Abstract
Background
Community pharmacies have the potential to reduce the prevalence of tobacco use, yet most pharmacies do not integrate cessation activities into routine practice. Acquiring participation of community pharmacies is difficult. Strategies detailing requirements by researchers to elicit such participation have not been established.
Objectives
The objective of this study was to describe the recruitment strategy and participant yield for a two-state, randomized trial evaluating two intervention approaches for increasing pharmacy-based referrals to tobacco quitlines.
Methods
Detailed study recruitment tracking forms were used to document all contact attempts between the study investigators and each potential study site. These data were analyzed to characterize the overall recruitment and consent process for community pharmacies and pharmacy personnel (pharmacists, technicians).
Results
Achieving the target sample size of 64 study sites required contacting a total of 150 pharmacies (84 independent and 66 chain). Excluding 22 ineligible pharmacies, participation rates were 49% (32 of 65) for independent pharmacies and 51% (32 of 63) for chain pharmacies (50% overall). Across the 64 participating pharmacies, a total of 124 pharmacists (of 171; 73%) and 127 pharmacy technicians (of 215; 59%) were enrolled in the study. Pharmacies that chose not to participate most often cited time constraints as the primary reason. Overall, combining both the recruitment and consent process, a median of 5 contacts were made with each participating pharmacy (range, 2–19; IQR, 4–7), and the median overall duration of time elapsed from initial contact to consent was 25 days (range, 3–122 days; IQR, 12–47 days).
Conclusions
Results from this study suggest that pharmacy personnel are willing to provide brief tobacco cessation interventions in a community pharmacy setting and are receptive to participation in multi-site clinical research trials. However, execution of a representative sampling and recruitment scheme for a multi-state study in this practice setting is a time and labor intensive process.
Keywords: Tobacco, tobacco cessation, smoking, smoking cessation, pharmacist, community pharmacy, quitline, brief intervention
BACKGROUND
Smoking is the primary preventable cause of morbidity and mortality in the United States,1 resulting in enormous, yet avoidable, health care expenditures.2 Despite the fact that health professionals have a proven, positive impact on the tobacco use of their patients3 few clinicians routinely provide tobacco cessation counseling to patients, often citing lack of time as a key barrier to doing so.3,4 However, even the busiest of clinicians can serve an important role by simply identifying tobacco users and providing these patients with referrals to other smoking cessation resources, such as toll-free tobacco quitlines.4 Quitlines, which have been proven to be effective in helping patients achieve sustained abstinence5,6 are available to all Americans with telephone access. Unfortunately, many clinicians are unaware of quitlines as a resource for patients, and clinician referrals to quitlines are low.7,8
Community pharmacies, as a key interface between the health care system and the general public, have the potential to reduce the prevalence of tobacco use substantially—particularly among key population groups for which tobacco use is a major risk factor for development or exacerbation of diseases requiring prescription medications (e.g., cardiovascular disease, pulmonary disease, and diabetes. Pharmacies are easily accessed by all segments of the population including uninsured and underinsured individuals. While it is not always possible to provide comprehensive tobacco cessation counseling in community pharmacies,9 this setting might be ideal for widespread implementation of brief interventions, such as asking patients about tobacco use, advising tobacco users to quit, and referring patients to the tobacco quitline.
The Ask-Advise-Refer: Promoting Pharmacy-Based Referrals to Tobacco Quitlines study is a randomized trial comparing two intervention approaches to engage community pharmacies in providing patient referrals to state tobacco quitlines. Although there are numerous reports describing recruitment strategies used in randomized controlled trials,10 very limited published information is available to inform recruitment in community pharmacy settings.11–14 This paper describes the Ask-Advise-Refer recruitment strategies and an evaluation of the recruitment rates and reasons for non-participation in a large (n=64 pharmacies), multi-state randomized trial to generate patient referrals to tobacco quitlines.
