Abstract
Attention Deficit Hyperactivity Disorder (ADHD) and Autism Spectrum Disorder (ASD) are often comorbid and share behavioural-cognitive abnormalities in sustained attention. A key question is whether this shared cognitive phenotype is based on common or different underlying pathophysiologies. To elucidate this question, we compared 20 boys with ADHD to 20 age and IQ matched ASD and 20 healthy boys using functional magnetic resonance imaging (fMRI) during a parametrically modulated vigilance task with a progressively increasing load of sustained attention. ADHD and ASD boys had significantly reduced activation relative to controls in bilateral striato–thalamic regions, left dorsolateral prefrontal cortex (DLPFC) and superior parietal cortex. Both groups also displayed significantly increased precuneus activation relative to controls. Precuneus was negatively correlated with the DLPFC activation, and progressively more deactivated with increasing attention load in controls, but not patients, suggesting problems with deactivation of a task-related default mode network in both disorders. However, left DLPFC underactivation was significantly more pronounced in ADHD relative to ASD boys, which furthermore was associated with sustained performance measures that were only impaired in ADHD patients. ASD boys, on the other hand, had disorder-specific enhanced cerebellar activation relative to both ADHD and control boys, presumably reflecting compensation. The findings show that ADHD and ASD boys have both shared and disorder-specific abnormalities in brain function during sustained attention. Shared deficits were in fronto–striato–parietal activation and default mode suppression. Differences were a more severe DLPFC dysfunction in ADHD and a disorder-specific fronto–striato–cerebellar dysregulation in ASD.
Keywords: ADHD, ASD, attention, dorsolateral prefrontal cortex, fMRI
Introduction
Autism spectrum disorder (ASD) is characterised by abnormalities in social interaction, communication and stereotyped/repetitive behaviours (DSM-IV-TR; ICD-10).1, 2 About 30% of ASD patients have comorbid Attention Deficit Hyperactivity Disorder (ADHD),3, 4 characterised by age-inappropriate inattention, impulsiveness and hyperactivity (DSM IV).1
A shared behavioural/cognitive phenotype is inattention.4, 5 Sustained attention deficits are among the most consistent cognitive deficits in ADHD.6, 7 In ASD, there is evidence for similar impairment,8, 9, 10, 11 albeit with also negative findings.12
Sustained attention/vigilance is defined here as the ability to voluntarily maintain the focus of attention to infrequently occurring critical events,13, 14 as opposed to the definition of a decrement of vigilance/sustained attention over time, which has been influential in the ADHD literature.15
It has been debated whether the phenotypically similar attention and other ADHD-related deficits in ASD are secondary to ASD and a phenocopy, preventing a co-diagnosis in the DSM-IV,1 or whether they reflect true comorbidity, reflected in the allowance for co-diagnosis in the upcoming DSM-V (http://www.dsm5.org).
Functional imaging could shed light on the debate by elucidating whether attention deficits in both disorders are based on common (‘true comorbidity') or dissociated (‘not true comorbidity') underlying brain dysfunctions. The identification of differences in the objectively measurable ‘biomarkers' that underlie clinically overlapping behaviour could furthermore help with future differential diagnosis.
The few fMRI studies that have measured sustained attention in ADHD, found reduced activation relative to healthy controls in inferior prefrontal cortex, striato–thalamic, parieto–temporal and cerebellar regions.16, 17, 18 No fMRI study, however, has tested sustained attention in ASD, despite evidence for fronto–striatal, parietal and cerebellar dysfunctions during related selective and flexible attention tasks.19, 20, 21, 22, 23, 24 Importantly, to our knowledge, no fMRI study has directly compared between disorders to elucidate disorder-specific or shared neurofunctional biomarkers. Only one structural MRI study compared both disorders, finding shared deficits in temporo–parietal structure as well as unique differences in ASD patients in increased supramarginal grey matter.25
The aim of this study was therefore to compare brain function of age and IQ-matched boys with non-comorbid ADHD, non-comorbid ASD and healthy controls while they performed a parametrically modified fMRI vigilance task with an increasingly more difficult load of sustained attention.
We hypothesised that (1) across all subjects progressively increased sustained attention load would be associated with progressively increasing activation in typical sustained attention regions comprising dorsolateral prefrontal cortex (DLPFC), striato–thalamic, parieto–temporal and cerebellar areas,16, 17, 26, 27 but (2) both disorder groups would show underactivation in these brain regions relative to healthy controls; and (3) given stronger evidence for sustained attention deficits in ADHD9, 12 and for disorder-specific dysfunctions in DLPFC and caudate relative to conduct and obsessive-compulsive disorders during attention tasks,7, 17, 28, 29, 30 ADHD boys would show more pronounced underactivation in fronto–striatal regions than ASD boys.
Materials and methods
A total of 60 male, right handed (Edinburgh Handedness Inventory31) medication-naïve boys (20 controls, 20 with ADHD and 20 with ASD), 11–17 years, IQ⩾70, participated. ADHD boys met the DSM-IV diagnosis of inattentive/hyperactive-impulsive combined type ADHD and scored above clinical threshold for ADHD symptoms on both the Strength and Difficulty Questionnaire (SDQ)32 and the Conners' Parent Rating Scale-Revised33 and had no comorbid condition. ASD diagnosis was made using ICD-10 research diagnostic criteria,2 confirmed by the autism diagnostic interview-revised34 and the autism diagnostic observation schedule.35 Comorbidity with other psychiatric disorders was excluded. All ASD participants underwent a structured physical and medical examination to exclude comorbid medical disorders and biochemical, haematological or chromosomal abnormalities associated with ASD. Boys with ASD were excluded if they scored above 7 on the Hyperactivity/Inattention ratings on the SDQ. ASD boys had to score above and ADHD patients below the clinical cut-off on the social communication questionnaire.36 Patients were recruited through local clinical services.
In all 20 healthy IQ, handedness, sex and age-matched controls were recruited locally by advertisement and scored below clinical threshold on the SDQ and social communication questionnaire.
Exclusion criteria for all were neurological disorders and drug/alcohol dependency. Intellectual ability was measured using the Wechsler Abbreviated Intelligence Scale–Revised short form.37 Ethical approval was obtained from the local Research Ethics Committee and written informed consent/assent was obtained for all participants. (For details on participants see Supplementary Material).
fMRI task: sustained attention task
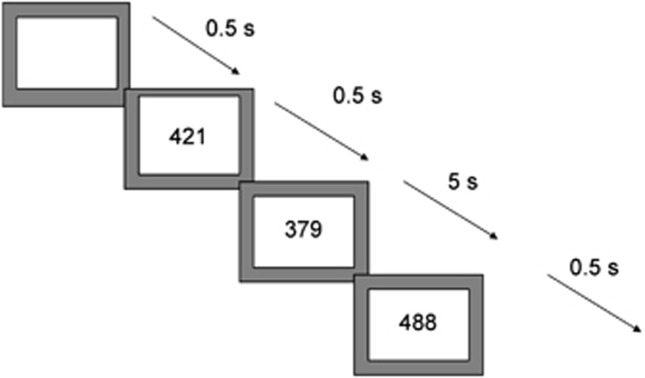
Subjects practised the task once in a mock scanner. The 12-min sustained attention task is a variant of psychomotor vigilance and delay tasks.38, 39 Subjects need to respond as quickly as possible to the appearance of a visual timer counting up in milliseconds via a right hand button response within 1 s. The visual stimuli appear either after short, predictable consecutive delays of 0.5 s, in series of 3–5 stimuli (260 in total) or after unpredictable time delays of 2, 5 or 8 s (20 each), pseudo-randomly interspersed into the blocks of 3–5 delays of 0.5 s. The long, infrequent, unpredictable delays place a higher load on sustained attention/vigilance, whereas the short, predictable 0.5 s delays are typically anticipated40 placing a higher demand on sensorimotor synchronisation39 (Figure 1).
Figure 1.
Schematic representation of the sustained attention task (SAT). Subjects are required to press a right-hand button as soon as they see a timer appear on the screen counting seconds. The counter appears after either predictable short delays of 0.5 s in blocks of 3–5 stimuli, or after unpredictable long delays of 2,5 or 8 s, pseudo-randomly interspersed into the blocks of 0.5 s delays. The long second delays have a progressively higher load on sustained attention than the short 0.5 s delays that are typically anticipated and have a higher load on sensorimotor synchronisation.
Analysis of performance data
Multiple repeated measures univariate analysis of variances (ANOVAs) with group as independent and delay as repeated measures were conducted to test for group differences in performance (that is, mean reaction time, intrasubject standard deviation of reaction time (SD intrasubject), omission and premature errors).
fMRI Image acquisition
fMRI images were acquired on a 3T General Electric Signa HDx TwinSpeed (General Electric, Milwaukee, Wisconsin, WI, USA) MRI scanner using a quadrature birdcage headcoil. In each of 22 non-contiguous planes parallel to the anterior–posterior commissure, 480 T2*-weighted MR images depicting BOLD (Blood Oxygen Level Dependent) contrast covering the whole brain were acquired with echo time (TE)=30 ms, repetition time (TR)=1.5 s, flip angle=60° in-plane resolution=3.75 mm, slice thickness=5.0 mm, slice skip=0.5 mm). A whole-brain high resolution structural scan (inversion recovery gradient echo planar image) on which to superimpose the activation maps, was also acquired in the inter-commissural plane, with TE=40 ms, TR=3 s, flip angle=90° 43 slices, slice thickness=3.0 mm, slice skip=0.3 mm, providing complete brain coverage.
fMRI image analysis
Event-related activation data were acquired in randomized trial presentation, and analysed using non-parametric data analysis (XBAM).41, 42 After preprocessing (see Supplementary text), time series analysis for each individual subject was based on a previously published wavelet-based data resampling method for functional MRI data.42, 43 Using rigid body and affine transformation, the individual maps were then registered into Talairach standard space.44 A group of brain activation map was then produced for each experimental condition (that is, long delays of 2, 5, 8 s, each contrasted with the implicit baseline, that is, 0.5 s delay) and hypothesis testing was carried out at the cluster level. In essence, a voxel-wise test at P<0.05 was conducted to identify any voxels that might plausibly be activated followed by a subsequent test at a cluster-level threshold of P<0.01 to remove the false-positive clusters produced by the voxel-level test. This combined voxel/cluster tests coupled with permutation testing allow for excellent type I error control at the cluster level.42, 43 For each task, <1 false-positive activated 3D clusters were expected at a P-value of <0.05 at the voxel-level and <0.01 at the cluster-level.
For the between-group comparisons, an ANOVA 3 × 3 split-plot design (three time delays, three groups) was conducted testing for group, delay and group by delay interaction effects, using a randomisation-based test for voxel or cluster-wise differences as described elsewhere.42 Less than one false-positive activation cluster was expected at P<0.05 at voxel level and P<0.01 at cluster level. Statistical measures of BOLD response for each participant were then extracted in each of the significant clusters and post-hoc least significance difference t-tests (correcting for multiple comparisons) were conducted to identify between-group differences.
Results
Subjects characteristics
There were no significant group differences for age (F(df=2,59)=0.8, P=0.4) or IQ (F(df=2,59)=1.6, P=0.2), but, as expected, for SDQ hyperactivity (F(df=2,59)=69, P<0.0001) and social communication questionnaire (F(df=2,59)=0.30, P<0.0001). Post-hoc analyses showed that ADHD scored significantly higher than ASD patients and controls on the SDQ hyperactivity (P<0.0001), while ASD patients scored significantly higher than controls (P<0.0001). In the social communication questionnaire, ASD boys scored significantly higher than control and ADHD boys (P<0.0001), and ADHD boys scored higher than controls (P<0.0001). Multivariate ANOVAs also showed a group effect for all other SDQ measures (F(df=2,57)=11, P<0.0001). Post-hoc analyses showed that both patient groups were more impaired than controls in all scales (P<0.05). They did not differ from each other in emotional and prosocial ratings; for conduct problems, however, ADHD boys were more impaired than ASD boys (P<0.0001), and for peer problems, ASD boys were more impaired than ADHD boys (P<0.0001) (Supplementary Table 1).
Performance data
Repeated measures univariate ANOVAs showed a significant within-subject effect of delay on mean reaction time (F(df=3,55)=179, P<0.0001), which were longer in all subjects with increasing delays; on SDintrasubject (df=3,55); F=6, P<0.001), which were reduced with longer delays; and on premature errors (F(df=3,55)=4, P<0.008), which were largest in the short delays of 0.5 s, in line with evidence for anticipation of short intervals.40
There was a significant group effect on SDintrasubject (F(df=2,57)=6.5, P=0.003). Post-hoc analyses showed that ADHD boys had significantly higher SDintrasubject than both control and ASD boys (P<0.02).
There was a group by delay interaction effect on mean reaction time (df=6,54; F=2, P<0.05), which was due to ADHD being slower than control boys only in all long delays (P<0.05) and there was a trend for ASD patients to differ from controls (P<0.1), due to ASD patients being slower in the 2- and 8-s delays (P<0.05). No other significant effects were observed (see Supplementary Table 2).
Movement
There were no group differences in maximum or mean displacement of x, y, z movement parameters and none of the subjects exceeded mean displacements of more than 1.5 mm (F(df=2,45)=0.4, P=0.6).
Delay effect
All subjects showed increased activation with increasing delay in a bilateral network comprising DLPFC and right inferior prefrontal cortex, cingulate, Supplementary motor area, parieto-temporal, cerebellum, basal ganglia, thalamus and hippocampal gyri (Supplementary Figure 1).
Group effect
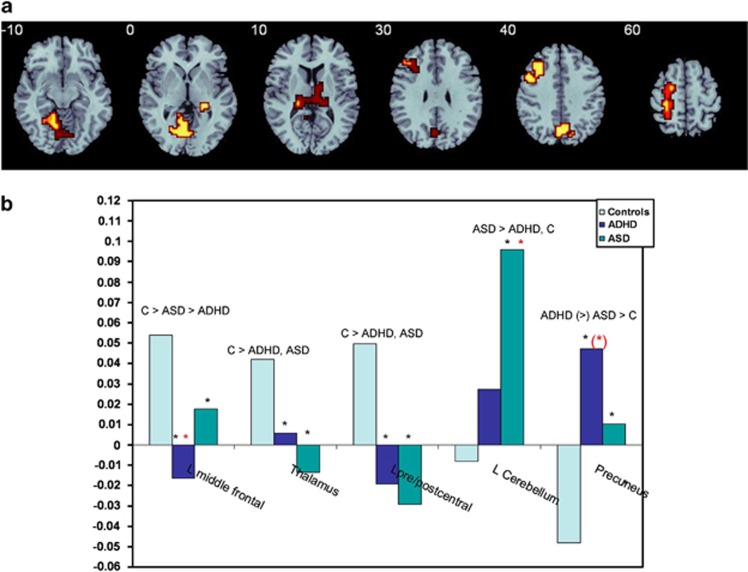
ANOVA split-plot analysis showed that there were significant group effects in five regions, in bilateral thalamus, reaching into caudate tail and in left-hemispheric middle frontal, pre and postcentral and superior parietal gyri, mid-precuneus and cerebellum/occipital cortex (Figure 2a, Table 1).
Figure 2.
(a) Horizontal slices showing split plot analysis of variance (ANOVA) effects of group on brain activation across the three delays. The right side corresponds to the right side of the image. (b) Statistical measures of BOLD response are shown for each of the three groups for each of the brain region that showed a significant group effect. *indicates significant differences between patient group and controls. *indicates a significant difference between the two patient groups. (*) indicates a trend-wise difference.
Table 1. ANOVA differences in brain activation between adolescents with ADHD, with ASD and healthy controls.
| Subject contrast | Brain regions of activation (Brodman area (BA)) | Talairach coordinates (x; y; z) | Voxels | Cluster P-value |
|---|---|---|---|---|
| C>ASD > ADHD | L Middle frontal (BA 46/9/8) | −25; 22; 26 | 72 | 0.004 |
| C>ADHD, ASD | L Pre/Postcentral/sup. parietal (BA 6/4/1/2/7) | −22; −30; 53 | 60 | 0.005 |
| C>ADHD, ASD | R + L Thalamus/putamen/hippocampus | 25; −26; −7 | 59 | 0.002 |
| ADHD (>) ASD>C | M Precuneus/cuneus (BA 7/19) | 0; −67; 31 | 42 | 0.005 |
| ASD>ADHD, C | L Cerebellum/hippocampal/lingual (BA 30/18/19) | −7; −48; −7 | 85 | 0.003 |
Abbreviations: ADHD, Attention Deficit Hyperactivity Disorder; ANOVA, analysis of variance; ASD, autism spectrum disorder; C, healthy controls.
Post-hoc analyses showed that in left DLPFC, controls had increased activation relative to both ASD (P<0.03) and ADHD boys (P<0.001), while ADHD boys also had reduced activation compared with ASD boys (P<0.04). In thalamus and caudate and in pre and postcentral gyrus, both disorder groups had reduced activation relative to controls (P<0.006), but did not differ from each other. In cerebellum, ASD boys showed increased activation compared with both control (P<0.0001) and ADHD boys (P<0.01), who did not differ from each other. In precuneus, both disorder groups showed increased activation relative to healthy boys (P<0.005), with a trend for ADHD boys to have enhanced activation relative to ASD boys (P<0.09) (Figure 2b, Table 1).
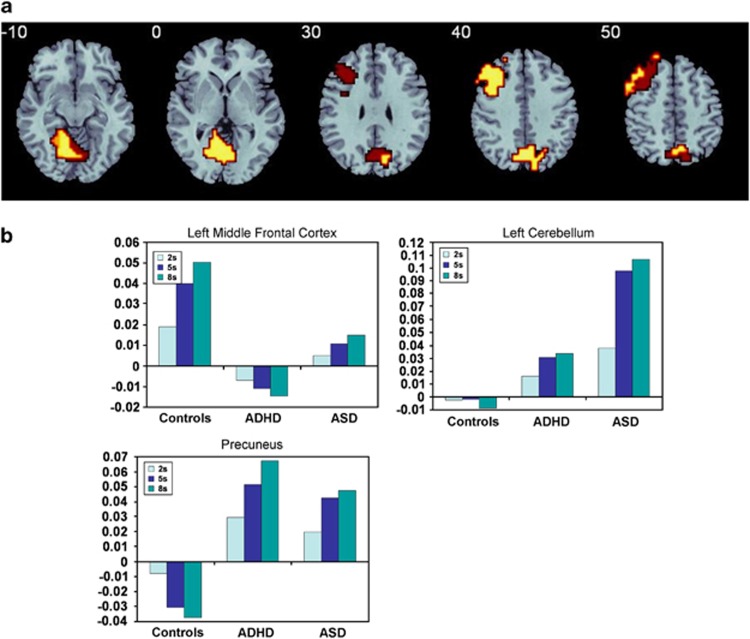
Group by delay effects
Split plot ANOVA showed that there was a group by delay interaction effect in three clusters, which overlapped with three of the group effect activation clusters, but were more extensive: in left middle frontal gyrus, cerebellum/occipital gyrus and precuneus (Table 2, Figure 3a). Post-hoc analyses showed that in the left DLPFC, both control and ASD boys showed increased activation with increasing delay, with ASD boys showing lower activation in all three delays (P<0.02). ADHD boys, in contrast, showed progressively more deactivation in DLPFC with increasing delays, which differed from both control (P<0.0001) and ASD boys (P<0.05). In the cerebellum, both disorder groups showed increased activation with increasing delays, but ASD showed overall greater activation than ADHD (P<0.03) and control boys (P<0.001) who showed increasing deactivation with increasing delays. In precuneus, both disorder groups showed increased activation with increasing delays, with no differences between each other, and which overall was increased relative to controls (P<0.001) –who showed progressively more pronounced deactivation with increasing delays (Figure 3b).
Table 2. ANOVA Group by delay interaction effect for brain activation between ADHD, ASD and healthy boys.
| Subject contrast | Brain regions of activation (BA) | Talairach coordinates (x; y; z) | Voxels | Cluster P-value |
|---|---|---|---|---|
| C > ASD >ADHD | L Middle frontal (BA 46/9/8/6) | −43; 33; 37 | 165 | 0.006 |
| ADHD, ASD >C | M Precuneus/cuneus (BA 7/19) | 0; −63; 31 | 138 | 0.008 |
| ASD >AD, C | L Cerebellum/hippocampal/lingual (BA 30/18/19) | −7; −52; −13 | 174 | 0.004 |
Abbeviations: ADHD, Attention Deficit Hyperactivity Disorder; ANOVA, analysis of variance; ASD, autism spectrum disorder; BA, Brodman area; C, healthy controls.
Figure 3.
(a) Horizontal slices showing split plot analysis of variance (ANOVA) effects of group by delay interactions on brain activation. The right side corresponds to the right side of the image. (b) Statistical measures of BOLD response are shown for each of the three groups for each of the brain region that showed a significant group by delay effect.
Although none of the ASD patients met an ADHD diagnosis, some of them (that is, 12) scored above clinical threshold in the Conners' Parent Rating Scale-Revised. In order to test whether findings would remain, we re-analysed the group and group by delay interactions without them. All main clusters and post-hoc comparisons remained significant with the exception of the L DLPFC for the group by delay interaction analysis, which was now only trend-wise significantly underactivated in ASD relative to controls (P<0.054) (but remained significantly underactivated for the group analysis).
Brain-behaviour and brain-performance correlations
In order to investigate whether regions that showed a significant group by delay effect were associated with performance or behaviour, the statistical BOLD response in these regions for the longest delay—with the greatest group differences—was extracted for each subject. Then the BOLD response measures were correlated (two-tailed) within each group separately with main performance measures of mean reaction time and SD intrasubject. Within each patient group, those BOLD measures that were abnormal (that is, DLPFC and precuneus in both disorders and cerebellum in ASD), were correlated with selected disorder-relevant behavioural measures, that is, with the SDQ hyperactivity for ADHD patients and with the autism diagnostic observation schedule communication and social skill scores and the SDQ prosocial score for ASD patients.
Within ASD there was a positive correlation between the autism diagnostic observation schedule communication and social scores and precuneus activation (r=0.6, P<0.007) and between DLPFC activation and SDQ prosocial scores (r=0.5, P<0.04). No significant correlations were observed for ADHD.
For performance, in controls, medial frontal activation was significantly negatively correlated with SDintrasubject (r=−0.6 P<0.007) and premature responses (r=−0.5, P<0.04). For ADHD boys, precuneus activation was significantly negatively correlated with premature errors (r=−0.4, P<0.05). No correlations were significant in ASD.
To determine if the enhanced precuneus brain activation cluster in patients was associated with the reduced DLPFC activation, we tested for inter-correlations. Within each group, the precuneus cluster was significantly negatively correlated with the prefrontal activation cluster (r<−0.5, P<0.01).
Discussion
To our knowledge, this is the first fMRI study elucidating commonalities or differences in the underlying neurofunctional substrates of patients with ADHD and ASD. During a parametrically modulated vigilance task, both disorders displayed shared underactivation relative to healthy controls in bilateral striato–thalamic and left-hemispheric DLPFC, pre and postcentral and superior parietal areas. They also shared enhanced precuneus activation, which in all groups correlated negatively with the prefrontal cluster. Precuneus was progressively more deactivated in controls with increased sustained attention load, but progressively more activated in patients, suggesting shared deficits in suppressing the default mode network (DMN). Disorder-specific effects were also observed: left DLPFC under activation was significantly more pronounced in ADHD relative to ASD boys, and associated with task performance measures that were only impaired in ADHD. ASD boys, in contrast, showed enhanced cerebellar activation relative to both ADHD and controls, suggesting a disorder-specific dysregulation of a fronto–striato–cerebellar attention network.
Brain activation data showed that across all subjects, increasing load of sustained attention was associated with progressively stronger activation in a typical sustained attention network comprising frontal, striato–thalamic, cingulate, temporo–parietal and cerebellar regions,16, 17, 26, 27 suggesting that the parametric vigilance task had the expected neural effects.
ADHD boys had significantly higher intra-subject response variability across all delays relative to both control and ASD boys and were slower in reaction times relative to controls in the long delays with the highest sustained attention load. The findings are in line with consistent evidence for higher response variability and slower reaction times in ADHD, thought to reflect poor concentration, attention lapses and poor stimulus anticipation.6, 45, 46, 47, 48, 49 Only for some of the longer delays, ASD patients also showed slower reaction times, suggesting some degree of performance impairment, in line with evidence for more severe attention deficits in ADHD than ASD.12, 50
Despite ASD boys having no performance deficits, both disorder groups, however, shared activation deficits in bilateral striato–thalamic, and left-hemispheric DLPFC, pre and postcentral and superior parietal areas. However, DLPFC was significantly more underactivated in ADHD relative to ASD boys.
While right lateral prefrontal underactivation has consistently been observed in ADHD patients during response inhibition,7 left DLPFC dysfunction is more typically observed during sustained attention17, 18 and oddball tasks.30, 51, 52, 53 The disorder-specific finding of more pronounced left frontal dysfunction in ADHD than ASD parallels more consistent evidence for frontal dysfunctions in ADHD7 than ASD54 and extends findings of disorder-specific left DLPFC underfunction in ADHD relative to conduct disorder.7 The findings are also parallel to a structural imaging study that showed that left DLPFC structural deficits were associated with attention problems in both patient groups.25
Interestingly, patients showed enhanced posterior activation, in the cerebellar vermis in patients with ASD (disorder-specific effect) and in precuneus in both disorders (trend-wise more pronounced in ADHD). The disorder-specific overactivation in ASD of the cerebellar part of a fronto–striato–parieto–cerebellar attention network16, 17, 26, 27 may have been a compensation for the reduced fronto–striatal activation, perhaps explaining the unimpaired performance.
The fronto–cerebellar dysregulation deficits in ASD may be associated with structural brain abnormalities in ASD in cerebellar vermis and left frontal regions,54, 55, 56, 57, 58 as well as with evidence for abnormal functional and structural fronto–cerebellar connectivity.59
The precuneus overactivation in both disorder groups is likely to reflect problems with deactivation of the DMN. With increasing attention load, healthy adults typically deactivate the DMN, comprising precuneus and posterior cingulate that mediate self-referential thoughts,60 which in turn is associated with optimal task performance and fewer attention lapses.60, 61 This interpretation is supported by our group by delay interaction findings, where controls, as expected,61 progressively deactivated precuneus with increasing sustained attention load, while in both disorders it was progressively more activated. The negative correlation between the DLPFC (that was progressively more activated in controls) and the precuneus clusters within all groups, further supports this interpretation of abnormal task-related DMN suppression in both disorders. The findings are in line with previous records of abnormal DMN activity in ASD59, 62 and ADHD, where this has been associated with attentional lapses.61, 63, 64 We have previously observed precuneus overactivation in ADHD patients during sustained attention tasks that was anti-correlated with reduced left lateral frontal activation and performance.17, 18 The more pronounced problems with the suppression of the precuneus DMN region in ADHD, together with a more pronounced DLPFC underactivation, may have been responsible for the disorder-specific performance deficits. This is further supported by the negative correlation in controls between DLPFC activation and response variability, and by the positive correlation between premature response errors in ADHD patients and precuneus activation, suggesting a link between the anti-correlated reduced DLPFC and increased precuneus activation and worse attention performance in ADHD. The less severe deficits in both of these regions in ASD together with a compensatory increase in cerebellum activation may thus explain the less severe performance deficits.
In ASD, furthermore, the deficits in precuneus deactivation were significantly positively correlated with autism diagnostic observation schedule social reciprocity and communication problem severity, while the DLPFC underactivation was positively associated with the prosocial SDQ scores. This could suggest that problems with reducing self-referential thoughts that interfere with frontal attention networks may be either a cause or a consequence of abnormalities in reciprocal social communication.
Other shared deficits were observed in thalamo–striatal and parietal areas, which in ADHD patients are typically abnormal during attention tasks,16, 17, 18, 30, 51, 53, 65 while in ASD, they are dysfunctional during selective attention and other cognitive tasks.20, 22, 23, 24, 55 The findings of shared parietal dysfunction are parallel to shared parietal structural deficits.25
The shared deficits in fronto–striato–parietal attention networks together with shared abnormalities in switching off the task-anticorrelated DMN may underlie shared attention deficits in both disorders but possibly also their shared deficits in other cognitive domains such as executive functions,50, 66 known to be mediated by the interplay between these task-positive and task-negative DMN networks.67
A strength of this study is the ‘medication-naïvety' of patients given long-term effects of psychotropic medication on brain structure and function68, 69 and the robust diagnostic characterisation of IQ matched, non-comorbid patient groups. However, although ASD patients did not meet clinical ADHD criteria, several of them scored high in attention problems on the Conners' Parent Rating Scale-Revised. Nevertheless, all findings remained when ‘purer' groups were compared.
In conclusion, this is, to our knowledge, the first comparative fMRI study between patients with ADHD and ASD. We demonstrate that the commonly compromised function of sustained attention is associated with both shared and disorder-specific dysfunctions and compensatory mechanisms. The findings suggest a more severe frontal lobe activation impairment in ADHD in association with more severe performance deficits, but also highlight shared striato–parietal and DMN dysfunctions that could explain and underlie the phenotypical overlap of poor attentive behaviours in both disorders.
Acknowledgments
This study was supported by grants from the Medical Research Council (MRC GO300155) to Prof Katya Rubia and the Medical Research Council UK Autism Imaging Multicentre Study (G0400061) to Prof Declan Murphy. Dr Anastasia Christakou was supported by a post-doctoral fellowship from MRC G0300155. Ana Cubillo and Dr Anna Smith were supported by PHD studentship/post-doctoral fellowships by the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) for Mental Health at the South London and Maudsley NHS Foundation Trust (SLaM) and the Institute of Psychiatry at King's College, London. We would like to thank all the individuals and their families who participated in this study and the National Autistic Society for their help in recruiting subjects.
KR has received funding from Lilly for another project and speaker's honoraria from Lilly, Shire, Novartis and Medice. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders4th edn.American Psychiatric Association: Washington DC; 2000 [Google Scholar]
- World Health Organisation The ICD-10 classification of mental and behavioural disordersIn: World Health Organisation (ed):Clinical Descriptions and Diagnostic Guidelines World Health Organisation; Geneva, 1994 [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. J Autism Dev Disord. 2006;36:849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Sturm H, Fernell E, Gillberg C. Autism spectrum disorders in children with normal intellectual levels: associated impairments and subgroup. Dev Med Child Neurol. 2004;46:444–447. doi: 10.1017/s0012162204000738. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Rubia K. Cool' inferior fronto-striatal dysfunction in Attention Deficit Hyperactivity Disorder (ADHD) versus ‘hot' ventromedial orbitofronto-limbic dysfunction in conduct disorder: a review. Biol Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ. Autism and attention deficit hyperactivity disorder: Assessing attention and response control with the Integrated Visual and Auditory Continuous Performance Test. Child Neuropsychol. 2006;12:335–348. doi: 10.1080/09297040500350938. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 2009;166:210–222. doi: 10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garretson HB, Fein D, Waterhouse L. Sustained attention in children with autism. J Autism Dev Disord. 1990;20:101–114. doi: 10.1007/BF02206860. [DOI] [PubMed] [Google Scholar]
- Schatz AM, Weimer AK, Trauner DA. Brief report Attention differences in Asperger syndrome. J Autism Dev Disord. 2002;32:333–336. doi: 10.1023/a:1016339104165. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Robertson IH, Kelly SP, Silk TJ, Barry E, Dáibhis A, et al. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia. 2007;45:2234–2245. doi: 10.1016/j.neuropsychologia.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman R, Warm JS, See JE.Brain systems of vigilanceIn: Parasuraman R (ed).The Attentive Brain MIT Press: Cambridge, MA; 1998221–256. [Google Scholar]
- Warm JS.An introduction to vigilanceIn: Warm JS (ed).Sustained Attention in Human Performance Wiley: Chichester; 19841–14. [Google Scholar]
- Sergeant JA.A theory of attention: An information processing perspective. Attention, memory, and executive functionIn: Reid LG, Krasnegor NA (eds).Attention, Memory, and Executive function Paul H Brookes Publishing: Baltimore, MD; 199657–69. [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a Rewarded Continuous Performance Task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A, Halari R, Matukura F, Mohammad M, Taylor E, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure Conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure Attention-Deficit/Hyperactivity Disorder during sustained attention. Am J Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Smith A, Giampietro V, Taylor E, Rubia K.Fronto-cortical and fronto-subcortical brain abnormalities in children and adults with ADHD: a review and evidence for fronto-striatal dysfunctions in adults with ADHD followed up from childhood during motivation and attention Cortex 2012(in press); doi: 10.1016/j.cortex.2011.04.007 [DOI] [PubMed]
- Allen G, Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: An fMRI study of autism. Am J Psychiatry. 2003;160:262–273. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain res Cogn brain res. 2003;2003-17:651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- Gomot M, Bernard FA, Davis MH, Belmonte MK, Ashwin C, Bullmore ET, et al. Change detection in children with autism: An auditory event-related fMRI study. Neuroimage. 2006;29:475–484. doi: 10.1016/j.neuroimage.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biol Psychiatry. 2008;63:974–980. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy D. Neural correlates of executive function in Autistic Spectrum Disorders. Biol Psychiatry. 2006;59:7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Silk TJ, Rinehart N, Bradshaw JL, Tonge B, Egan G, O'Boyle MW, et al. Visuospatial processing and the function of prefrontal-parietal networks in autism spectrum disorders: a functional MRI study. Am J Psychiatry. 2006;163:1440–U1443. doi: 10.1176/ajp.2006.163.8.1440. [DOI] [PubMed] [Google Scholar]
- Brieber S, Neufang S, Bruning N, Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, et al. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2007;48:1251–1258. doi: 10.1111/j.1469-7610.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- Voisin J, Bidet-Caulet A, Bertrand O, Fonlupt P. Listening in silence activates auditory areas: A functional magnetic resonance imaging study. J Neuroscience. 2006;26:273–278. doi: 10.1523/JNEUROSCI.2967-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Halari R, Giampietro V, Brammer M, Rubia K. Developmental effects of reward on sustained attention networks. Neuroimage. 2011;56:1693–1704. doi: 10.1016/j.neuroimage.2011.01.072. [DOI] [PubMed] [Google Scholar]
- Rubia K, Cubillo A, Smith AB, Woolley J, Heyman I, Brammer MJ. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum Brain Mapp. 2010;31:287–299. doi: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Cubillo A, Woolley J, Brammer MJ, Smith AB. Disorder-specific dysfunctions in patients with Attention-Deficit/Hyperactivity Disorder compared to patients with Obsessive-compulsive disorder during interference inhibition and attention allocation. Hum Brain Mapp. 2011;32:601–611. doi: 10.1002/hbm.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Smith AB, Mohammad M, Scott S, Brammer MJ. Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J Child Psychol Psychiatry. 2009;50:669–678. doi: 10.1111/j.1469-7610.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Goodman R. The strengths and difficulties questionnaire: A research note. J Child Psychol Psychiatry Allied Disciplines. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): Factor structure, reliability, and criterion validity. J Abnormal Child Psychol. 1998;26:279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Lecouteur A. Autism diagnostic interview-revised - a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, et al. Autism diagnostic observation schedule - a standardized observation of communicative and social-behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey L, Lord C.(eds)Social Communication Question Western Psychological Services: Los Angeles, USA; 2003 [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation: San Antonio Texas; 1999. [Google Scholar]
- Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–1068. [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S, Simmons A, et al. Prefrontal involvement in ″temporal bridging″ and timing movement. Neuropsychologia. 1998;36:1283–1293. doi: 10.1016/s0028-3932(98)00038-4. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Onishi Y, Poppel E. Two types of anticipation in synchronization tapping. Acta Neurobiol Experimentalis. 2004;64:415–426. doi: 10.55782/ane-2004-1524. [DOI] [PubMed] [Google Scholar]
- Brammer MJ, Bullmore ET, Simmons A, Williams SC, Grasby PM, Howard RJ, et al. Generic brain activation mapping in functional magnetic resonance imaging: a nonparametric approach. Magn Reson Imaging. 1997;15:763–770. doi: 10.1016/s0730-725x(97)00135-5. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Long C, Suckling J, Fadili J, Calvert G, Zelaya F, et al. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp. 2001;12:61–78. doi: 10.1002/1097-0193(200102)12:2<61::AID-HBM1004>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Brain. Thieme: New York; 1988. [Google Scholar]
- Rubia K, Smith A, Brammer M, Taylor E. Performance of children with Attention Deficit Hyperactivity Disorder (ADHD) on a test battery for impulsiveness. Child Neuropsychol. 2007;30:659–695. doi: 10.1080/09297040600770761. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Christakou A, Taylor E. Impulsiveness as a timing disturbance: neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos Trans R Soc Lond B Biol Sci. 2009;364:1919–1931. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Noorloos J, Smith A, Gunning B, Sergeant J. Motor timing deficits in community and clinical boys with hyperactive behavior: The effect of methylphenidate on motor timing. J Abnormal Child Psychol. 2003;31:301–313. doi: 10.1023/a:1023233630774. [DOI] [PubMed] [Google Scholar]
- Rommelse NNJ, Franke B, Geurts HM, Hartman CA, Buitelaar JK. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. European Child Adoles Psychiatry. 2010;19:281–295. doi: 10.1007/s00787-010-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse NNJ, Geurts HM, Franke B, Buitelaar JK, Hartman CA. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci Biobehav Rev. 2011;35:1363–1396. doi: 10.1016/j.neubiorev.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Giampietro V, Taylor E, