present in the unseeded scaffolds.
Although this study provides evidence that cell-seeded tubularized scaffolds can be used in the reconstruction of long urethral defects, one limitation may be the small number of experimental animals that were evaluated per time point. Further investigations are necessary to translate this technology into the clinic.
5. Conclusions
This study provides preclinical evidence that cell-seeded tubularized scaffolds can be used in the reconstruction of long urethral defects. Acellular collagen matrix derived from the bladder combined with autologous cells resulted in the development of normal-appearing urethral tissue layers over time. This study provides some of the information needed to move toward clinical studies of this technique. More studies are required to establish the feasibility of using the same concept for longer and more complicated defects. However, the use of cell-seeded tubularized urethral scaffolds is feasible for defects up to 6–7 cm in length, and it is likely that this same technique could be used for even longer defects.
Take-home message.
The treatment options for patients requiring repair of a long segment of the urethra are limited. We show that tissue engineering techniques can be used in this situation. We demonstrate that cell-seeded tubularized collagen scaffolds can be used for urethroplasty in patients.
Acknowledgments
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors wish to thank Dr. Jennifer Olson for editorial assistance with this manuscript. We also thank Cindy Andrews, Mandy Lockard, Paul Sikoski, and Sergio Rodriguez for surgical assistance.
Footnotes
Author contributions: Anthony Atala had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Orabi, AbouShwareb, Zhang, Yoo, Atala.
Acquisition of data: Orabi, Aboushwareb.
Analysis and interpretation of data: Orabi, AbouShwareb, Zhang, Yoo, Atala.
Drafting of the manuscript: Orabi, Yoo, Atala.
Critical revision of the manuscript for important intellectual content: Yoo, Atala.
Statistical analysis: Orabi, AbouShwareb, Yoo.
Obtaining funding: Yoo, Atala.
Administrative, technical, or material support: Yoo, Atala.
Supervision: Yoo, Atala.
Other (specify): None.
Financial disclosures: Anthony Atala certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhandari M, Dubey D, Verma BS. Dorsal or ventral placement of the preputial/penile skin onlay flap for anterior urethral strictures: does it make a difference? BJU Int. 2001;88:39–43. doi: 10.1046/j.1464-410x.2001.02257.x. [DOI] [PubMed] [Google Scholar]
- 2.Quartey JK. One-stage transverse distal penile/preputial island flap urethroplasty for urethral stricture. Ann Urol (Paris) 1993;27:228–32. [PubMed] [Google Scholar]
- 3.Venn SN, Mundy AR. Urethroplasty for balanitis xerotica obliterans. Br J Urol. 1998;81:735–7. doi: 10.1046/j.1464-410x.1998.00634.x. [DOI] [PubMed] [Google Scholar]
- 4.Wu S, Liu Y, Bharadwaj S, Atala A, Zhang Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials. 2011;32:1317–26. doi: 10.1016/j.biomaterials.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangera A, Chapple CR. Tissue engineering in urethral reconstruction. F1000 Med Rep. 2010;2:65. doi: 10.3410/M2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan Y, Ou L, Hu G, et al. Tissue engineering of urethra using human vascular endothelial growth factor gene-modified bladder urothelial cells. Artif Organs. 2008;32:91–9. doi: 10.1111/j.1525-1594.2007.00502.x. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Xu YM, Song LJ, Fu Q, Cui L, Yin S. Urethral reconstruction using oral keratinocyte seeded bladder acellular matrix grafts. J Urol. 2008;180:1538–42. doi: 10.1016/j.juro.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Fu Q, Deng CL, Liu W, Cao YL. Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int. 2007;99:1162–5. doi: 10.1111/j.1464-410X.2006.06691.x. [DOI] [PubMed] [Google Scholar]
