Abstract
Calcitriol [1,25(OH)2D3], the hormonally active form of vitamin D exerts anti-proliferative, pro-apoptotic, anti-inflammatory effects and other anticancer actions in breast cancer (BCa) cell cultures and animal models of BCa. Our research is focused on investigating the potential beneficial effects of dietary vitamin D3 compared to calcitriol and the underlying mechanisms in BCa treatment and chemoprevention. We recently found that dietary vitamin D3 exhibits significant tumor inhibitory effects in xenograft models of BCa that are equivalent to those elicited by the administration of the active hormone calcitriol. At the easily achievable dose tested in our studies, dietary vitamin D3 exhibited substantial tumor inhibitory activity and, unlike calcitriol, did not cause hypercalcemia demonstrating its relative safety. We found elevations in circulating calcitriol as well as increased CYP27B1 expression in the tumor and the intestine in tumor-bearing mice ingesting a vitamin D3-supplemented diet. We hypothesize that the elevation in circulating 25(OH)D induced by dietary vitamin D3 supplements stimulates local synthesis of calcitriol in the mammary tumor microenvironment and the ensuing paracrine/autocrine actions play a major role in the anticancer activity of dietary vitamin D3. Our findings suggest that the endocrine activity of calcitriol derived from tumor and other extra-renal sources such as the intestine, probably also plays a role in mediating the anticancer effects of dietary vitamin D3. Thus it appears that multiple sites of 1α-hydroxylation contribute to the anticancer effects of dietary vitamin D3. Our data strongly suggest that dietary vitamin D will be useful in the chemoprevention and treatment of BCa since it is a safe, economical and easily available nutritional agent that is equivalent to calcitriol in exerting anticancer effects, at least in mouse models. Furthermore, adequate vitamin D nutrition and avoidance of vitamin D deficiency appear to be important in reducing BCa risk. These findings warrant clinical trials in BCa patients and in women at high risk for BCa to evaluate the benefits of dietary vitamin D3 supplementation.
Introduction
Vitamin D plays an important role in calcium homeostasis through its actions in intestine, kidney, parathyroid glands and bone [1]. When written without a subscript the D indicates either D2 or D3. Vitamin D is not really a vitamin but the precursor to the potent steroid hormone 1,25-dihydroxyvitamin D3 (1,25(OH)2D3 or calcitriol). Vitamin D derived from the diet or synthesized in the skin gets hydroxylated to 25(OH)D by liver 25-hydroxylase enzymes (CYP2R1 and CYP27A1) [1]. 25(OH)D, the circulating prohormone, is converted to 1,25(OH)2D3 by the kidney in a tightly controlled enzymatic step catalyzed by 1α-hydroxylase (CYP27B1) [1]. Calcitriol binds to the vitamin D receptor (VDR), a member of the steroid receptor superfamily to regulate gene expression in all tissues of the body. In addition to its actions on calcium and bone homeostasis, calcitriol also exhibits anti-proliferative and pro-differentiation activities indicating its potential use in the prevention and treatment of multiple cancers, including breast cancer (BCa) [2–9].
Vitamin D and Breast Cancer
Recent meta-analyses of epidemiological data support a protective role for vitamin D in BCa development [10,11]. An analysis of pooled data from observational studies suggests that serum 25(OH)D levels of 52 ng/ml or more are associated with 50% reduction in BCa risk [12]. Although the recent report by the Institute of Medicine (IOM) [13] concluded that further evidence is required to establish a cause-effect relationship between vitamin D levels and cancer prevention, the IOM committee report as well as the Endocrine Society statement have concluded that there is a strong biological basis for a role of vitamin D in cancer prevention supported by data from basic science studies in cell culture and animal models of BCa [13,14]. Vitamin D plays an important role in the development of normal mammary glands by regulating proliferation, differentiation and apoptosis and modulating estrogen regulated processes [9]. Importantly, normal and malignant human mammary tissue express CYP27B1, the enzyme that catalyzes calcitriol synthesis [15]. CYP27B1 and the megalin-cubulin complex that mediates internalization of circulating 25(OH)D are present in mammary cells and therefore the active hormone calcitriol can be synthesized locally within the breast [9].
Mechanisms of the anticancer actions of calcitriol in BCa cell cultures
The active hormone calcitriol inhibits the growth of both estrogen receptor-positive (ER+) and ER-negative human BCa cell lines by regulating many molecular pathways (reviewed in [5,6,9]). These regulatory actions include the induction of cell cycle arrest [16–18] and apoptosis [3,19,20] and promoting differentiation [21–23]. In addition, calcitriol reduces the invasive and metastatic potential of several BCa cells [24–26]. Calcitriol also exhibits potent anti-angiogenic activity that could contribute to its actions to inhibit invasion and metastasis in vivo (reviewed in [27]). Calcitriol evokes significant anti-inflammatory actions in several cancer cells including BCa cells [5,6]. Calcitriol also mediates actions that would be especially effective in ER+ BCa [28]. These actions are the inhibition of both estrogen synthesis due to the repression of aromatase transcription [29] and estrogen signaling due to the down-regulation of ERα expression [18,30–32] culminating in a significant suppression of the proliferative stimulus provided by estrogens [28].
Studies of calcitriol efficacy in animal models of BCa
The anticancer effects of calcitriol in cell culture models have been observed at high concentrations of the hormone. Achievement of adequate concentrations of calcitriol to demonstrate anticancer effects in vivo runs the risk of causing hypercalcemia and hypercalciuria leading to renal stone formation. The following alternate approaches have been used to minimize the toxicity: (1) Several academic investigators and pharmaceutical companies have focused their efforts on designing calcitriol analogs that exhibit decreased calcemic effects while maintaining equal or increased anti-proliferative activity [reviewed in [1]]. (2) Another approach to increase efficacy and decrease toxicity is to use lower doses of calcitriol in combination with other anticancer agents that act by different mechanisms [6]. The drug combination strategy has the advantage of limiting the toxicity associated with individual drugs while obtaining additive and potentially synergistic therapeutic effects. (3) A third approach, which is the focus of current studies in our laboratory, involves raising the blood levels of 25(OH)D through dietary vitamin D3 supplementation to achieve increased local production of calcitriol and ensuing paracrine anticancer effects at tumor sites. In the following sections we will discuss the findings of studies in animal models of BCa that evaluate the administration of various vitamin D compounds with a specific emphasis on the anticancer benefits of dietary vitamin D3 in BCa therapy and chemoprevention.
