Abstract
Objective To assess the main risk factors associated with stillbirth in a multiethnic English maternity population.
Design Cohort study.
Setting National Health Service region in England.
Population 92 218 normally formed singletons including 389 stillbirths from 24 weeks of gestation, delivered during 2009-11.
Main outcome measure Risk of stillbirth.
Results Multivariable analysis identified a significant risk of stillbirth for parity (para 0 and para ≥3), ethnicity (African, African-Caribbean, Indian, and Pakistani), maternal obesity (body mass index ≥30), smoking, pre-existing diabetes, and history of mental health problems, antepartum haemorrhage, and fetal growth restriction (birth weight below 10th customised birthweight centile). As potentially modifiable risk factors, maternal obesity, smoking in pregnancy, and fetal growth restriction together accounted for 56.1% of the stillbirths. Presence of fetal growth restriction constituted the highest risk, and this applied to pregnancies where mothers did not smoke (adjusted relative risk 7.8, 95% confidence interval 6.6 to 10.9), did smoke (5.7, 3.6 to 10.9), and were exposed to passive smoke only (10.0, 6.6 to 15.8). Fetal growth restriction also had the largest population attributable risk for stillbirth and was fivefold greater if it was not detected antenatally than when it was (32.0% v 6.2%). In total, 195 of the 389 stillbirths in this cohort had fetal growth restriction, but in 160 (82%) it had not been detected antenatally. Antenatal recognition of fetal growth restriction resulted in delivery 10 days earlier than when it was not detected: median 270 (interquartile range 261-279) days v 280 (interquartile range 273-287) days. The overall stillbirth rate (per 1000 births) was 4.2, but only 2.4 in pregnancies without fetal growth restriction, increasing to 9.7 with antenatally detected fetal growth restriction and 19.8 when it was not detected.
Conclusion Most normally formed singleton stillbirths are potentially avoidable. The single largest risk factor is unrecognised fetal growth restriction, and preventive strategies need to focus on improving antenatal detection.
Introduction
Stillbirths represent a devastating pregnancy outcome, and the need for increased efforts in prevention has been highlighted by SANDS (Stillbirth and Neonatal Death Charity)1 and other parent led campaigns. Stillbirth rates in the United Kingdom are among the highest in high income countries2 and have shown little improvement since the early 1990s.3
Despite free availability of postmortem investigations in the English National Health Service as many as 50-70% of stillbirths have until recently been categorised as unclassified or unexplained4 and are, by implication, often considered unavoidable.5 Further investigation found that many stillborn fetuses had failed to reach their growth potential,6 7 8 and inclusion of fetal growth restriction as a category in stillbirth classifications resulted in a substantial drop to around 15% in the proportion of cases considered unexplained.8 9 This encouraged a renewed focus on understanding the underlying, mostly placental disease related, causes of fetal growth restriction. Recent reports of laboratory investigations10 and postmortem studies11 have confirmed the large contribution of placental failure in the cause of stillbirth.
The clinical relevance of such findings is that although there are limited means by which to treat fetal growth restriction in utero, recognition that the fetus is at risk after appropriate maternal-fetal investigations can lead to well timed delivery and improved perinatal outcome.12 13 However, there are currently no established and routinely used means to predict stillbirth, and risk factors known at the beginning of pregnancy are considered weak predictors of outcome.14
We investigated the role of demographic, social, and medical risk factors that can be ascertained at the beginning of pregnancy together with those that become apparent as pregnancy progresses, and their respective contributions to the incidence of stillbirths in an NHS region in England with a multiethnic population.15 To focus on potentially avoidable factors we excluded congenital anomalies, as their contribution to stillbirth is contingent on incidence as well as cultural choices on antenatal screening, prenatal diagnosis, and decisions in response to positive results. We therefore explored the risk factors in pregnancies with normally formed singletons and estimated the respective contribution of these factors to the overall burden of stillbirth in our population.
Methods
Maternity data
The database was derived from the regional, NHSnet based perinatal episode electronic record (PEER) hosted and managed by the West Midlands Perinatal Institute.16 This electronic record was first implemented in April 2009 in the 19 maternity units in the West Midlands. For this study we used the data collected over a two year period, representing births between June 2009 and May 2011.
The data originated from prospective records created in the standardised hand held maternity notes during pregnancy by the midwives and doctors.17 Trained data clerks in the respective hospitals transfer information from the notes on to the perinatal episode electronic record at the end of pregnancy. Quality was assured by central project staff through training workshops and regular on-site data quality audits. The dataset contained 87 regionally agreed and defined data items,18 with information about maternal characteristics, including age, parity, ethnic origin, and maternal height and weight (expressed as body mass index); social factors, including employment status of the mother and her partner, consanguinity with the partner, and index of multiple deprivation; history of mental health problems, pre-existing diabetes or hypertensive disease, or previous stillbirth; smoking status, alcohol consumption, non-prescription drugs, folic acid intake, and time of first visit in pregnancy (the information for all these previous variables was usually recorded at the early pregnancy booking visit); complications in pregnancy, including gestational diabetes, antepartum haemorrhage, pregnancy induced hypertension, and pre-eclampsia (defined as pregnancy induced hypertension with proteinuria); and fetal or neonatal characteristics, including sex, gestational age and weight at birth, and estimated weight during pregnancy. Gestational age was determined on the basis of routinely offered dating scans, which were carried out in the first or second trimester (<22 weeks) in 96.5% of pregnancies in this population, with the remaining pregnancies dated by the last menstrual period.
The presence of intrauterine growth restriction was established on the basis of a birth weight below the 10th weight for gestational age centile, using the gestation related optimal weight standard (GROW),19 with coefficients derived from the West Midlands population. This method defines the fetal growth potential by excluding pathological factors such as smoking and diabetes, and individual adjustment or “customisation” for the baby’s sex and the mother’s height, weight, ethnic origin, and parity.20 21 A weight that is small for gestational age after such adjustment by growth potential has been shown to represent pathological smallness22 and is referred to as fetal growth restriction. We applied the 10th centile as the cut-off, as it is in standard clinical use and has been validated through receiver operator curves as being close to optimal for predicting pathology by customised centiles.23
The case notes were also examined for any recording of fetal growth problems. Fetal growth surveillance is usually done by a protocol of measuring fundal height at each antenatal visit in the third trimester, with referral for ultrasound assessment of estimated fetal weight when the serially plotted fundal height measurements do not follow the predicted curve. In pregnancies where assessment of fundal height is difficult (for example, maternal obesity) or where the risk of fetal growth restriction is considered increased (for example, due to obstetric history) serial estimated fetal weight measurements are done according to local protocols of varying scan frequency. Antenatal recognition of fetal growth restriction in pregnancies with birth weights below the 10th customised centile was defined as at least one antenatal entry with this diagnosis, usually based on one or more estimated fetal weights below the 10th customised centile, or an abnormal umbilical artery Doppler, or both. This assessment of the maternity record is part of routine ascertainment, as the proportion of pregnancies with antenatally detected fetal growth restriction is a regionally agreed key performance indicator.