METHODS
Overview of Study Design
The Ask-Advise-Refer study compares two intervention approaches to engage community pharmacy personnel in providing patient referrals to tobacco quitlines: academic detailing and mailed materials. Detailed information about the study design is published elsewhere.15 Study sites include 64 community pharmacies in Connecticut (n=32) and Washington (n=32). These two states were chosen because: (a) they are geographically disparate and offer rural, suburban, and urban locations, (b) the study team had established relationships with personnel at state professional associations, and (c) their quitlines are serviced by Alere Wellbeing (formerly known as Free & Clear and a partner in this study), which provides tobacco cessation counseling services to state residents by telephone and is contracted to provide these services for both states.
Identification of pharmacies
To identify participating pharmacies, a complete, current listing of licensed pharmacies (n=2,069) was obtained from the State Boards of Pharmacy in Connecticut (n=639) and Washington (n=1,430). From each state list, pharmacies that were not clearly community-based (e.g., hospital, mail order, or clinic-based pharmacies) were omitted by reviewing names and associated websites, yielding a pool of 1,678 community pharmacies. This remaining sample was stratified by pharmacy type (retail chain or independent), and their corresponding zip codes were linked to year 2000 census tract data based on racial/ethnic composition. Consistent with federal mandates for inclusion of minority populations, pharmacies were further stratified according to zip codes with at least one community-based pharmacy categorized as exhibiting a high proportion (i.e., within the upper 20th percentile, relative to the rest of the state) or a low proportion (i.e., within the lowest 20th percentile, relative to the rest of the state) of the following racial/ethnic groups: white, black, Hispanic, or Asian. A descriptive analysis was performed to cross-tabulate these categories (e.g., high vs. low white, high vs. low black, high vs. low Hispanic, high vs. low Asian) to determine associations between the ethnic distributions. In some zip codes, there was a high proportion (upper 20th percentile) of more than one minority group; in these instances, minority groups were combined into one sampling strata (e.g., Asian Black). After completing this process, four racial/ethnic groups were formed for CT and five groups were formed for WA, with deliberate over-sampling of pharmacies in zip codes with higher minority: in CT, White (n=12), Black-Hispanic-Asian (n=8), Black-Asian (n=8), and Asian (n=4); in WA, White (n=12), Hispanic (n=8), Black (n=4), Asian (n=4), and Asian-Black (n=4). The underlying rationale for this approach was to ensure selection of pharmacies from geographic areas that serve minority populations. It should be noted, however, that in cases for which a zip code was deemed to be high minority, the predominant group was nonetheless white in each case.
A random sample of pharmacies, stratified by type (chain or independent), was then generated for recruitment. A two-stage random selection process ensued, whereby (a) zip codes were selected at random, from within each racial/ethnic category, and (b) one pharmacy within each zip code was selected at random. To maximize geographic distribution and to minimize contamination across study sites, only one pharmacy was enrolled per zip code. If a pharmacy did not agree to participate, another pharmacy within the same zip code was randomly selected for recruitment. If no pharmacies located within the zip code chose to participate, an alternative zip code was randomly selected as a replacement. This process continued until all recruitment race/ethnicity categories were saturated.
Pharmacy Recruitment Process
The pharmacies selected for initial contact were recruited using a tiered approach with assistance from state pharmacy association partners. First, the chief executive officer of the state pharmacy association (or their designee) contacted the owner or pharmacy manager of selected pharmacies, notified them of their preliminary selection in the study, provided a brief overview of the study goals, and informed them that study personnel would contact them within 1–2 weeks to determine interest in participating.
Next, a pharmacist member of the study team (R.L.C., K.S.H., A.J.Z.) contacted each pharmacy via telephone, using the pharmacy manager (or designee) as the primary point of contact. Once the decision maker (usually the pharmacy owner or pharmacy manager) was reached, a brief overview of the study was provided along with a description of the intervention and expectations of time commitment of pharmacy personnel. Pharmacies were informed that the study was funded by the National Cancer Institute and approved by their State’s Department of Health. A 1-page description of the study was faxed or emailed to the primary contact. During this initial conversation, inclusion and exclusion criteria for participation were confirmed. Inclusion criteria required that each pharmacy: (a) have one or more full-time pharmacist(s) and technician(s) (if employed by the pharmacy) willing to participate; (b) have outgoing fax capability; (c) be accessible to study staff by automobile (thereby excluding island-based pharmacies in Washington State). For all participating pharmacies, one pharmacist was designated as the “primary contact” (even when multiple pharmacists participated). As such, there were no pharmacies with only technician participants and no sites where a technician was the primary contact.