BCa xenograft models
A frequently used experimental model to investigate the inhibitory effects of vitamin D compounds in vivo is the establishment of human BCa cell lines as xenografts in immunocompromised mice typically in the flanks or sometimes in the mammary fat pads. Calcitriol and its analogs have been shown to inhibit the growth of xenografts of both ER-positive cell lines such as MCF-7 cells [33,34] and the more aggressive ER-negative cell lines like MDA-MB-231 [24,35,36]. In the case of ER-positive xenografts, the anti-tumor effects of calcitriol and its analogs are due to both increased expression of mediators of growth arrest such as the CDK inhibitor p21 and IGFBP-3 and the disruption of estrogen-mediated survival signals following the suppression of estrogen synthesis and signaling [33]. The inhibition of ER-negative tumors by calcitriol analogs is associated with decreased proliferation and apoptosis as evidenced by increased DNA fragmentation [24,36]. In comparison to calcitriol, the analogs appear to exert similar anti-tumor activity while causing less hypercalcemia [24,34,36]. Combinations of calcitriol or its analogs with other anticancer agents have also been tested for anti-tumor activity in xenograft models. Combinations of calcitriol with aromatase inhibitors [33] or the analog EB1089 with all-trans-retinoic acid [37] exhibit enhanced inhibition of the growth of MCF-7 xenografts. Calcitriol analogs sensitize MCF-7 xenografts to the tumor inhibitory effects of chemotherapy drugs such as paclitaxel [38] or ionizing radiation, partly through promotion of apoptosis [39]. Advanced stage disease is commonly associated with BCa metastases to bone. Recent studies have investigated the effect of vitamin D status in models of bone metastasis established by intra-tibial injections of BCa cell lines such as MCF-7 or MDA-MB-231. In both models, vitamin D deficiency enhances both the growth of the tumors as well as tumor-induced osteosclerotic changes in the tibia [40,41], suggesting an anti-metastatic effect of vitamin D in advanced BCa.
Chemically induced mammary carcinogenesis models
Differentiation-inducing agents such as vitamin D are potentially promising as cancer chemopreventive agents. The chemopreventive potential of calcitriol and its analogs have been examined in mammary carcinogenesis models wherein the animals are exposed to a chemical carcinogen such as N-nitoso-N-methylurea (NMU) or 7, 12-dimethylbenz(a)anthracene (DMBA) to induce mammary tumors. Treatment with calcitriol analogs significantly extended tumor latency, decreased tumor incidence and multiplicity and caused substantial regression of tumors that did develop in rats exposed to NMU [42–45]. In DMBA-induced mammary carcinogenesis in rats, the calcitriol analog 1α-hydroxyvitamin D5 exhibited stage-specific inhibition of tumorigenesis with more pronounced inhibition of tumor incidence in the promotion or progression phase of carcinogenesis [45].
Genetically engineered mouse models
Many genetically engineered mouse models of mammary cancer utilize the mouse mammary tumor virus (MMTV) promoter that is primarily expressed in the mammary gland to drive the expression of cancer promoting genes such as the IGF-I receptor, Her2/neu, TGFa or Wnt-1 [46]. Use of such a transgenic mouse model enables the assessment of inhibitory effects of vitamin D compounds in a spontaneously arising tumor model. In the MMTV-neu model it was found that VDR was highly expressed in neu-positive preneoplastic lesions, established tumors as well as lung metastasis in these mice [47]. Compared with MMTV-neu mice with two copies of the VDR gene, haploinsufficiency of VDR shortened the latency and increased the incidence of mammary tumor formation, suggesting a protective role for vitamin D in mammary carcinogenesis. A recent study showed that MMTV-neu transgenic mice fed a vitamin D-supplemented diet induced signaling of vitamin D regulated genes involved in pathways that drive differentiation, alter metabolism, remodel the extracellular matrix and trigger innate immunity in the mammary tissue [48]. In another transgenic model of hormone-induced mammary cancer, the LH-overexpressing mouse, treatment with the analog EB1089 decreased proliferation of the mammary epithelial cells in preneoplastic glands [49]. Further, half of the hormone-induced tumors in EB1089-treated mice demonstrated a decreased rate of growth while a subset of tumors regressed. Collectively the findings of these studies suggest that vitamin D compounds may have utility as cancer chemopreventive agents.