Linkage to mortality data
Data on fetal, neonatal, and infant deaths and congenital anomalies were notified to the Perinatal Institute by a network of coordinators, and had a consistently high level of ascertainment. The information is held on secure NHS servers and is pseudonymised before any further analysis. Data linkage was established with the electronic maternity database, using the NHS number as unique identifier.
Stillbirth was defined as a child born after the 24th week of pregnancy who did not, at any time after delivery, breathe or show any other signs of life. Along the lines of previously applied methods,6 we adjusted the gestational age of each stillborn fetus by deducting two days from the length of pregnancy at delivery, to correct for the estimated 48 hour average delay between intrauterine death and delivery.24
Confidentiality and consent
Maternal consent was obtained by provision of information and opt-out. Mothers are informed at their first antenatal visit about the intention of collection and analysis of secondary data, and this is also explained in text printed in their standardised, hand held pregnancy notes. That this information has been given and explained is recorded in the notes and signed by the midwife. A mother can opt out at any time from her data being used, in which case her NHS number is added to an “opt-out register” and further analysis is blocked. The Perinatal Institute’s confidentiality and consent protocol has been reviewed and passed as appropriate by UK Connecting for Health, the NHS, and the Information Commissioner.
Statistical analyses
After initial exploratory analysis, we assessed the independent and multiple variable effects of explanatory variables on stillbirths in Poisson regression models. Variables entered in the multivariable analysis are those of known clinical relevance and from previous publications2 14 and included maternal age, parity, ethnic origin, place of birth, body mass index, history of mental health problems, pre-existing hypertension, pre-existing diabetes, cardiac disease, previous stillbirths, smoking in pregnancy, alcohol consumption, antenatal folic acid intake, late booking (≥13 weeks), gestational diabetes, pregnancy induced hypertension, pre-eclampsia, antepartum haemorrhage, and fetal growth restriction. In addition to the index of multiple deprivation, we included maternal and paternal employment status as social factors in the multivariable analysis. We entered variables using the manual stepwise (forward-backward) method. To reduce the over-reliance in the estimates and the selection mechanism that may arise due to rarity of stillbirths, we used the bootstrapping approach to calculate standard errors. In the multivariable analysis all variables reaching a 0.05 significance level were retained in the model. We also considered all two factor interactions between the explanatory variables and used empirical probability plots to check the final model to assess whether the modelling assumptions were met. We carried out sensitivity analyses to assess the influence of factors excluded because of P>0.05, potential clustering by maternity unit, and the effect of repeat pregnancies from the same mother.
To assess the proportion of stillbirths that could be potentially prevented if risk factors were removed, we calculated adjusted population attributable risk estimates using standard methods25: population attributable risk=expected number of cases in the population−expected number of cases if nobody in the population has the risk factor of interest/expected number of cases in the population ×100
The analyses were done using statistical package STATA version 11.
Results
A total of 105 476 cases were entered during the two year collection period. Of these, 13 258 were excluded because of congenital anomaly or multiple pregnancy, which left 92 218 normally formed singleton pregnancies leading to 91 829 live births and 389 stillbirths. This represented a stillbirth rate of 4.2/1000 births and compares with nationally reported stillbirth rates of normally formed singletons of 3.9-4.1/1000 over the same period.26 The analysis included 841 (0.9%) repeat pregnancies during the two year period of the 92 218 mothers in the cohort.
Univariate analysis
Table 1 lists the variables, grouped according to maternal and fetal characteristics, social factors, medical history, and complications during pregnancy. Analysis was for complete cases only. The stillbirth rate is presented for subgroups, together with relative risks and 95% confidence intervals in relation to the respective reference values.
Table 1.
Univariate analysis of risk factors associated with stillbirths compared with live births
| Risk factors | No (%) of all births | No (%) of stillbirths | Rate/1000 births | Relative risk (95% CI) |
|---|---|---|---|---|
| Total | 92 218 (100) | 389 (100) | 4.2 | — |
| General maternal characteristics | ||||
| Age (years): | n=92 208 | n=389 | ||
| <20 | 6456 (7.0) | 33 (8.5) | 5.1 | 1.3 (0.9 to 1.9) |
| 20-24 | 20 834 (22.6) | 99 (25.5) | 4.8 | 1.2 (0.9 to 1.6) |
| 25-30 | 27 110 (29.4) | 107 (27.5) | 3.9 | Reference |
| 30-34 | 23 110 (25.1) | 79 (20.3) | 3.4 | 0.9 (0.6 to 1.2) |
| ≥35 | 14 698 (15.9) | 71 (18.3) | 4.8 | 1.2 (0.9 to 1.6) |
| Parity: | n=91 160 | n=377 | ||
| 0 | 38 653 (42.4) | 192 (50.9) | 5.0 | 1.9 (1.4 to 2.4) |
| 1 | 29 791 (32.7) | 79 (21.0) | 2.7 | Reference |
| 2 | 13 421 (14.7) | 56 (14.9) | 4.2 | 1.6 (1.1 to 2.2) |
| ≥3 | 9295 (10.2) | 50 (13.3) | 5.4 | 2.0 (1.4 to 2.9) |
| Body mass index: | n=90 350 | n=389 | ||
| <18.5 | 3109 (3.4) | 12 (3.1) | 3.9 | 1.0 (0.5 to 1.8) |
| 18.5-24.9 | 43 898 (48.6) | 173 (44.5) | 3.9 | Reference |
| 25-29.9 | 25 156 (27.8) | 101 (26.0) | 4.0 | 1.0 (0.8 to 1.3) |
| 30-34.9 | 11 427 (12.6) | 61 (15.7) | 5.3 | 1.4 (1.0 to 1.8) |
| ≥35 | 6760 (7.5) | 42 (10.8) | 6.2 | 1.6 (1.1 to 2.2) |
| Maternal ethnic origin: | n=87 911 | n=334 | ||
| European: | ||||
| UK | 60 130 (67.9) | 192 (57.5) | 3.2 | Reference |
| Non-UK | 4254 (4.8) | 17 (5.1) | 4.0 | 1.3 (0.8 to 2.1) |
| African* | 2986 (3.2) | 22 (5.7) | 7.4 | 2.3 (1.5 to 3.6) |
| African-Caribbean* | 1796 (2.0) | 12 (3.1) | 6.7 | 2.1 (1.2 to 3.7) |
| Bangladeshi* | 1674 (1.8) | 7 (1.8) | 4.2 | 1.3 (0.6 to 2.8) |
| Indian: | ||||
| UK | 2282 (2.6) | 9 (2.7) | 3.9 | 1.2 (0.6 to 2.4) |
| Non-UK | 2189 (2.5) | 14 (4.2) | 6.4 | 2.0 (1.2 to 3.4) |
| Pakistani: | ||||
| UK | 3412 (3.9) | 14 (4.2) | 4.1 | 1.3 (0.7 to 2.2) |
| Non-UK | 4378 (4.9) | 30 (9.0) | 6.9 | 2.1 (1.5 to 3.1) |
| Other: | ||||
| UK | 1535 (1.7) | 6 (1.8) | 3.9 | 1.2 (0.5 to 2.8) |
| Non-UK | 3275 (3.7) | 11 (3.3) | 3.4 | 1.1 (0.6 to 1.9) |
| Place of birth: | n=88 559 | n=334 | ||
| UK | 69 878 (78.9) | 236 (70.7) | 3.4 | Reference |
| Other | 18 681 (21.1) | 98 (29.3) | 5.2 | 1.6 (1.3 to 2.1) |
| Social factors | ||||
| Mother not employed: | n=86 969 | n=330 | ||
| No | 50 905 (58.5) | 176 (53.3) | 3.5 | Reference |
| Yes | 36 064 (41.5) | 154 (46.7) | 4.3 | 1.2 (1.0 to 1.5) |
| Partner not employed: | n=81 624 | n=310 | ||
| No | 63 212 (77.4) | 230 (74.2) | 3.6 | Reference |
| Yes | 11 950 (14.6) | 58 (18.7) | 4.9 | 1.3 (1.0 to 1.8) |
| No partner | 6462 (7.9) | 22 (7.1) | 3.4 | 0.9 (0.6 to 1.4) |
| Baby’s father blood relation: | n=81 627 | n=298 | ||
| No | 76 084 (93.2) | 273 (91.6) | 3.6 | Reference |
| Yes | 5543 (6.8) | 25 (8.4) | 4.5 | 1.3 (0.8 to 1.9) |
| Index of multiple deprivation (fifths): | n=92 218 | n=389 | ||
| 1-3 (least deprived) | 34 863 (37.8) | 115 (29.6) | 3.3 | Reference |
| 4 | 19 230 (20.9) | 69 (17.7) | 3.6 | 1.1 (0.8 to 1.5) |
| 5 (most deprived) | 38 125 (41.3) | 205 (52.7) | 5.4 | 1.6 (1.3 to 2.0) |
| Maternal history | ||||
| Mental health problems: | n=89 985 | n=340 | ||
| No | 79 553 (88.4) | 285 (83.8) | 3.6 | Reference |
| Yes | 10 432 (11.6) | 55 (16.2) | 5.3 | 1.5 (1.1 to 2.0) |
| Pre-existing hypertension: | n=90 965 | n=342 | ||
| No | 88 528 (97.3) | 329 (96.2) | 3.7 | Reference |
| Yes | 2437 (2.7) | 13 (3.8) | 5.3 | 1.4 (0.8 to 2.5) |
| Pre-existing diabetes: | n=90 965 | n=342 | ||
| No | 90 238 (99.2) | 332 (97.1) | 3.7 | Reference |
| Yes | 727 (0.8) | 10 (2.9) | 13.8 | 3.7 (2.0 to 6.9) |
| Cardiac disease: | n=90 965 | n=342 | ||
| No | 89 051 (97.9) | 331 (96.8) | 3.7 | Reference |
| Yes | 1914 (2.1) | 11 (3.2) | 5.7 | 1.5 (0.8 to 2.8) |
| Previous stillbirth para ≥1 (n=53 565): | n=52 475 | n=184 | ||
| No | 51 482 (98.1) | 173 (94.0) | 3.4 | Reference |
| Yes | 993 (1.9) | 11 (6.0) | 11.1 | 3.3 (1.8 to 6.0) |
| Pregnancy related factors | ||||
| Smoking in pregnancy: | n=85 337 | n=333 | ||
| Non-smoker | 52 473 (61.5) | 166 (49.8) | 3.2 | Reference |
| Smoker | 17 834 (20.9) | 104 (31.2) | 5.8 | 1.8 (1.4 to 2.3) |
| Passive smoker | 15 030 (17.6) | 63 (18.9) | 4.2 | 1.3 (1.0 to 1.8) |
| Alcohol consumption: | n=88 569 | n=329 | ||
| No | 81 288 (91.8) | 305 (92.7) | 3.8 | Reference |
| Yes | 7281 (8.2) | 24 (7.3) | 3.3 | 0.9 (0.6 to 1.3) |
| Antenatal folic acid: | n=88 173 | n=326 | ||
| Yes | 74 526 (84.5) | 262 (80.4) | 3.5 | Reference |
| No | 13 647 (15.5) | 64 (19.6) | 4.7 | 1.3 (1.0 to 1.8) |
| Late booking (≥13 weeks): | n=89 886 | n=338 | ||
| No | 74 644 (83.0) | 269 (79.6) | 3.6 | Reference |
| Yes | 15 242 (17.0) | 69 (20.4) | 4.5 | 1.3 (1.0 to 1.6) |
| Complications in pregnancy | ||||
| Gestational diabetes: | n=91 010 | n=339 | ||
| No | 88 134 (96.8) | 326 (96.2) | 3.7 | Reference |
| Yes | 2876 (3.2) | 13 (3.8) | 4.5 | 1.2 (0.7 to 2.1) |
| Pregnancy induced hypertension: | n=91 010 | n=339 | ||
| No | 86 640 (95.2) | 322 (95.0) | 3.7 | Reference |
| Yes | 4370 (4.8) | 17 (5.0) | 3.9 | 1.0 (0.6 to 1.7) |
| Pre-eclampsia: | n=91 010 | n=339 | ||
| No | 89 938 (98.8) | 328 (96.8) | 3.6 | Reference |
| Yes | 1072 (1.2) | 11 (3.2) | 10.3 | 2.8 (1.5 to 5.1) |
| Antepartum haemorrhage: | n=91 010 | n=339 | ||
| No | 83 902 (92.2) | 277 (81.7) | 3.3 | Reference |
| Yes | 7108 (7.8) | 62 (18.3) | 8.7 | 2.6 (2.0 to 3.5) |
| Fetal/neonatal characteristics | ||||
| Sex: | n=92 136 | n=383 | ||
| Boy | 47 308 (51.3) | 209 (54.6) | 4.4 | Reference |
| Girl | 44 828 (48.7) | 174 (45.4) | 3.9 | 0.9 (0.7 to 1.1) |
| Gestational age at delivery | n=92 218 | n=389 | ||
| Median (interquartile range) days | 280 (272-286) | 240 (198-271) | ||
| Birth weight (g): | n=91 858 | n=389 | ||
| Mean (SD) | 3343.3 (569.1) | 1931.6 (1146.2) | ||
| Gestation related optimal weight centile: | n=88 053 | n= 380 | ||
| Median (interquartile range) | 41.5 (19.5-67.5) | 9.7 (0.6-37.3) | ||
| ≤10 | 69 840 (75.7) | 168 (43.2) | 16.7 | 6.8 (5.6 to 8.4) |
| 10-90 | 11 697 (12.7) | 195 (50.1) | 2.4 | Reference |
| >90 | 6516 (7.1) | 17 (4.4) | 2.6 | 1.1 (0.7 to 1.8) |
| Fetal growth restriction†: | n=88 053 | n=380 | ||
| No | 76 356 (86.7) | 185 (48.7) | 2.4 | Reference |
| Yes | ||||
| Detected antenatally | 3601 (4.1) | 35 (9.2) | 9.7 | 4.0 (2.8 to 5.7) |
| Not detected antenatally | 8096 (9.2) | 160 (42.1) | 19.8 | 8.0 (6.5 to 9.9) |
*Place of birth combined if either subgroup <1000 women.