Pharmacies that were not traditional community pharmacies were excluded, including mail-order or closed-door (e.g., not open to the public) pharmacies, specialty pharmacies (e.g., mental health, compounding only), and clinic-affiliated pharmacies located inside hospitals or medical buildings. The primary contact also was informed that pharmacists and technicians who participated in the program and completed 3 brief written surveys (at baseline and 3 and 6 months) would receive $80 in gift cards as compensation for their time.
Typically, the process to determine interest in participation and confirm inclusion/exclusion criteria occurred over a period of time whereby the pharmacy contact was presented with the information and given time to consider participation and discuss with their pharmacy staff (pharmacists and pharmacy technicians). Study investigators used a recruitment tracking form to capture all contacts and contact attempts with each pharmacy. This tracking form included the name, address, telephone and fax number of the pharmacy, the name of the primary contact, as well as the date, time, and comments related to each contact (or attempted contact if no one was reached). The form also collected data regarding inclusion and exclusion criteria, the number of pharmacists and technicians employed by the pharmacy and interested in participating, as well as any reasons for declining participation in the study (recruitment log is available from the study investigators).
With most chain pharmacies, additional approval from district, regional, and/or national representatives was necessary before the individual stores could agree to participate. These corporate approvals were obtained by either state association partners (who used existing personal contacts within the chain) or by the study investigators. When a blanket corporate approval was not readily obtainable from a key decision-maker, the approval process typically began with store managers of the randomly-selected store and proceeded up the chain of command. Each step involved the same process to identify and contact (via telephone) the appropriate individual, describe the study and time commitment of the pharmacy (or pharmacies), and obtain written approval.
During the initial contact process, if one pharmacy declined participation, another pharmacy within the same zip code from the randomly-ordered list was selected for initial contact. Similarly, if a chain corporation declined to participate, all subsequent pharmacies from that chain were removed from the list and were no longer eligible for additional contacts. With each decline, replacement and initial contact with the next randomly-selected pharmacy continued until the target sample size of 64 pharmacies agreed to participate.
Pharmacist and Technician Recruitment and Consent process
Once willingness and approval of individual pharmacists and technicians within the store to participate was confirmed, study staff began the informed consent process. A packet of materials containing a cover letter, study fact sheet, and an individualized informed consent document for each pharmacy staff member who expressed interest in participating was faxed (or emailed) to the primary contact. Follow-up contact between research staff and pharmacy staff occurred until all informed consents were received or a refusal to participate was received from an individual pharmacy staff member. All follow-up contacts were documented on the tracking form. During the informed consent phase, if a pharmacy decided not to participate, they were replaced with the next pharmacy on the randomly-ordered list, and the recruitment process was restarted by a pharmacist member of the study team. All study procedures were approved by Purdue University Institutional Review Board.
Analysis of recruitment process
Data analysis included frequencies and summary statistics to characterize the recruitment and consent process. Categorical comparisons were performed using chi-squared statistics. Analyses were conducted using SPSS Version 18.0 (Chicago, IL).