Dietary vitamin D supplementation as a therapeutic approach in BCa
Ongoing studies in our laboratory are investigating the potential benefit of dietary vitamin D3 in the treatment and chemoprevention of BCa. Our rationale is based on studies that have demonstrated the presence of the enzyme CYP27B1 in normal and malignant mammary tissue [9,15,50,51], which converts the circulating prohormone 25(OH)D3 to the active hormone calcitriol locally within the breast. Recent studies show that breast adipocytes also express CYP27B1 [52]. These observations raise the possibility that dietary vitamin D [the precursor to 25(OH)D] rather than the active hormone calcitriol could be used in BCa therapy. Raising the blood levels of 25(OH)D through dietary vitamin D supplementation provides increased levels of the substrate for CYP27B1 in target tissues like breast leading to the local production of increased calcitriol, which then exerts autocrine/paracrine actions to inhibit BCa growth. This therapeutic approach avoids the systemic side effect of hypercalcemia, which may be associated with high dose calcitriol [1–4]. The ability of 25(OH)D to cause hypercalcemia is very much reduced because of its significantly lower affinity for the VDR [53,54]. However, extremely elevated serum levels of 25(OH)D can act through the VDR to influence calcium metabolism independent of its conversion to calcitriol as has been demonstrated by studies in CYP27B1 knockout mice [55]. Hypercalcemia due to vitamin D compounds results from elevated circulating levels of the active hormone calcitriol that cause increased intestinal calcium absorption and increased bone resorption. The expression of renal CYP27B1 is tightly controlled by parathyroid hormone (PTH) [1]. Following an initial rise in serum calcium concentration after ingestion of vitamin D, PTH levels would be suppressed leading to a decrease in renal CYP27B1expression and the restriction of further renal conversion of 25(OH)D to calcitriol thereby limiting the ability of vitamin D supplementation to cause hypercalcemia [1]. Treatment with calcitriol bypasses this checkpoint thus increasing the risk of hypercalcemia. Since extra-renal CYP27B1within cancer cells is not regulated by PTH [56], the extent of 25(OH)D conversion to calcitriol in the extra-renal tissues is not restricted by the normal feedback system for renal CYP27B1. Therefore local production of calcitriol within the BCa seems to be predominantly dependent on the levels of the circulating substrate 25(OH)D, although other regulators are being studied. Thus dietary vitamin D ingestion will retain the beneficial paracrine effects of calcitriol within the breast, while avoiding systemic hypercalcemia that is much more likely to be associated with high dose calcitriol administration [1–4].
Dietary vitamin D3 exhibits anticancer activity equivalent to calcitriol
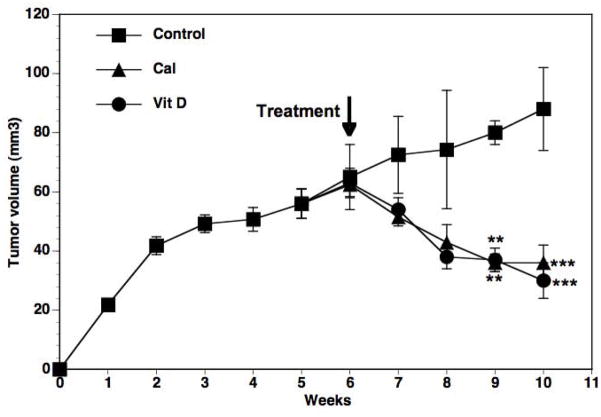
Our recent study [57] demonstrated significant tumor shrinkage (>50%) in female nude mice bearing MCF-7 xenografts following ingestion of a vitamin D3-supplemented diet (5000 IU/kg) compared to the standard diet (1000 IU vitamin D3/kg) (Fig 1). Reduction in tumor growth was noticed within one week of starting the supplemented diet and by 4 weeks the tumors were reduced to less than half the size of mice fed the control diet. In parallel the effects of graded concentrations of calcitriol administered as intraperitoneal (i.p.) injections were tested. The inhibition of tumor growth by dietary vitamin D3 was equivalent to that of i.p. calcitriol at the dose of 50 ng/mouse, three times a week (Fig 1). Calcitriol at 50 ng and higher caused modest but statistically significant increases in serum calcium levels (Table 1) indicating that the dietary vitamin D3 comparison was to a maximally safe calcitriol dose. Dietary vitamin D3 did not increase serum calcium (Table 1), demonstrating its safety at the concentration tested. Both calcitriol and dietary vitamin D3 were equipotent in suppressing estrogen synthesis and signaling and other pro-inflammatory and growth signaling pathways [57]. Continuing studies in our laboratory also indicate that exposure to a vitamin D3-supplemented diet prior to tumor inoculation delays the appearance of tumors in some xenograft and transgenic mouse models of BCa (unpublished observations), demonstrating the potential utility of dietary vitamin D supplementation in cancer chemoprevention.
Fig 1. Effect of calcitriol and dietary vitamin D3 on the growth of MCF-7 xenograft tumors.
MCF-7 xenografts were established in the flanks of intact female nude mice. After 6 weeks of tumor growth the mice were given a vitamin D3-supplemented diet (Vit D; 5000 IU vitamin D3/kg) or the standard diet (1000 IU vitamin D3/kg) + i.p. injections of calcitriol (50 ng/mouse), three times a week (Cal) for the following 4 weeks. Mice in the control group (Control) were on the standard diet and received i.p. injections of sterile saline vehicle during this time period. Tumor volumes were measured weekly. ** p<0.01 and *** p < 0.001 as compared to the corresponding control values. Reproduced with permission from [57].
TABLE 1.
Body weight and serum levels of vitamin D metabolites and calcium
| GROUPS | Body Weight (g) | Serum 25-Hydroxy- vitamin D+ (ng/ml) | Serum 1,25-dihydroxy- vitamin D+ (pg/ml) | Serum Calcium (mg/dl) |
|---|---|---|---|---|
| Tumor Bearing Mice | ||||
| Control | 22±0.4 | 21±2 | 77+9 | 9.7±0.2 |
| Calcitriol (0.05 ug) | 23.8±0.8 | 15±3 | 32+3* | 10.9±0.2* |
| Vit D | 23±0.4 | 38±2*** ^^^ | 117+11* ^ | 9.5±0.2 |
| Non-Tumor Bearing Mice | ||||
| Control | 22±1 | 24±2 | 61+7 | 9.5±0.1 |
| Calcitriol (0.05 ug) | 21±2 | 11±0.6*** | 13+1*** | ND |
| Vit D | 23±1 | 40±1*** ^^^ | 72+6^^^ | 9.9±0.2 |
p < 0.05;
p < 0.001 when compare to control and
p <0.05;
p < 0.001 when compared to calcitriol.
ND – not determined.
The assay did not distinguish between D2 and D3 metabolites in the serum. Adapted from [57].