†Birth weight <10th gestation related optimal weight centile.
For maternal characteristics, stillbirth rates were increased in first as well as third and subsequent pregnancies compared with second pregnancies, and in mothers of African, African-Caribbean, and South Asian ethnic origin compared with their European counterparts. First generation migrants had an overall higher risk of stillbirth. Maternal age indicated a slight increase in younger (<25) and older (≥35) mothers, suggesting a U-shaped distribution, but this trend did not reach significance.
Social factors with significant associations included deprivation and unemployment of the mother or her partner. Pregnancies in which the parents were blood relations were not at significantly increased risk. Obesity (body mass index ≥30), active as well as passive smoking, lack of antenatal folic acid, and booking after 13 weeks were all associated with an increased risk of stillbirth. A history of mental health problems, diabetes, and stillbirth increased the risk. In the current pregnancy, pre-eclampsia and antepartum haemorrhage were strongly associated, whereas gestational diabetes was not.
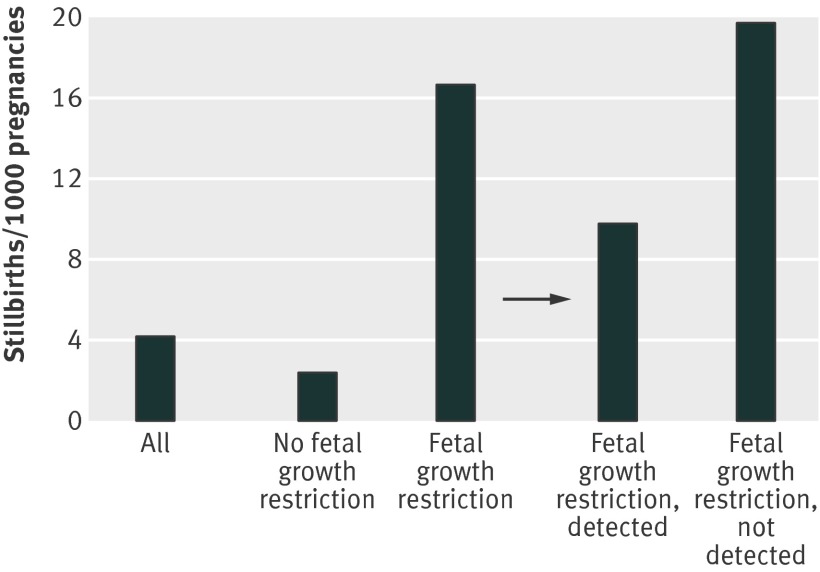
The strongest factor was fetal growth restriction, with a relative risk of 4.0 (95% confidence interval 2.8 to 5.7) when fetal growth restriction was detected antenatally, doubling to 8.0 (6.5 to 9.9) when it was not detected. The overall stillbirth rate (per 1000 births) was 4.2, which was a composite of a rate of 2.4 (185/76 356) in pregnancies without fetal growth restriction and 16.7 (195/11 697) in pregnancies with fetal growth restriction (table 1). Of pregnancies with fetal growth restriction, the stillbirth rate for cases detected antenatally was 9.7 (35/3601), whereas the rate increased to 19.8 (160/8096) when cases were not detected (fig 1).
Fig 1 Stillbirth rates in relation to fetal growth restriction and whether it was detected antenatally
Because of the strong interaction between smoking and fetal growth restriction, stillbirth rates for pregnancies with maternal smoking are also presented for subgroups of fetal growth restriction (table 2). The overall stillbirth rate (per 1000 births) was higher in mothers who smoked (5.8 v 3.8), but this was only the case for pregnancies with fetal growth restriction (13.0), whereas the risk of stillbirth in pregnancies without fetal growth restriction (3.7) was similar to that where the mother did not smoke (3.8). The highest risk of stillbirth was in pregnancies with fetal growth restriction where the mother did not smoke. Antenatal detection of fetal growth restriction during the study period was 31% overall (table 1) and higher in pregnancies where the mother smoked (1451/4012, 36.2%) than where she did not (1480/5280, 28.0%).
Table 2.
Smoking and fetal growth restriction (birth weight <10th gestation related optimal weight centile)
| Variables | Proportion of total (%) | Stillbirth rate/1000 births |
|---|---|---|
| All | 100.0 | 4.2 |
| Smokers: | 18.7 | 5.8 |
| Fetal growth restriction | 4.3 | 13.0 |
| No fetal growth restriction | 13.7 | 3.7 |
| Non-smokers: | 81.3 | 3.8 |
| Fetal growth restriction | 8.3 | 18.3 |
| No fetal growth restriction | 68.7 | 2.1 |
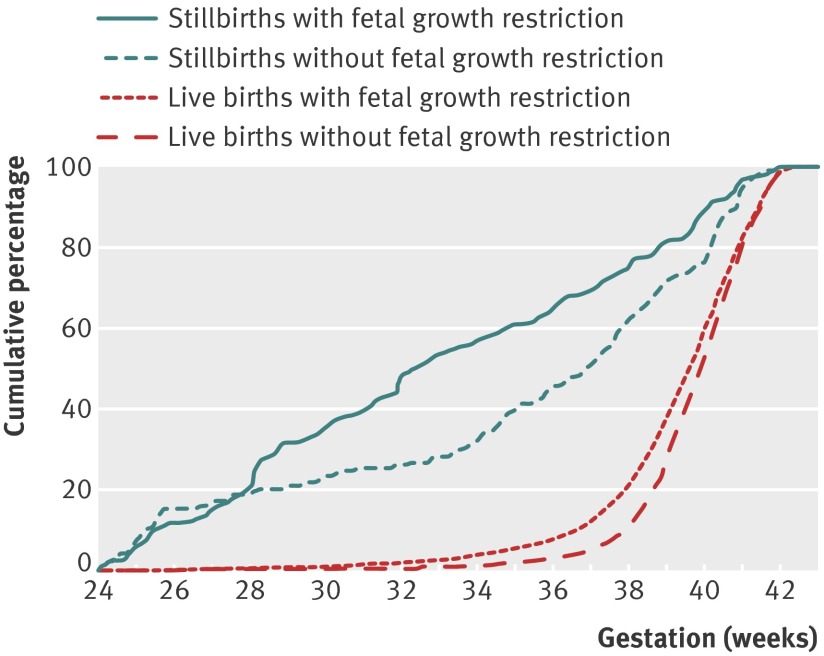
Fig 2 shows cumulative frequency graphs for pregnancies with and without fetal growth restriction for stillbirths and live births. For both outcomes, pregnancies with fetal growth restriction tended to be delivered earlier. The median gestational age for stillbirths with fetal growth restriction was 32 weeks and three days compared with 36 weeks and six days for stillbirths without fetal growth restriction.