RESULTS
Community pharmacy study sites
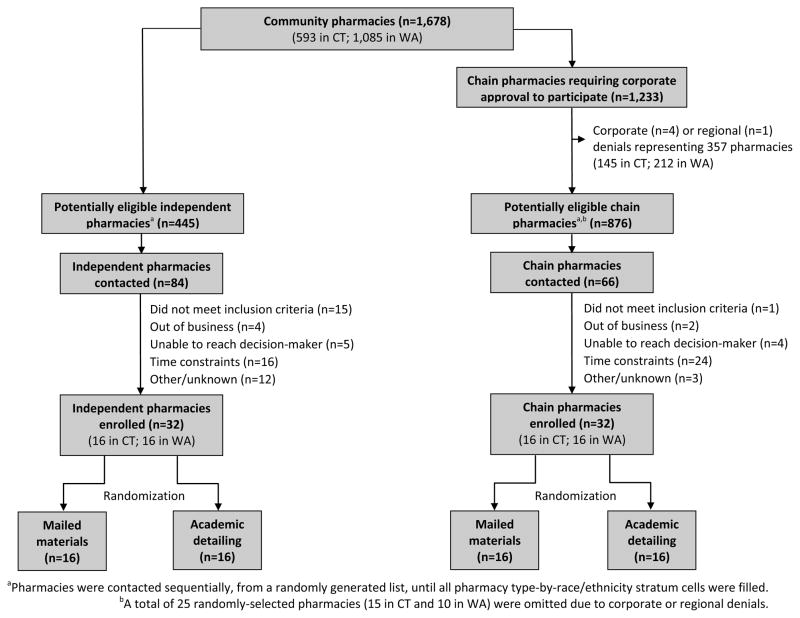
Of 1,678 potential study sites in Connecticut and Washington, 445 were independent pharmacies and 876 were chain pharmacies (Figure 1). During the recruitment period, 16 chain corporations were approached, and 12 (75%) agreed to participate, however one chain that had received corporate approval declined participation regionally, in Connecticut, but participated in Washington. The chains that declined approval either corporately or regionally represented a total of 357 potentially eligible pharmacies, of which 25 had been randomly selected for recruitment according to the study sampling strategy.
Figure 1.
Recruitment and enrollment of participating pharmacies.
Achieving a target sample size of 64 study sites (32 in each state; Figure 2) required contacting a total of 150 pharmacies (84 independent and 66 chain); of these, 16 did not meet the inclusion criteria and 6 were no longer in business. Excluding these 22 ineligible pharmacies, participation rates were 49% (32 of 65) for independent pharmacies and 51% (32 of 63) for chain pharmacies (50% overall).
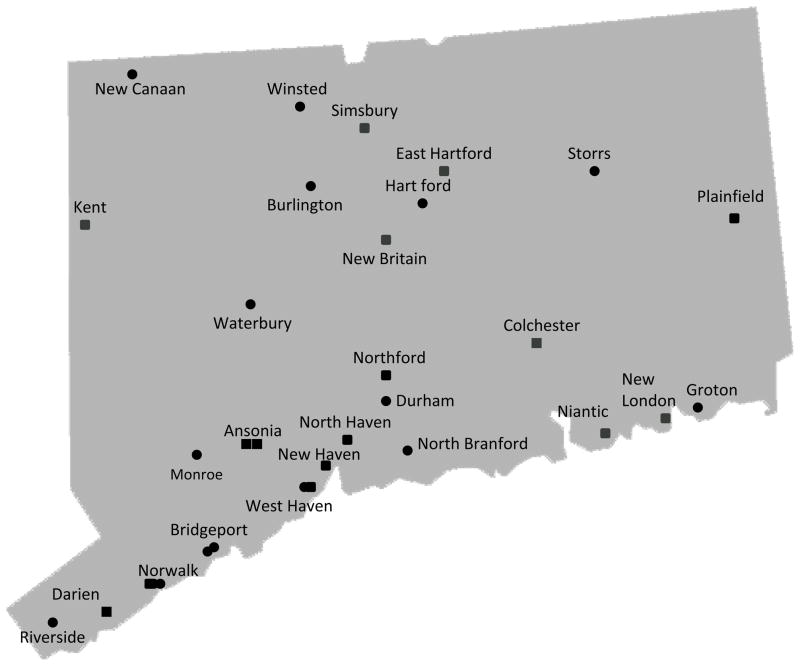
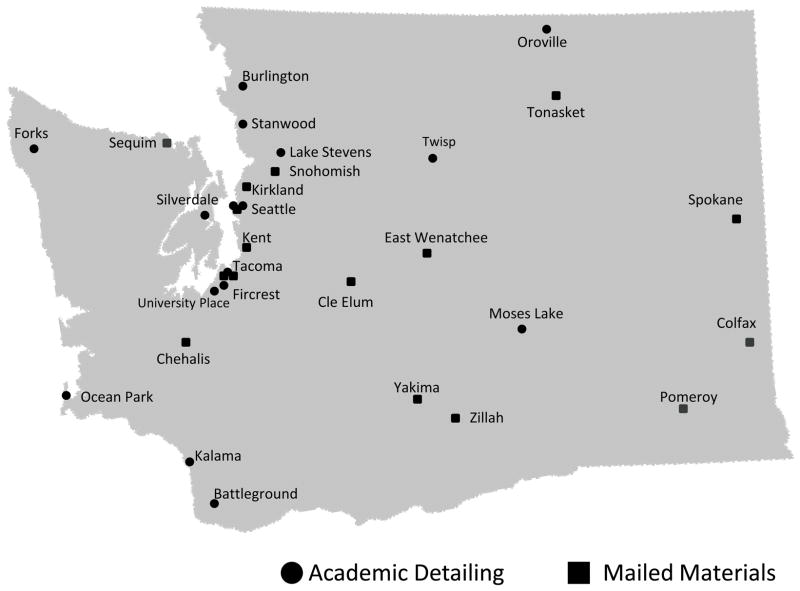
Figure 2.