Importance of tumor CYP27B1 expression
We and others have shown that supplementation of the mouse diet to increase vitamin D content 5-fold over the standard diet resulted in an elevation in the levels of circulating 25(OH)D as expected [48,57]. Interestingly, comparison of mice receiving a dietary vitamin D supplement to mice on the standard diet revealed the following two unexpected findings that warrant discussion: (1) an increase in the expression of CYP27B1 mRNA in the tumors (Fig 2A) and (2) a significant elevation in serum 1,25(OH)2D levels (Table 1). Calcitriol is a known negative regulator of CYP27B1 transcription in the kidney [58]. However, calcitriol regulation of CYP27B1 appears to be variable and published studies report either no alteration [51,59] or an increase [40] in CYP27B1 expression in MCF-7 cells treated with calcitriol. In our in vivo study, no significant changes were seen in tumor CYP27B1 mRNA levels in mice treated with calcitriol. However, an appreciable up-regulation (~5-fold) of CYP27B1 mRNA in MCF-7 tumors was observed following dietary vitamin D3 ingestion. An increase in CYP27B1 mRNA was reported in mouse mammary gland organ cultures exposed to 25(OH)D [60]. The mechanism underlying the differential regulation of CYP27B1 expression by the active hormone calcitriol compared to feeding the precursor vitamin D3 remains unclear at present and further research is required to elucidate the mechanism of this effect. It should be emphasized that in the tumors where dietary vitamin D3 induced up-regulation of CYP27B1 expression, calcitriol did not exert this action suggesting a unique effect of dietary vitamin D3 in the same tissue. Other genes responded similarly to both calcitriol and dietary vitamin D3.
Fig 2. CYP27 B1 expression in xenograft tumors, kidneys and intestines of nude mice fed a vitamin D3-supplemented diet.
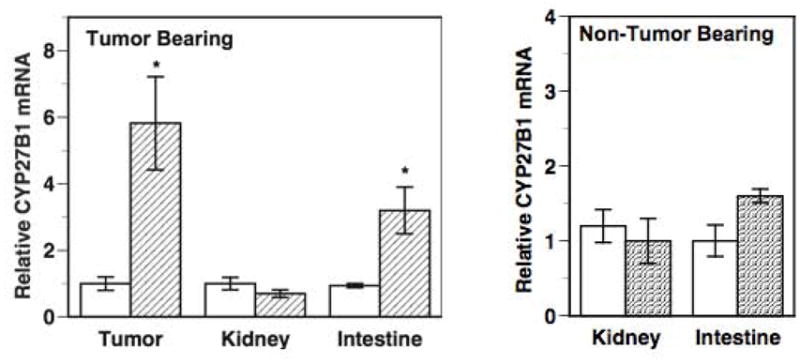
CYP27B1 mRNA levels were determined by qRT-PCR in the tumors, kidneys and intestines of nude mice bearing MCF-7 xenograft tumors (Fig 2A) and in the kidneys and intestines of non-tumor bearing mice (Fig 2B). Relative CYP27B1 mRNA levels in each tissue from mice fed the standard diet (open bars) were set at 1 and compared to the corresponding levels in mice receiving the vitamin D3-supplemented diet (hatched bars). * p < 0.05 as compared to the corresponding controls on the standard diet. Adapted from [57].
Rise in circulating concentrations of calcitriol following dietary supplementation with vitamin D3
In spite of the elevations in serum 25(OH)D levels following dietary vitamin D3 ingestion, we did not expect an increase in circulating calcitriol levels because of the tight regulation of renal CYP27B1 by PTH and serum calcium that restricts renal 25(OH)D conversion to calcitriol [1]. Since extra-renal CYP27B1 is not regulated by PTH [56], we hypothesized that the extent of 25(OH)D conversion to calcitriol in the tumors would not be restricted and would be proportional to the dietary levels of vitamin D3. We expected that the locally synthesized calcitriol would be sufficient to exert significant autocrine/paracrine anticancer effects, but not high enough to “spill” into the circulation to raise circulating calcitriol levels. However, contrary to our expectation, we observed significant elevations in serum calcitriol levels in tumor-bearing mice ingesting dietary vitamin D3 supplements. Dietary vitamin D3 administration to mice bearing MCF-7 tumors did not cause an increase in renal CYP27B1 expression (Fig 2A), suggesting that in these mice the origin of the elevated circulating serum calcitriol levels was not renal. Interestingly in parallel experiments, non-tumor bearing female nude mice fed the same vitamin D3-supplemented diet did not show elevations in circulating calcitriol (Table 1). This finding suggests that the elevated serum calcitriol levels in the tumor-bearing mice receiving the vitamin D3-supplemented diet was likely from extra-renal sources and could, at least in part, be tumor-derived. The finding in tumor-bearing mice is similar to the phenomena noted in some patients with sarcoid that exhibit elevated concentrations of calcitriol especially after precursor 25(OH)D is increased by sunlight or diet [50].
The elevation in tumor CYP27B1 expression caused by dietary vitamin D3 is likely to be a factor contributing to the rise in the serum calcitriol concentration in mice receiving vitamin D3 supplementation. In these mice the CYP27B1 mRNA level was also increased significantly (~3.5-fold) in the intestine (Fig 2A), which could be an additional factor contributing to the elevated serum calcitriol levels. Interestingly, in non-tumor bearing mice receiving the same vitamin D3-supplemented diet, there were no significant changes in intestinal CYP27B1 mRNA expression (Fig 2B) and as expected the kidney CYP24 mRNA levels remained unchanged (Fig 2B).