Fig 2 Stillbirths and live births with and without fetal growth restriction: cumulative percentage graph of gestational age at delivery
Multivariable analysis
Table 3 lists the results of the Poisson regression model, which included all significant factors shown in the univariate analysis. Overall, 25 021 births, including 103 stillbirths, were excluded because of incomplete data. This represented a rate of 4.1/1000 births, which was similar to the overall rate of stillbirth (4.2/1000, table 1). Sensitivity analyses of the excluded factors (P>0.05 in univariate analysis), of clustering of births within maternity units (see supplementary appendices 1 and 2), and of repeat pregnancies by the same mother (results not shown) indicated only minor differences in confidence intervals and resulted in no changes in relative risks.
Table 3.
Multivariable analysis of significant (P<0.1) risk factors in table 1
| Variables | Adjusted relative risk (95% CI) | P value | Prevalence (%) | Population attributable risk (%) |
|---|---|---|---|---|
| Parity: | ||||
| 0 | 1.8 (1.3 to 2.5) | <0.01 | 43.4 | 21.3 |
| ≥3 | 1.6 (1.0 to 2.5) | 0.05 | 9.4 | 4.6 |
| Ethnic origin, place of birth: | ||||
| African* | 2.4 (1.2 to 4.6) | 0.01 | 3.0 | 2.9 |
| African-Caribbean* | 2.3 (1.3 to 4.1) | 0.01 | 2.0 | 2.2 |
| Indian* | 2.1 (1.3 to 3.5) | <0.01 | 5.2 | 3.9 |
| Pakistani, non-UK | 3.0 (1.9 to 4.8) | <0.01 | 4.6 | 6.4 |
| Body mass index: | ||||
| 30-34.9 | 1.4 (1.0 to 2.0) | 0.07 | 12.5 | 4.5 |
| ≥35 | 1.6 (1.1 to 2.4) | 0.03 | 7.3 | 4.2 |
| Mental health history | 1.4 (1.0 to 1.9) | 0.06 | 11.7 | 4.7 |
| Pre-existing diabetes | 3.9 (1.7 to 8.9) | <0.01 | 0.7 | 2.0 |
| Antepartum haemorrhage | 3.4 (2.6 to 4.5) | <0.01 | 8.1 | 15.5 |
| Maternal smoking, no fetal growth restriction† | ||||
| Active smoker | 2.5 (1.7 to 3.6) | <0.01 | 14.9 | 9.4 |
| Passive smoker | 1.3 (0.8 to 2.0) | 0.28 | 15.7 | — |
| Maternal smoking, fetal growth restriction†: | ||||
| Active smoker | 5.7 (3.6 to 8.9) | <0.01 | 4.6 | 6.1 |
| Passive smoker | 10.0 (6.6 to 15.8) | <0.01 | 2.1 | 9.1 |
| Fetal growth restriction†, non-smoker | 7.8 (5.6 to 10.9) | <0.01 | 6.5 | 22.2 |
Reference group: para 1, UK born, non-smoking European mother; body mass index 18.5-24.9.
*UK and non-UK groups combined because of small numbers.
†Birth weight <10th gestation related optimal weight centile.
First, third, and higher order pregnancies were significantly associated with stillbirth as were pregnancies in African, African-Caribbean, and Indian mothers and first generation migrants from Pakistan. Obesity (body mass index >30), pre-existing diabetes, history of mental health problems, and antepartum haemorrhage in the index pregnancy were associated with an increased risk of stillbirth.
Interactions between all variables were tested and were found to be non-significant, with the exception of a strong interaction between smoking and fetal growth restriction. Therefore the results are presented separately for smokers with pregnancies that did or did not have fetal growth restriction. Active smoking was associated with an increased risk of stillbirth (adjusted relative risk 2.5, 95% confidence interval 1.7 to 3.6), but the association became substantially stronger (5.7, 3.6 to 8.9) for pregnancies where the fetus was also growth restricted. There was no association between passive smoking and stillbirth unless fetal growth restriction was also present, in which case the relative risk was even higher than with active smoking (10.0, 6.6 to 15.8). The risk of stillbirth was increased for all pregnancies with fetal growth restriction, but was highest when the mother did not smoke (7.8, 5.6 to 10.9).
Table 3 also lists the population attributable risk derived from prevalence and relative risk of each significant factor. The model was able to attribute risk factors to 80.6% of the stillbirths in this cohort. The highest population attributable risks were associated with fetal growth restriction, primiparity, and antepartum haemorrhage.
In pregnancies with fetal growth restriction, the adjusted risk of stillbirth was 3.4 (2.2 to 5.2) if fetal growth restriction was detected antenatally. The risk increased to 6.5 (4.9 to 8.4) if fetal growth restriction was not detected, and 32% of the stillbirths could be attributed to this group. Pregnancies with fetal growth restriction detected antenatally were delivered on average 10 days earlier than those not detected antenatally (table 4).
Table 4.
Gestational age at delivery and risk of stillbirths in pregnancies with fetal growth restriction*, with and without antenatal detection
| Variables | Prevalence (%) | Median (interquartile range) gestational age at delivery (days) | Adjusted relative risk† (95% CI) | P value | Population attributable risk (%) |
|---|---|---|---|---|---|
| No fetal growth restriction | 86.8 | 280 (273-286) | Reference | ||
| Fetal growth restriction: | |||||
| Detected antenatally | 4.0 | 270 (261-279) | 3.4 (2.2 to 5.2) | <0.01 | 6.2 |
| Not detected antenatally | 9.2 | 280 (273-287) | 6.5 (4.9 to 8.4) | <0.01 | 32.0 |
*Adjusted for ethnicity, parity, body mass index, pre-eclampsia, antepartum haemorrhage, history of mental health problems, and smoking.
†Birth weight <10th gestation related optimal weight centile.
Just over half of the stillbirths (203/389, 52%) occurred after 34 weeks of gestation. Table 5 presents the significant pathological factors of the model, adjusted for maternal characteristics, for stillbirths before and after 34 weeks of gestation. For stillbirths between 24 and 33 weeks of gestation, the only significant factor was fetal growth restriction (adjusted relative risk 4.0, 2.9 to 5.6), which accounted for just under half of the stillbirths (population attributable risk 49.5%). At the same time, pre-eclampsia emerged as a significant protective factor. From 34 weeks of gestation, fetal growth restriction again represented the strongest risk, and was highest in pregnancies where the mother did not smoke.