Distribution of participating community pharmacies in Connecticut (n=32) and Washington State (n=32).
Participation rates were comparable between states (49% in CT and 51% in WA; p > 0.05). In WA, the participation rates by racial/ethnic census category ranged from 27% in the Asian-Black zip codes to 100% in the Black zip codes. In CT, the participation rates by racial/ethnic census category ranged from 47% in Black-Hispanic-Asian zip codes to 50% in Black-Hispanic zip codes and in Asian zip codes.
State association partners made the initial telephone contact with 27 independent pharmacies that had been selected at random for study participation. The recruitment success rate associated with independently-owned pharmacies first contacted by state association partners was 67%, compared with a 42% success rate for independent pharmacies that received an initial contact by one of the pharmacy faculty investigators (χ2, 1df = 3.51; p=0.06).
Pharmacies that chose not to participate (i.e., not including those determined to be ineligible or those for which the decision-maker was unable to be reached) most often cited time constraints as the primary reason (73% of 55 pharmacies).
Characterization of the recruitment and consent process
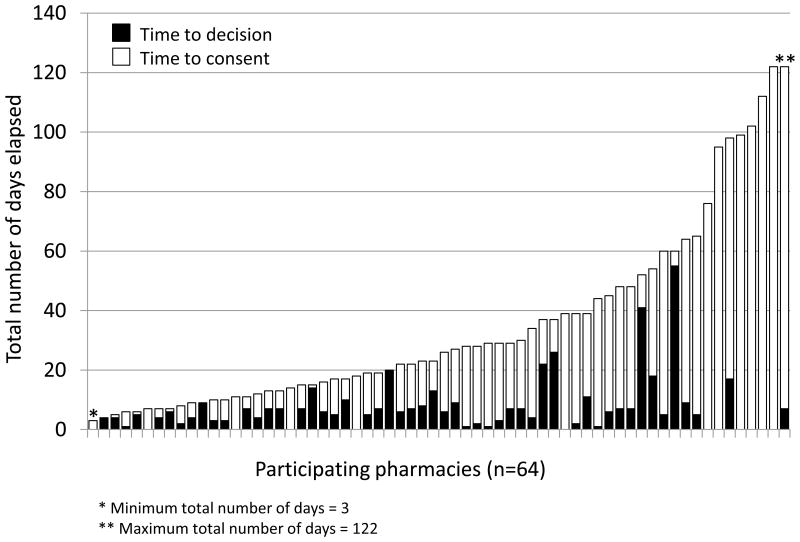
The median number of contacts (telephone, facsimile, e-mail) made by the study team from the initial contact until a participation decision was rendered was 3 (range, 1–15; IQR, 1.5–4.0). Among the 55 pharmacies that elected not to participate, 21 (38%) declined upon first contact; for all other non-participating pharmacies, the median overall duration of time elapsed between first contact and decision to decline was 6.5 days (range, 1–95 days; IQR, 3–13.5 days). Among pharmacies that agreed to participate, 12 (19%) agreed upon first contact; for all other participating pharmacies, the median overall duration of time elapsed between first contact and decision to participate was 6.5 days (range, 1–55 days; IQR, 4–9.0 days) (Figure 3).
Figure 3.
Time to decision and consent (in days) for participating pharmacies.
Immediately following a verbal commitment to participate, the consent process was initiated. The median number of days required to obtain consent forms from participating pharmacists and technicians was 15 days (range, 0–122 days; IQR, 6–39 days). During this time, one or more consent forms were sent to stores via facsimile or email a median of 2 times (range, 1–5 times; IQR, 1–2 times). Overall, combining both the recruitment and consent process, a median of 5 contacts were made with each participating pharmacy (range, 2–19; IQR, 4–7) and the median overall duration of time elapsed from initial contact to consent was 25 days (range, 3–122 days; IQR, 12–47 days). Across the 64 participating pharmacies, a total of 124 pharmacists (of 171; 73%) and 127 pharmacy technicians (of 215; 59%) enrolled in the study. There were no differences in median time to decision, time to consent, or overall duration of time elapsed from initial contact to consent for chain versus independent pharmacies.
DISCUSSION
Community pharmacy participation in translational research efforts is necessary to demonstrate the value of pharmacy services in the healthcare delivery system. To determine the feasibility and effectiveness of community pharmacy-based interventions, the engagement and participation of pharmacy personnel practicing in ‘real-world’ community settings is imperative. While the importance of effectiveness trials in health services research is appreciated, there are few reports evaluating the recruitment of community-based clinicians for these studies16,17 and studies within pharmacy-based research are rarely published.11
This report provides important information to achieve success in engaging community pharmacies in research efforts. First, identification of community pharmacies can be challenging. To identify the community pharmacy study sites, contact lists of all registered pharmacies were obtained from the Connecticut and Washington State Boards of Pharmacy. Within these states (and many other states), there is no specific identifier to designate a community pharmacy versus a hospital or clinic-based pharmacy. As a result, study investigators manually reviewed more than 2,000 listings, noting which facilities were unlikely to be a community pharmacy based on the name of the licensee (e.g., St. Francis Hospital Pharmacy). In cases where the name did not readily identify the type of pharmacy location, investigators used information from internet websites (cross checking the names, addresses, telephone numbers of these facilities) to categorize the pharmacy location. This process was a time consuming, yet necessary initial step to simply identify “potentially eligible traditional community pharmacies.”
Recruitment and consent of pharmacy personnel was another labor-intensive and time-consuming process requiring a median of 5 contacts over a median of 27 days. For independently owned community pharmacies, the process was generally straightforward and involved a call to the pharmacist-in-charge (who was often also the pharmacy owner/manager). In contrast, commitment from chain pharmacies was a more complicated process. The study recruitment approach (e.g., random sampling with replacement) made it impossible to know a priori which chains would be randomly selected for participation. Therefore corporate approval was not sought for all chain pharmacies operating in the states of CT and WA prior to contacting the individual stores. Instead, selected large national/regional chains (n=5) that were likely to be randomly selected were targeted based on market penetration within each state.
Other strategies that appeared to enhance study site recruitment included (a) endorsement and initial contact by state professional association partners, (b) rapid recruitment contact by pharmacist members of the study team who were supported by study staff dedicated to the consent process, and (c) modest financial incentives for participating pharmacy personnel. Several of these approaches, including direct recruitment of clinicians by clinicians,18–20 endorsement by professional organizations20 and incentives that recognize the value of clinicians’ time,17 have been found to be effective in recruiting physicians for community-based research studies. Furthermore, it is possible that pharmacy personnel perceived the Ask-Advise-Refer study to be highly relevant and the interventions applicable to their practice setting. Indeed, during the recruitment process, many of the pharmacists expressed interest in the study because they believed that brief tobacco cessation interventions had a significant potential to improve patient care.