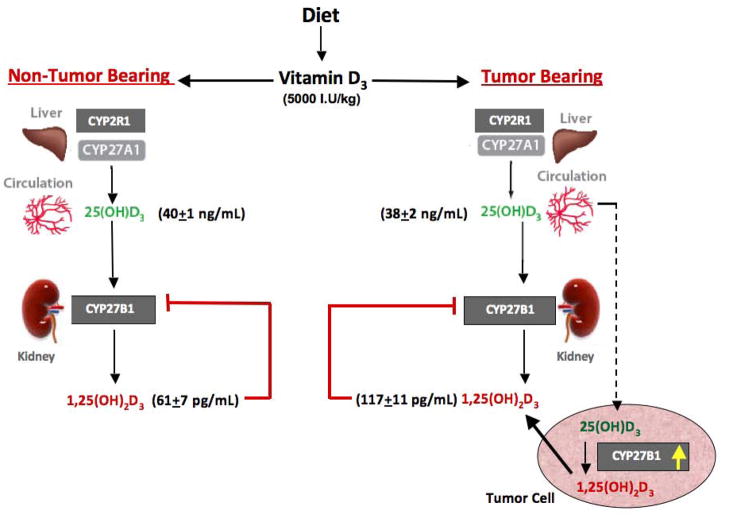
Fig 3 depicts a model summarizing our findings on vitamin D metabolism in tumor bearing mice compared to non-tumor bearing mice receiving the same vitamin D3-supplemented diet. Collectively our data suggest that the tumor or tumor-derived factors play an important role in increasing circulating calcitriol levels seen in the tumor bearing mice receiving dietary vitamin D3, since the phenomenon is not seen in non-tumor bearing mice. In the absence of changes in renal CYP27B1 expression, we hypothesize that the increase in tumor CYP27B1 expression following dietary vitamin D3 ingestion contributes to the inhibition of tumor growth as well as the elevated serum calcitriol. The increase in CYP27B1 expression in other extra-renal sites such as the intestine may also be a contributing factor. It is of interest that the increase in intestinal CYP27B1 is not seen in mice without tumors fed the same vitamin D3-supplemented diet. Further studies are clearly needed to elucidate the mechanism underlying the phenomenon of tumor and intestine CYP27B1 stimulation rather than suppression in the face of elevated circulating calcitriol. Other genes in the tumor respond as expected to calcitriol. Also additional study is needed to better understand the role of local calcitriol synthesis in mediating the anticancer effects of dietary vitamin D3. Our current data suggest that both the autocrine/paracrine activity of locally synthesized calcitriol in the breast as well as the endocrine activity of the elevated serum calcitriol play a role in the anticancer activity of dietary vitamin D3.
Fig 3. A model depicting changes in vitamin D metabolism following dietary vitamin D3 administration to nude mice.
Changes in serum vitamin D metabolite levels and tumor and renal CYP27B1 expression are shown following the administration of the vitamin D3-supplemented diet to tumor bearing mice as compared to non-tumor bearing mice. Dietary vitamin D administration increases circulating 25(OH)D levels and up-regulates CYP27B1 expression within the tumors, without altering renal CYP27B1 levels. Circulating 1,25(OH)2D levels are elevated in tumor bearing mice but not in mice without tumors. Tumor-derived factors, including the elevated CYP27B1 expression, may play an important role in increasing circulating calcitriol levels seen in the tumor bearing mice receiving dietary vitamin D3. We hypothesize that both the autocrine/paracrine activity ensuing from local synthesis of calcitriol in the tumors and the endocrine activity of elevated circulating calcitriol play a role in mediating the anticancer effects of dietary vitamin D3.
Safety of dietary vitamin D intervention
Another interesting observation from our studies was that the ingestion of dietary vitamin D3 at 5000 IU/kg did not increase serum calcium levels, in spite of the elevations detected in the serum calcitriol concentration. Interestingly, the elevated serum calcitriol levels assayed in blood of mice receiving calcitriol, drawn 14 hours after the final calcitriol injections, were lower than the concentrations in mice receiving the vitamin D3-supplemented diet. However, the time-point of the blood draw was delayed and represents the nadir serum calcitriol levels measured in mice receiving calcitriol injections. It is likely that the serum calcitriol concentrations peaked within the first few hours after calcitriol injections [61] and they were transiently but substantially higher than the levels in the mice receiving dietary vitamin D3 supplements. These peak calcitriol concentrations likely caused the observed increases in serum calcium concentrations in the calcitriol-treated mice. Other investigators have shown that the ingestion of diets supplemented with vitamin D3 at levels up to 20,000 IU/kg for up to 7 weeks in non-tumor bearing mice does not cause hypercalcemia [62]. However, these studies in non-tumor bearing mice may not hold true in the presence of tumors that appear to alter the usual pattern of CYP27B1 regulation. It is possible that hypercalcemia would occur if dietary vitamin D3 were supplemented to tumor bearing mice at higher doses further raising the circulating calcitriol concentration. However, at the supplement levels that we tested, our data support the hypothesis that dietary vitamin D3 is useful in the chemoprevention and treatment of BCa since it is an economical and easily available nutritional agent that is as active as calcitriol in inhibiting tumor growth with minimal hypercalcemic side effects. However the use of very high doses of dietary vitamin D3 in the presence of cancers will require careful monitoring for hypercalcemia.
Summary and Conclusions
Numerous studies in various cell culture and animal models of BCa demonstrate the anticancer effects of calcitriol. Our recent studies in mouse tumor models highlight the potential utility of dietary vitamin D3 in BCa treatment and chemoprevention. We have shown that dietary vitamin D3 exhibits significant tumor inhibitory effects in xenograft models of BCa, which are equivalent to the anticancer benefits mediated by the active hormone calcitriol. At moderate, achievable doses dietary vitamin D3 unlike calcitriol, does not cause hypercalcemia demonstrating its relative safety. The autocrine/paracrine activity ensuing from local synthesis of calcitriol in the tumors following elevations in serum 25(OH)D3 due to dietary vitamin D3 supplementation probably plays a key role in the anticancer activity of dietary vitamin D3. However, the elevations in circulating calcitriol concentrations in tumor-bearing mice ingesting a vitamin D3-supplemented diet suggests that the endocrine activity of the supplement-induced calcitriol derived from tumors as well as renal and other extra-renal sources may also play a role. Our data do not resolve the relative importance of each source and probably all contribute to the anticancer effects of dietary vitamin D3.
These data strongly suggest that dietary vitamin D3 will be useful in the chemoprevention and treatment of BCa since it is a safe, economical and easily available nutritional agent that is as active as calcitriol in exerting anticancer effects. Furthermore, adequate vitamin D nutrition and avoidance of vitamin D deficiency appear to be important in reducing cancer risk. We are keenly aware that similar findings remain to be demonstrated in women. However, these findings and others in preclinical models, strongly indicate that clinical trials in women at risk of BCa or who already have BCa are clearly warranted to evaluate the benefits of dietary vitamin D3 supplementation.