Table 5.
Fetal growth restriction and other pathological factors before and after 34 weeks of gestation
| Variables | Adjusted relative risk* (95% CI) | P value | Prevalence (%) | Population attributable risk (%) |
|---|---|---|---|---|
| 24-33 weeks | ||||
| Pre-eclampsia | 0.3 (0.1 to 0.6) | <0.01 | 10.5 | −13.2 |
| Fetal growth restriction† | 4.0 (2.9 to 5.6) | <0.01 | 14.3 | 49.5 |
| ≥34 weeks | ||||
| Body mass index: | ||||
| 30-34 | 1.9 (1.3 to 3.0) | <0.01 | 12.0 | 9.9 |
| ≥35 | 1.8 (1.1 to 3.0) | 0.02 | 7.3 | 5.1 |
| Mental health history | 1.7 (1.0 to 2.7) | 0.04 | 11.3 | 7.6 |
| Late booking | 1.6 (1.1 to 2.3) | 0.01 | 16.6 | 9.1 |
| Antepartum haemorrhage | 3.1 (2.0 to 4.7) | <0.01 | 7.5 | 13.0 |
| Smoker, no fetal growth restriction† | 1.7 (1.1 to 2.7) | 0.02 | 14.1 | 7.3 |
| Smoker, fetal growth restriction† | 2.5 (1.3 to 5.1) | 0.01 | 4.3 | 2.5 |
| Fetal growth restriction†, non-smoker | 5.1 (3.4 to 7.6) | <0.01 | 8.0 | 22.7 |
*Adjusted for ethnicity, maternal age, parity, and body mass index.
†Birth weight <10th gestation related optimal weight centile.
Discussion
Our study shows that while there are several risk factors for stillbirth that can be ascertained from the outset of pregnancy, the single largest factor is fetal growth restriction, which is currently not well predicted and not recognised antenatally in most pregnancies. Considering that most instances are missed, a retrospective definition of fetal growth restriction using growth potential 19 applied to birth weight was important to be able to quantify the link with stillbirth. The findings indicate the importance of improving current strategies and protocols for improved surveillance of fetal growth throughout the antenatal period.13
Early pregnancy risk factors
The population based data derived from a whole NHS region allowed us to assess several known as well as new risk factors that will require further investigation in future studies. These factors have varying clinical implications.
Parity, ethnicity, and previous mental health problems are indicators of risk elicited at the beginning of pregnancy. The association between nulliparity and stillbirth reflects other reports,14 but we also observed a 60% increase in risk for mothers with a parity of 3 or higher, suggesting a U-shaped relation between parity and risk of stillbirth (table 2), as previously reported.27 Contrary to a systematic review,28 we found no significant increase in risk of stillbirth with older maternal age (table 1). This may be because we excluded congenital anomalies from our cohort, which are known to be increased in older mothers.29 This is consistent with a recent report which found that the association between stillbirth and maternal age disappears when congenital anomalies are excluded.14
Mothers living in the most deprived areas had an increased risk of stillbirth, as did women who were or had a partner who was unemployed. Ethnic or racial disparities in stillbirth rates have been highlighted14 30 and were also independent risk factors in our study. Each of the main ethnic minority groups in our population had an increased risk of stillbirth (table 3). Further work is required to help understand the reasons for the increased risk in these groups. A limitation of our study is that consent rates for postmortem examinations are low in some ethnic groups, as a result of which some cases with undiagnosed congenital anomalies may have been inadvertently included in our cohort.
Among pre-existing conditions, diabetes is a known risk factor31 and had a higher risk (adjusted relative risk 3.9, 1.7 to 8.9), but a population attributable risk of only 2.0% because of the low prevalence in this cohort. A history of mental health problems was also a significant risk factor (adjusted relative risk 1.4, 1.0 to 1.9), reported by 11.7% of mothers. It has been suggested that the link between mental illness and adverse outcome could be mediated through general factors such as insufficient attendance at antenatal clinics or unhealthy lifestyles.32 Antenatal haemorrhage including placental abruption has a known association with stillbirth28; our results confirm this link and emphasise the need for immediate, thorough investigation of any antepartum bleeding.
Body mass index categories of 30-34.9 and 35 or more represented a 40% and 60% increased risk of stillbirth, respectively, and together were prevalent in just under 20% of this cohort, resulting in a population attributable risk of 8.7%. Contrary to some reports,14 we found no increase in risk of stillbirth associated with overweight mothers (body mass index 25-29.9) in our population.
Active and passive smoking
Maternal smoking in early pregnancy is associated with stillbirth, with an average risk of 1.36 based on four studies.28 We found the risk to be higher (relative risk 1.8, 95% confidence interval 1.4 to 2.3, table 1), possibly as a result of high levels of social deprivation in our population, which is strongly linked to smoking.33
However, the database also allowed us to examine the interaction between smoking and fetal growth, and the effect on stillbirth risk when fetal growth restriction is detected antenatally. The increased risk of smoking works mostly through fetal growth restriction, and the rate of stillbirth in pregnancies of mothers who smoked but had no fetal growth restriction was similar (3.7) to that of mothers who were non-smokers (3.8, table 2). This highlights the importance of early pregnancy smoking cessation programmes, which have been found to reduce smallness for gestational age and prematurity.34 Interestingly, the highest stillbirth rate was in non-smoking pregnant women with fetal growth restriction, which could be because these pregnancies are considered low risk and fetal growth restriction is less likely to be detected antenatally.
We were also able to quantify the effect of passive or environmental smoking, and found it to be associated with an increase in risk of still birth by 30% (table 1)—an effect corresponding to the 23% reported in a recent meta analysis of four trials.35 However in the multivariable model this factor was significant only when there was fetal growth restriction, in which case the risk even exceeded that associated with the mother being a smoker (adjusted relative risk 10.0, 95% confidence interval 6.6 to 15.8; table 3). This observation needs confirmation in future studies but could have several plausible explanations. Passive smoking, resulting from the “side stream” effect of smoke produced between puffs, has been shown in animal studies to be four times as toxic as “mainstream” smoke,36 and this may extend to fetuses during pregnancy. Also, that a non-smoking mother lives in a smoking environment may be missed, resulting in the pregnancy not being afforded the same clinical attention, including concern about fetal growth.
Modifiable factors
Overall, the largest population attributable risk (37.4%) was associated with fetal growth restriction with and without smoking, followed by maternal smoking without fetal growth restriction (9.4%) and obesity (body mass index ≥30; population attributable risk 8.7%, table 3). These three variables could be considered as the potentially most modifiable factors and, allowing for overlap, together accounted for 55.5% of stillbirths in our cohort. However they have different implications for prevention. Reduction of smoking and obesity require concerted public health efforts and education programmes for women of childbearing age, as well as the mothers’ cooperation with smoking cessation programmes during pregnancy. The effectiveness of such programmes is not always clear. On the other hand, the risk of stillbirth as a result of fetal growth restriction can be reduced by early identification of the condition and referral for investigations by ultrasound and Doppler imaging.12
Fetal growth restriction and antenatal detection
The overall detection rate of fetal growth restriction was only 31%, lower than that achieved in our controlled study in Nottingham using customised charts (48%)37 and a recent report from Australia (51%).38 However, we found that the detection rate in our population ranged widely between maternity units (12.5-50.0%),39 varying with the amount of staff training and adherence to protocols. This highlights the importance of a standardised and quality assured approach to the antenatal surveillance of fetal growth in routine clinical practice. Among the cohort of 389 stillbirths, the detection rate was even lower: 195 (50.1%) of the cases had fetal growth restriction and in 160 (82.1%) fetal growth restriction was not detected antenatally (table 1).
The potential preventability of stillbirths associated with fetal growth restriction is illustrated by the reduced risk after antenatal detection (fig 1 and table 1). Although pregnancies with fetal growth restriction have an eight times higher rate of stillbirth (16.7 v 2.1, fig 1), this risk is reduced when fetal growth restriction is detected (9.7), although not to the same levels as in non-fetal growth restricted pregnancies. This is likely to be because the baby is often not delivered immediately, either due to inappropriate delays or because of concerns about neonatal immaturity. Conversely, the risk is higher still if fetal growth restriction is present but not detected.
These associations are also shown when all other factors are adjusted for in the multivariable analysis (table 4): the population attributable risk of 32.0% indicates that five times as many stillbirths are associated with undetected antenatal fetal growth restriction than with detected fetal growth restriction (6.2). Pregnancies with fetal growth restriction were delivered on average 10 days earlier than those without, but still at relatively mature gestations when detected than when not (median 270 v 280 days; table 4). Based on the prevalence of undetected fetal growth restriction (9.2%, table 4) and the lower risk of stillbirth when it is detected (adjusted relative risk 3.4), 18.2% or 71 stillbirths in our cohort could have been avoided through improved antenatal detection. Extrapolated to the UK population, this would represent 600 fewer stillbirths per year.
The subanalysis of the multivariable model by early and late gestational age categories (table 5) showed fetal growth restriction again to be the strongest factor for risk of stillbirth after 34 weeks of gestation, with the highest risk in pregnancies where the mother did not smoke. Before 34 weeks of gestation, fetal growth restriction was the single significant factor and accounted for close to half of all stillbirths (population attributable risk 49.5%). These results support the notion of differing patterns of placental disease in early and late onset fetal growth restriction.10 13 The protective effect of pre-eclampsia (table 5) is interesting but not surprising: the disease is often associated with fetal growth restriction, but as these pregnancies are often delivered early for maternal reasons, the baby is removed from a high risk intrauterine environment.
Implications for clinicians and commissioners
The increased risk of stillbirth after undetected fetal growth problems is consistent with a nine year review of deliveries in a single unit in Malmö40 and a recent case-control study from Auckland.41 In our database from a large NHS region, we were able to quantify the importance of this risk factor and establish its pre-eminent association with stillbirth even after adjustment for medical, social, and demographic risk factors that can be ascertained in early pregnancy. Most cases of fetal growth restriction do not manifest until the third trimester of pregnancy, and in the absence of effective screening tests, prevention strategies need to include an enhanced level of surveillance throughout pregnancy. Our findings suggest that early detection of fetal growth problems can substantially reduce the risk of stillbirth, and needs to become a cornerstone and key indicator of safety and effectiveness in antenatal care.
What is already known on this topic
Stillbirth rates have changed little in the English National Health Service over the past two decades
Many have conventionally been considered unexplained and unavoidable
Risk factors have generally been considered weak predictors of stillbirth
What this study adds
Maternal obesity, smoking, and fetal growth restriction, potentially modifiable risk factors, together account for the majority of normally formed stillbirths
Compared with pregnancies with normal growth, the stillbirth rate in those with fetal growth restriction increased fourfold, and increased further to eightfold if growth restriction was not detected antenatally
Fetal growth restriction is currently missed in most pregnancies, and better antenatal detection needs to become a cornerstone and key indicator of safety and effectiveness in maternity care
We thank our regional network of bereavement midwives who submit notifications on all perinatal deaths; Ann Tonks, Catherine Franklin, and Robyn Caley who run the regional perinatal mortality registers for ensuring high quality ascertainment; the network of unit based data clerks who enter the regional maternity dataset for each pregnancy on the PEER electronic record (www.pi.nhs.uk/peer); and Michelle Southam and Lorraine Ecclestone for ensuring data quality through a rolling programme of training and accuracy audits.
Contributors: All authors had full access to the data, assisted with its analysis and interpretation of the results, and reviewed and approved the final manuscript. JG developed the study design, assisted with the analysis and interpretation, and wrote the paper. He has final responsibility to submit for publication, and is the guarantor.
Funding: Data collection and analytical staff were financially supported by NHS West Midlands Strategic Health Authority and all primary care trusts. The funders had no influence on the study design, analysis, interpretation, writing up of the manuscript, or the decision to submit for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required; data were collected with patient consent and were pseudonymised before analysis.
Data sharing: No additional data available.
Cite this as: BMJ 2013;346:f108
Web Extra. Extra material supplied by the author
Sensitivity analysis of excluded non-significant risk factors
Sensitivity analysis with variance estimates adjusted for clustering maternity units
References
- 1.Saving Babies Lives Report. Stillbirth and Neonatal Death Charity (SANDS), 2009. 2012. www.why17.org/fileadmin/content/About_Sands/03520_Saving_babies_lives_watermarked.pdf.
- 2.Flenady V, Middleton P, Smith GC, Duke W, Erwich JJ, Yee Khong T, et al. Stillbirths: the way forward in high-income countries. Lancet 2011;377:1703-17. [DOI] [PubMed] [Google Scholar]
- 3.Office for National Statistics (2011). Death registration summary tables, England & Wales, 2010. 2012. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-230730.
- 4.Confidential Enquiry into Maternal and Child Health (CEMACH). Perinatal mortality 2006: England, Wales and Northern Ireland. CEMACH, 2008. (Table 4.2).
- 5.Gardosi J. Clinical implications of ‘unexplained’ stillbirths. In: Maternal and Child Health Research Consortium, ed: CESDI 8th annual report.Confidential Enquiry into Stillbirths and Deaths in Infancy, 2001:40-7.