The most frequently specified reason for declining to participate in the study reflected time constraints associated with the busy practice environment in many community pharmacies. Despite the brief nature of the study intervention, 73% of the pharmacists who declined study participation cited lack of’ time ‘(e.g., heavy prescription volume, other competing priorities such as seasonal influenza outbreaks and immunization campaigns, and concerns about workflow disruption) as the primary reason for declining study participation. These findings are consistent with the experience of other investigators in community pharmacy-based research.12–14
This is the first report to characterize the effort required to (a) recruit pharmacies and (b) consent pharmacy personnel for a community pharmacy-based, randomized trial. Achieving a sample size of 64 pharmacies, using a complex stratified random sampling scheme, was a lengthy (overall, approximately 6 months), time-intensive, and costly process. Strengths of this study design include the potential for broad scale dissemination of the findings given the various types of community pharmacies participating in this study, including independent and chain pharmacies as well as grocery and mass merchant stores with pharmacies. The study’s sampling method attempted to ensure representativeness of pharmacies in racially/ethnically diverse locations, which is crucial for generalizing study findings to other geographic regions with similar characteristics.
The study site recruitment strategies employed in the Ask-Advise-Refer study yielded a 50% participation rate in a randomized trial to promote community-pharmacy based patient referrals to tobacco quitlines. This recruitment rate is comparable to the 55.7% rate observed by McIntosh and colleagues19 in an adolescent smoking cessation trial in community-based physician practices and higher than the 24% rate obtained by Cockerill and colleagues,11 who used a combined mail/facsimile approach to recruit pharmacies to a study providing emergency contraception in a community setting. One potential strategy for streamlining identification and recruitment of community pharmacies is to draw sites from a practice-based research network (PBRN). PBRNs, which bring together a network of health professionals and practice sites to participate in improving patient outcomes through research and practice improvement, are common in primary care and ambulatory care settings.21,22 Several pharmacy-based PBRNs currently exist or are in the early phases of development.23–25 While these networks provide pharmacies with a mechanism to collaborate and likely lead to reduced recruitment costs, they typically are not conducive to sampling strategies that aim to achieve representativeness of patient or pharmacy populations, such as the stratified random sampling strategy applied here.
In summary, although the recruitment phase was time-intensive and costly, each step was necessary to yield a viable and representative sample of pharmacies. Results of this study suggest that pharmacists and pharmacy technicians are receptive to the idea of providing brief tobacco cessation interventions in a community pharmacy setting and to participation in multi-site clinical research trials. If the results of this study are positive, the potential for broad scale dissemination of this approach within community pharmacy settings is substantial and could significantly increase rates of smoking cessation nationwide.
Acknowledgments
The authors thank the Connecticut State Department of Public Health and Washington State Department of Health for their support of the study and provision of service to pharmacy patients referred to the quitline. Andy Schmelz, PharmD assisted with development of the recruitment protocol. Funded by the National Cancer Institute, grant R01 CA 129312 to K Hudmon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.CDC. Smoking-attributable mortality, years of potential life lost, and productivity losses -- United States, 2000–2004. MMWR. 2008;57:1226–1228. [PubMed] [Google Scholar]
- 3.Fiore MC, Jaén CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- 4.Schroeder SA. What to do with a patient who smokes. JAMA. 2005;294:482–487. doi: 10.1001/jama.294.4.482. [DOI] [PubMed] [Google Scholar]
- 5.Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2006;3:CD002850. doi: 10.1002/14651858.CD002850.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Zhu SH, Anderson CM, Tedeschi GJ, et al. Evidence of real-world effectiveness of a telephone quitline for smokers. N Engl J Med. 2002;347:1087–1093. doi: 10.1056/NEJMsa020660. [DOI] [PubMed] [Google Scholar]