Highlights.
Dietary vitamin D3 and calcitriol exhibit equivalent tumor inhibition in mouse models of breast cancer
At moderate, achievable doses dietary vitamin D3 does not cause hypercalcemia, demonstrating safety
Tumor CYP27B1 and local calcitriol synthesis play a major role in the anticancer activity of vitamin D3
Endocrine activity of calcitriol derived from tumor and other extra-renal sources also contribute
Avoidance of vitamin D deficiency is important for reducing breast cancer risk
Acknowledgments
Grant Support: This work was supported by NCI grant CA13099, Komen Foundation grant 070101 and CBCRP grant # 17OB-0071 to D.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feldman D, Malloy PJ, Krishnan AV, Balint E. Vitamin D: biology, action and clinical implications. In: Marcus R, Feldman D, Nelson DA, Rosen CJ, editors. Osteoporosis. 3. Vol. 1. Elsevier Academic Press; San Diego: 2007. pp. 317–382. [Google Scholar]
- 2.Beer TM, Myrthue A. Calcitriol in cancer treatment: from the lab to the clinic. Mol Cancer Ther. 2004;3(3):373–381. [PubMed] [Google Scholar]
- 3.Colston KW, Hansen CM. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr Relat Cancer. 2002;9(1):45–59. doi: 10.1677/erc.0.0090045. [DOI] [PubMed] [Google Scholar]
- 4.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–336. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan AV, Trump DL, Johnson CS, Feldman D. The role of vitamin D in cancer prevention and treatment. Endocrinol Metab Clin North Am. 2010;39(2):401–418. doi: 10.1016/j.ecl.2010.02.011. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luong QT, Koeffler HP. Vitamin D compounds in leukemia. J Steroid Biochem Mol Biol. 2005;97(1–2):195–202. doi: 10.1016/j.jsbmb.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Pereira F, Larriba MJ, Munoz A. Vitamin D and colon cancer. Endocr Relat Cancer. 2012;19(3):R51–71. doi: 10.1530/ERC-11-0388. [DOI] [PubMed] [Google Scholar]
- 9.Welsh J. Vitamin D metabolism in mammary gland and breast cancer. Mol Cell Endocrinol. 2011;347(1–2):55–60. doi: 10.1016/j.mce.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121(2):469–477. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 11.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 2010;46(12):2196–2205. doi: 10.1016/j.ejca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, Newmark H, Holick MF, Garland FC. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 2007;103(3–5):708–711. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. The National Academic Press; Washington, DC: 2011. [Google Scholar]
- 14.Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The nonskeletal effects of vitamin d: an endocrine society scientific statement. Endocr Rev. 2012;33(3):456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Townsend K, Banwell CM, Guy M, Colston KW, Mansi JL, Stewart PM, Campbell MJ, Hewison M. Autocrine metabolism of vitamin D in normal and malignant breast tissue. Clin Cancer Res. 2005;11(9):3579–3586. doi: 10.1158/1078-0432.CCR-04-2359. [DOI] [PubMed] [Google Scholar]
- 16.Fan FS, Yu WC. 1,25-Dihydroxyvitamin D3 suppresses cell growth, DNA synthesis, and phosphorylation of retinoblastoma protein in a breast cancer cell line. Cancer Invest. 1995;13(3):280–286. doi: 10.3109/07357909509094463. [DOI] [PubMed] [Google Scholar]
- 17.Jensen SS, Madsen MW, Lukas J, Binderup L, Bartek J. Inhibitory effects of 1alpha,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol Endocrinol. 2001;15(8):1370–1380. doi: 10.1210/mend.15.8.0673. [DOI] [PubMed] [Google Scholar]
- 18.Simboli-Campbell M, Narvaez CJ, van Weelden K, Tenniswood M, Welsh J. Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells. Breast Cancer Res Treat. 1997;42(1):31–41. doi: 10.1023/a:1005772432465. [DOI] [PubMed] [Google Scholar]
- 19.Narvaez CJ, Zinser G, Welsh J. Functions of 1alpha,25-dihydroxyvitamin D(3) in mammary gland: from normal development to breast cancer. Steroids. 2001;66(3–5):301–308. doi: 10.1016/s0039-128x(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 20.Pirianov G, Colston KW. Interactions of vitamin D analogue CB1093, TNFalpha and ceramide on breast cancer cell apoptosis. Mol Cell Endocrinol. 2001;172(1–2):69–78. doi: 10.1016/s0303-7207(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 21.Lazzaro G, Agadir A, Qing W, Poria M, Mehta RR, Moriarty RM, Das Gupta TK, Zhang XK, Mehta RG. Induction of differentiation by 1alpha-hydroxyvitamin D(5) in T47D human breast cancer cells and its interaction with vitamin D receptors. Eur J Cancer. 2000;36(6):780–786. doi: 10.1016/s0959-8049(00)00016-2. [DOI] [PubMed] [Google Scholar]
- 22.Pendas-Franco N, Gonzalez-Sancho JM, Suarez Y, Aguilera O, Steinmeyer A, Gamallo C, Berciano MT, Lafarga M, Munoz A. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation. 2007;75(3):193–207. doi: 10.1111/j.1432-0436.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Lee D, Sysounthone V, Chandraratna RAS, Christakos S, Korah R, Wieder R. 1,25-dihydroxyvitamin D3 and retonic acid analogues induce differentiation in breast cancer cells with function- and cell-specific additive effects. Breast Cancer Res Treat. 2001;67(2):157–168. doi: 10.1023/a:1010643323268. [DOI] [PubMed] [Google Scholar]