- 9.De Filippo RE, Yoo JJ, Atala A. Urethral replacement using cell seeded tubularized collagen matrices. J Urol. 2002;168:1789–92. doi: 10.1097/01.ju.0000027662.69103.72. discussion 1792–3. [DOI] [PubMed] [Google Scholar]
- 10.Dorin RP, Pohl HG, De Filippo RE, Yoo JJ, Atala A. Tubularized urethral replacement with unseeded matrices: what is the maximum distance for normal tissue regeneration? World J Urol. 2008;26:323–6. doi: 10.1007/s00345-008-0316-6. [DOI] [PubMed] [Google Scholar]
- 11.Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011;377:1175–82. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.el-Kassaby A, AbouShwareb T, Atala A. Randomized comparative study between buccal mucosal and acellular bladder matrix grafts in complex anterior urethral strictures. J Urol. 2008;179:1432–6. doi: 10.1016/j.juro.2007.11.101. [DOI] [PubMed] [Google Scholar]
- 13.el-Kassaby AW, Retik AB, Yoo JJ, Atala A. Urethral stricture repair with an off-the-shelf collagen matrix. J Urol. 2003;169:170–3. doi: 10.1016/S0022-5347(05)64060-8. discussion 173. [DOI] [PubMed] [Google Scholar]
- 14.Chun SY, Lim GJ, Kwon TG, et al. Identification and characterization of bioactive factors in bladder submucosa matrix. Biomaterials. 2007;28:4251–6. doi: 10.1016/j.biomaterials.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Atala A, Vacanti JP, Peters CA, Mandell J, Retik AB, Freeman MR. Formation of urothelial structures in vivo from dissociated cells attached to biodegradable polymer scaffolds in vitro. J Urol. 1992;148:658–62. doi: 10.1016/s0022-5347(17)36685-5. [DOI] [PubMed] [Google Scholar]
- 16.De Filippo RE, Yoo JJ, Atala A. Urethral replacement using cell seeded tubularized collagen matrices. J Urol. 2002;168:1789–92. doi: 10.1097/01.ju.0000027662.69103.72. discussion 1792–3. [DOI] [PubMed] [Google Scholar]
- 17.Andrich DE, Greenwell TJ, Mundy AR. The problems of penile urethroplasty with particular reference to 2-stage reconstructions. J Urol. 2003;170:87–9. doi: 10.1097/01.ju.0000069721.20193.fd. [DOI] [PubMed] [Google Scholar]
- 18.Mundy AR. The long-term results of skin inlay urethroplasty. Br J Urol. 1995;75:59–61. doi: 10.1111/j.1464-410x.1995.tb07233.x. [DOI] [PubMed] [Google Scholar]
- 19.Barbagli G, Lazzeri M. Urethral reconstruction. Curr Opin Urol. 2006;16:391–5. doi: 10.1097/01.mou.0000250277.44990.ab. [DOI] [PubMed] [Google Scholar]
- 20.Fichtner J, Filipas D, Fisch M, Hohenfellner R, Thuroff JW. Long-term followup of buccal mucosa onlay graft for hypospadias repair: analysis of complications. J Urol. 2004;172:1970–2. doi: 10.1097/01.ju.0000142451.78966.fb. discussion 1972. [DOI] [PubMed] [Google Scholar]
- 21.Dublin N, Stewart LH. Oral complications after buccal mucosal graft harvest for urethroplasty. BJU Int. 2004;94:867–9. doi: 10.1111/j.1464-410X.2004.05048.x. [DOI] [PubMed] [Google Scholar]
- 22.Kinkead TM, Borzi PA, Duffy PG, Ransley PG. Long-term followup of bladder mucosa graft for male urethral reconstruction. J Urol. 1994;151:1056–8. doi: 10.1016/s0022-5347(17)35179-0. [DOI] [PubMed] [Google Scholar]
- 23.Atala A, Guzman L, Retik AB. A novel inert collagen matrix for hypospadias repair. J Urol. 1999;162:1148–51. doi: 10.1016/S0022-5347(01)68105-9. [DOI] [PubMed] [Google Scholar]
- 24.Atala A. Bioengineered tissues for urogenital repair in children. Pediatr Res. 2008;63:569–75. doi: 10.1203/PDR.0b013e3181660639. [DOI] [PubMed] [Google Scholar]