- 6.Gardosi J, Mul T, Mongelli M, Fagan D. Analysis of birth weight and gestational age in antepartum stillbirths. BJOG 1998;105:524-30. [DOI] [PubMed] [Google Scholar]
- 7.Froen JF, Gardosi J, Thurmann A, Francis A, Stray-Pedersen B. Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet Gynecol Scand 2004;83:81-7. [DOI] [PubMed] [Google Scholar]
- 8.Gardosi J, Kady SM, McGeown P, Francis A, Tonks A. Classification of stillbirth by relevant condition at death (ReCoDe): population based cohort study. BMJ 2005;331:1113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergani P, Cozzolino S, Pozzi E, Cuttin MS, Greco M, Ornaghi S, et al. Identifying the causes of stillbirth: a comparison of four classification systems. Am J Obstet Gynecol 2008;199:319.e1-4. [DOI] [PubMed] [Google Scholar]
- 10.Korteweg FJ Gordijn SJ, Timmer A, Holm JP, Ravise JM, Erwich JJ. A placental cause of intra-uterine fetal death depends on the perinatal mortality classification system used. Placenta 2008;29:71-8. [DOI] [PubMed] [Google Scholar]
- 11.Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA 2011;306:2459-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfirevic Z, Nielson JP. Doppler ultrasonography in high risk pregnancies: systematic review with meta-analysis. Am J Obstet Gynecol 1995;172:1379-87. [DOI] [PubMed] [Google Scholar]
- 13.Figueras F, Gardosi J: Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol 2010; 10.1016/j.ajog.2010.08.055 [DOI] [PubMed] [Google Scholar]
- 14.Stillbirth Collaborative Research Network Writing Group. Association between stillbirth and risk factors known at pregnancy confirmation. JAMA 2011;306:2469-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Key Health Data for the West Midlands—2009. NHS Perinatal Institute. 2012. www.pi.nhs.uk/pnm.
- 16.Perinatal Episode Electronic Record (PEER). NHS Perinatal Institute. 2012. www.pi.nhs.uk/peer.
- 17.Pregnancy notes. NHS Perinatal Institute. 2012. www.preg.info.
- 18.West Midlands Core Maternity Dataset v 1.7, 2009. NHS Perinatal Institute. 2012. www.pi.nhs.uk/peer/WM_Maternity_Dataset_v1.7.pdf.
- 19.GROW (Gestation Related Optimal Weight) software version 6.5 (UK). Gestation Network. 2012. www.gestation.net.
- 20.Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet 1992;339:283-7. [DOI] [PubMed] [Google Scholar]
- 21.Gardosi J, Mongelli M, Wilcox M, Chang A. An adjustable fetal weight standard. Ultrasound Obstet Gynecol 1995;6:168-74. [DOI] [PubMed] [Google Scholar]
- 22.Gardosi J. Customised assessment of fetal growth potential: implications for perinatal care. Arch Dis Child Fetal Neonatal Ed 2012; doi:10.1136/fetalneonatal-2012-301708. [DOI] [PubMed] [Google Scholar]
- 23.De Jong CL, Francis A, Van Geijn HP, Gardosi J. Customized fetal weight limits for antenatal detection of fetal growth restriction. Ultrasound Obstet Gynecol 2000;15:36-40. [DOI] [PubMed] [Google Scholar]
- 24.Genest DR, Williams MA, Greene MF. Estimating the time of death in stillborn fetuses: I. Histologic evaluation of fetal organs; an autopsy study of 150 stillborns. Obstet Gynecol 1992;80:575-84. [PubMed] [Google Scholar]
- 25.Kooperberg C, Petitti DB. Using logistic regression to estimate the adjusted attributable risk of low birthweight in an unmatched case-control study. Epidemiology 1991;2:363-6. [DOI] [PubMed] [Google Scholar]
- 26.Confidential Enquiry into Maternal and Child Health. Perinatal mortality 2007. CEMACH, 2009:21.
- 27.Bai J, Wong FW, Bauman A, Mohsin M. Parity and pregnancy outcomes. Am J Obstet Gynecol 2002;186:274-8. [DOI] [PubMed] [Google Scholar]
- 28.Flenady V, Koopmans L, Middleton P, Frøen F, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet 2011;377:1331-40. [DOI] [PubMed] [Google Scholar]
- 29.Hollier LM, Leveno KJ, Kelly MA, McIntire DD, Cunningham FG. Maternal age and malformations in singleton births. Obstet Gynecol 2000;96:701-6. [DOI] [PubMed] [Google Scholar]
- 30.Reeske A, Kutschmann M, Razum O, Spallek J. Stillbirth differences according to regions of origin: an analysis of the German perinatal database, 2004-2007. BMC Pregnancy Childbirth 2011;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population-based study. Diabetes Care 2009;32:2005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King-Hele S, Webb RT, Mortensen PB, Appleby L, Pickles A, Abel KM. Risk of stillbirth and neonatal death linked with maternal mental illness: a national King-Helecohort study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F105-10. [DOI] [PubMed] [Google Scholar]
- 33.Goya J, Dodd L, Rosenberg MW Kinga WD Health-risk behaviours: examining social disparities in the occurrence of stillbirth Paediatr Perinatal Epidemiol 2008;22:314-20. [DOI] [PubMed] [Google Scholar]
- 34.McCowan LM, Dekker GA, Chan E, Stewart A, Chappell LC, Hunter M, et al. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ 2009;26;338:b1081. Erratum in: BMJ 2009;338. doi:10.1136/bmj.b1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonardi-Bee J, Britton J, Venn A. Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: a meta-analysis. Pediatrics 2011;127:734-41. [DOI] [PubMed] [Google Scholar]
- 36.Schick S, Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tob Control 2005;14:396-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardosi J, Francis A. Controlled trial of fundal height measurement plotted on customised antenatal growth charts. BJOG 1999;106:309-17. [DOI] [PubMed] [Google Scholar]
- 38.Roex A, Nikpoor P, van Eerd E, Hodyl N, Dekker G. Serial plotting on customised fundal height charts results in doubling of the antenatal detection of small for gestational age fetuses in nulliparous women. Aust N Z J Obstet Gynaecol 2012;52:78-82. doi:10.1111/j.1479-828X.2011.01408.x. [DOI] [PubMed] [Google Scholar]
- 39.West Midlands Perinatal KPI Report, Q2 2010/11. Perinatal Institute, 2011. 2012. www.pi.nhs.uk/pnm/maternitydata/Q2_2010-11_Perinatal_KPI_report.pdf.
- 40.Lindqvist PG, Molin J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol 2005;25:258-64. [DOI] [PubMed] [Google Scholar]
- 41.Stacey T, Thompson JM, Mitchell EA, Zuccollo JM, Ekeroma AJ, McCowan LM. Antenatal care, identification of suboptimal fetal growth and risk of late stillbirth: findings from the Auckland Stillbirth Study. Aust N Z J Obstet Gynaecol 2012. doi:10.1111/j.1479-828X.2011.01406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analysis of excluded non-significant risk factors
Sensitivity analysis with variance estimates adjusted for clustering maternity units