- 24.Flanagan L, Packman K, Juba B, O’Neill S, Tenniswood M, Welsh J. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. J Steroid Biochem Mol Biol. 2003;84(2–3):181–192. doi: 10.1016/s0960-0760(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 25.Hansen CM, Frandsen TL, Brunner N, Binderup L. 1 alpha,25-Dihydroxyvitamin D3 inhibits the invasive potential of human breast cancer cells in vitro. Clin Exp Metastasis. 1994;12(3):195–202. doi: 10.1007/BF01753887. [DOI] [PubMed] [Google Scholar]
- 26.Koli K, Keski-Oja J. 1alpha,25-dihydroxyvitamin D3 and its analogues down-regulate cell invasion-associated proteases in cultured malignant cells. Cell Growth Differ. 2000;11(4):221–229. [PubMed] [Google Scholar]
- 27.Welsh J. Vitamin D actions in mammary gland and breast cancer. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. 3. Vol. 2. Elsevier Academic Press; San Diego: 2011. pp. 1657–1673. [Google Scholar]
- 28.Krishnan AV, Swami S, Feldman D. Vitamin D and breast cancer: inhibition of estrogen synthesis and signaling. J Steroid Biochem Mol Biol. 2010;121(1–2):343–348. doi: 10.1016/j.jsbmb.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan AV, Swami S, Peng L, Wang J, Moreno J, Feldman D. Tissue-selective regulation of aromatase expression by calcitriol: implications for breast cancer therapy. Endocrinology. 2010;151(1):32–42. doi: 10.1210/en.2009-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James SY, Mackay AG, Binderup L, Colston KW. Effects of a new synthetic vitamin D analogue, EB1089, on the oestrogen-responsive growth of human breast cancer cells. J Endocrinol. 1994;141(3):555–563. doi: 10.1677/joe.0.1410555. [DOI] [PubMed] [Google Scholar]
- 31.Stoica A, Saceda M, Fakhro A, Solomon HB, Fenster BD, Martin MB. Regulation of estrogen receptor-alpha gene expression by 1, 25-dihydroxyvitamin D in MCF-7 cells. J Cell Biochem. 1999;75(4):640–651. [PubMed] [Google Scholar]
- 32.Swami S, Krishnan AV, Feldman D. 1alpha,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res. 2000;6(8):3371–3379. [PubMed] [Google Scholar]
- 33.Swami S, Krishnan AV, Wang JY, Jensen K, Peng L, Albertelli MA, Feldman D. Inhibitory effects of calcitriol on the growth of MCF-7 breast cancer xenografts in nude mice: selective modulation of aromatase expression in vivo. Horm Cancer. 2011;2(3):190–202. doi: 10.1007/s12672-011-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VanWeelden K, Flanagan L, Binderup L, Tenniswood M, Welsh J. Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089. Endocrinology. 1998;139(4):2102–2110. doi: 10.1210/endo.139.4.5892. [DOI] [PubMed] [Google Scholar]
- 35.Lee HJ, Paul S, Atalla N, Thomas PE, Lin X, Yang I, Buckley B, Lu G, Zheng X, Lou YR, Conney AH, Maehr H, Adorini L, Uskokovic M, Suh N. Gemini vitamin D analogues inhibit estrogen receptor-positive and estrogen receptor-negative mammary tumorigenesis without hypercalcemic toxicity. Cancer Prev Res (Phila) 2008;1(6):476–484. doi: 10.1158/1940-6207.CAPR-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.So JY, Lee HJ, Smolarek AK, Paul S, Wang CX, Maehr H, Uskokovic M, Zheng X, Conney AH, Cai L, Liu F, Suh N. A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Mol Pharmacol. 2011;79(3):360–367. doi: 10.1124/mol.110.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koshizuka K, Kubota T, Said J, Koike M, Binderup L, Uskokovic M, Koeffler HP. Combination therapy of a vitamin D3 analog and all-trans-retinoic acid: effect on human breast cancer in nude mice. Anticancer Res. 1999;19(1A):519–524. [PubMed] [Google Scholar]
- 38.Koshizuka K, Koike M, Asou H, Cho SK, Stephen T, Rude RK, Binderup L, Uskokovic M, Koeffler HP. Combined effect of vitamin D3 analogs and paclitaxel on the growth of MCF-7 breast cancer cells in vivo. Breast Cancer Res Treat. 1999;53(2):113–120. doi: 10.1023/a:1006123819675. [DOI] [PubMed] [Google Scholar]
- 39.Sundaram S, Sea A, Feldman S, Strawbridge R, Hoopes PJ, Demidenko E, Binderup L, Gewirtz DA. The combination of a potent vitamin D3 analog, EB 1089, with ionizing radiation reduces tumor growth and induces apoptosis of MCF-7 breast tumor xenografts in nude mice. Clin Cancer Res. 2003;9(6):2350–2356. [PubMed] [Google Scholar]
- 40.Ooi LL, Zheng Y, Zhou H, Trivedi T, Conigrave AD, Seibel MJ, Dunstan CR. Vitamin D deficiency promotes growth of MCF-7 human breast cancer in a rodent model of osteosclerotic bone metastasis. Bone. 2010;47(4):795–803. doi: 10.1016/j.bone.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Ooi LL, Zhou H, Kalak R, Zheng Y, Conigrave AD, Seibel MJ, Dunstan CR. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Res. 2010;70(5):1835–1844. doi: 10.1158/0008-5472.CAN-09-3194. [DOI] [PubMed] [Google Scholar]
- 42.Anzano MA, Smith JM, Uskokovic MR, Peer CW, Mullen LT, Letterio JJ, Welsh MC, Shrader MW, Logsdon DL, Driver CL, et al. 1 alpha,25-Dihydroxy-16-ene-23-yne-26,27-hexafluorocholecalciferol (Ro24-5531), a new deltanoid (vitamin D analogue) for prevention of breast cancer in the rat. Cancer Res. 1994;54(7):1653–1656. [PubMed] [Google Scholar]
- 43.Colston KW, Pirianov G, Bramm E, Hamberg KJ, Binderup L. Effects of Seocalcitol (EB1089) on nitrosomethyl urea-induced rat mammary tumors. Breast Cancer Res Treat. 2003;80(3):303–311. doi: 10.1023/A:1024962316691. [DOI] [PubMed] [Google Scholar]
- 44.James SY, Mercer E, Brady M, Binderup L, Colston KW. EB1089, a synthetic analogue of vitamin D, induces apoptosis in breast cancer cells in vivo and in vitro. Br J Pharmacol. 1998;125(5):953–962. doi: 10.1038/sj.bjp.0702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murillo G, Mehta RG. Chemoprevention of chemically-induced mammary and colon carcinogenesis by 1alpha-hydroxyvitamin D5. J Steroid Biochem Mol Biol. 2005;97(1–2):129–136. doi: 10.1016/j.jsbmb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Cleary MP, Grossmann ME, Ray A. Effect of obesity on breast cancer development. Vet Pathol. 2010;47(2):202–213. doi: 10.1177/0300985809357753. [DOI] [PubMed] [Google Scholar]
- 47.Zinser GM, Welsh J. Vitamin D receptor status alters mammary gland morphology and tumorigenesis in MMTV-neu mice. Carcinogenesis. 2004;25(12):2361–2372. doi: 10.1093/carcin/bgh271. [DOI] [PubMed] [Google Scholar]
- 48.Matthews D, LaPorta E, Zinser GM, Narvaez CJ, Welsh J. Genomic vitamin D signaling in breast cancer: Insights from animal models and human cells. J Steroid Biochem Mol Biol. 2010;121(1–2):362–367. doi: 10.1016/j.jsbmb.2010.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milliken EL, Zhang X, Flask C, Duerk JL, MacDonald PN, Keri RA. EB1089, a vitamin D receptor agonist, reduces proliferation and decreases tumor growth rate in a mouse model of hormone-induced mammary cancer. Cancer Lett. 2005;229(2):205–215. doi: 10.1016/j.canlet.2005.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hewison M, Adams JS. Extrarenal 1a-hydroxylase. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. Vol. 1. Elsevier Academic Press; San Diego: 2011. pp. 777–804. [Google Scholar]
- 51.Kemmis CM, Salvador SM, Smith KM, Welsh J. Human mammary epithelial cells express CYP27B1 and are growth inhibited by 25-hydroxyvitamin D-3, the major circulating form of vitamin D-3. J Nutr. 2006;136(4):887–892. doi: 10.1093/jn/136.4.887. [DOI] [PubMed] [Google Scholar]
- 52.Ching S, Kashinkunti S, Niehaus MD, Zinser GM. Mammary adipocytes bioactivate 25-hydroxyvitamin D(3) and signal via vitamin D(3) receptor, modulating mammary epithelial cell growth. J Cell Biochem. 2011;112(11):3393–3405. doi: 10.1002/jcb.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skowronski RJ, Peehl DM, Feldman D. Actions of vitamin D3, analogs on human prostate cancer cell lines: comparison with 1,25-dihydroxyvitamin D3. Endocrinology. 1995;136(1):20–26. doi: 10.1210/endo.136.1.7530193. [DOI] [PubMed] [Google Scholar]
- 54.Wecksler WR, Okamura WH, Norman AW. Studies on the mode of action of vitamin D--XIV. Quantitative assessment of the structural requirements for the interaction of 1 alpha, 25-dihydroxyvitamin D3 with its chick intestinal mucosa receptor system. J Steroid Biochem. 1978;9(10):929–937. doi: 10.1016/0022-4731(78)90053-5. [DOI] [PubMed] [Google Scholar]
- 55.Rowling MJ, Gliniak C, Welsh J, Fleet JC. High dietary vitamin D prevents hypocalcemia and osteomalacia in CYP27B1 knockout mice. J Nutr. 2007;137(12):2608–2615. doi: 10.1093/jn/137.12.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young MV, Schwartz GG, Wang L, Jamieson DP, Whitlatch LW, Flanagan JN, Lokeshwar BL, Holick MF, Chen TC. The prostate 25-hydroxyvitamin D-1 alpha-hydroxylase is not influenced by parathyroid hormone and calcium: implications for prostate cancer chemoprevention by vitamin D. Carcinogenesis. 2004;25(6):967–971. doi: 10.1093/carcin/bgh082. [DOI] [PubMed] [Google Scholar]
- 57.Swami S, Krishnan AV, Wang JY, Jensen K, Horst R, Albertelli MA, Feldman D. Dietary vitamin d3 and 1,25-dihydroxyvitamin d3 (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology. 2012;153(6):2576–2587. doi: 10.1210/en.2011-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murayama A, Kim MS, Yanagisawa J, Takeyama K, Kato S. Transrepression by a liganded nuclear receptor via a bHLH activator through co-regulator switching. Embo J. 2004;23(7):1598–1608. doi: 10.1038/sj.emboj.7600157. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Turunen MM, Dunlop TW, Carlberg C, Vaisanen S. Selective use of multiple vitamin D response elements underlies the 1 alpha,25-dihydroxyvitamin D3-mediated negative regulation of the human CYP27B1 gene. Nucleic Acids Res. 2007;35(8):2734–2747. doi: 10.1093/nar/gkm179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng X, Hawthorne M, Vaishnav A, St-Arnaud R, Mehta RG. 25-Hydroxyvitamin D3 is a natural chemopreventive agent against carcinogen induced precancerous lesions in mouse mammary gland organ culture. Breast Cancer Res Treat. 2009;113(1):31–41. doi: 10.1007/s10549-008-9900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muindi JR, Modzelewski RA, Peng Y, Trump DL, Johnson CS. Pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 in normal mice after systemic exposure to effective and safe antitumor doses. Oncology. 2004;66(1):62–66. doi: 10.1159/000076336. [DOI] [PubMed] [Google Scholar]
- 62.Fleet JC, Gliniak C, Zhang Z, Xue Y, Smith KB, McCreedy R, Adedokun SA. Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. J Nutr. 2008;138(6):1114–1120. doi: 10.1093/jn/138.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]