- 7.Wadland WC, Holtrop JS, Weismantel D, Pathak PK, Fadel H, Powell J. Practice-based referrals to a tobacco cessation quit line: assessing the impact of comparative feedback vs general reminders. Ann Fam Med. 2007;5:135–142. doi: 10.1370/afm.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Association of American Medical Colleges. A report prepared for the American Legacy Foundation. Washington, DC: 2007. [Accessed June 9, 2012]. Physician behavior and practice patterns related to smoking cessation. Available at: https://www.aamc.org/download/55438/data/smokingcessationsummary.pdf. [Google Scholar]
- 9.Prokhorov AV, Hudmon KS, Marani S, et al. Engaging physicians and pharmacists in providing smoking cessation counseling. Arch Intern Med. 2010;170:1640–1646. doi: 10.1001/archinternmed.2010.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treweek S, Pitkethly M, Cook J, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev. 2010;4:MR000013. doi: 10.1002/14651858.MR000013.pub5. [DOI] [PubMed] [Google Scholar]
- 11.Cockerill R, Cohen M, Dunn S, Brown T. Recruitment strategies. Pharmacists’ participation in an evaluation project to dispense emergency contraception. Eval Health Prof. 2004;27:70–79. doi: 10.1177/0163278703261202. [DOI] [PubMed] [Google Scholar]
- 12.Saini B, Brillant M, Filipovska J, et al. Factors influencing Austrailian community pharmacists’ willingness to participate in research projects-an exploratory study. International Journal of Pharmacy Practice. 2007;14:179–188. [Google Scholar]
- 13.Armour C, Brillant M, Krass I. Pharmacists’ views on involvement in pharmacy practice research: strategies for facilitating participation. Pharmacy Practice. 2007;5:59–66. doi: 10.4321/s1886-36552007000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson SH, Johnson JA, Biggs C, et al. Practice-based research: lessons from community pharmacist participants. Pharmacotherapy. 2001;21:731–739. doi: 10.1592/phco.21.7.731.34570. [DOI] [PubMed] [Google Scholar]
- 15.Zillich AJ, Corelli RL, Zbikowski SM, et al. A randomized trial evaluating 2 approaches for promoting pharmacy-based referrals to the tobacco quitline: Methods and baseline findings. Res Social Adm Pharm. 2012 doi: 10.1016/j.sapharm.2012.03.001. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52:1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 17.Asch S, Connor SE, Hamilton EG, Fox SA. Problems in recruiting community-based physicians for health services research. J Gen Intern Med. 2000;15:591–599. doi: 10.1046/j.1525-1497.2000.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kottke TE, Solberg LI, Conn S, et al. A comparison of two methods to recruit physicians to deliver smoking cessation interventions. Arch Intern Med. 1990;150:1477–1481. [PubMed] [Google Scholar]
- 19.McIntosh S, Ossip-Klein DJ, Hazel-Fernandez L, Spada J, McDonald PW, Klein JD. Recruitment of physician offices for an office-based adolescent smoking cessation study. Nicotine Tob Res. 2005;7:405–412. doi: 10.1080/14622200500125567. [DOI] [PubMed] [Google Scholar]
- 20.Ellis SD, Bertoni AG, Bonds DE, et al. Value of recruitment strategies used in a primary care practice-based trial. Contemp Clin Trials. 2007;28:258–267. doi: 10.1016/j.cct.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindbloom EJ, Ewigman BG, Hickner JM. Practice-based research networks: the laboratories of primary care research. Med Care. 2004;42(4 Suppl):45–49. [PubMed] [Google Scholar]
- 22.Green LA, Hickner J. A short history of primary care practice-based research networks: from concept to essential research laboratories. J Am Board Fam Med. 2006;19:1–10. doi: 10.3122/jabfm.19.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Carr MB, Divine H, Hanna C, Freeman PR, Blumenschein K. Independent community pharmacist interest in participating in community pharmacy research networks. J Am Pharm Assoc. 2011;51:727–733. doi: 10.1331/JAPhA.2011.10099. [DOI] [PubMed] [Google Scholar]
- 24.Marinac JS, Kuo GM. Characterizing the American college of clinical pharmacy practice-based research network. Pharmacotherapy. 2010;30:865. doi: 10.1592/phco.30.8.865. [DOI] [PubMed] [Google Scholar]
- 25.Schommer JC. Establishing pharmacist practice-based research networks. [Accessed June 9, 2012];APhA Foundation White Paper. 2010 May; Available at: http://www.pharmacist.com/AM/Template.cfm?Section=Professional_Advancement&Template=/CM/ContentDisplay.cfm&ContentID=23805.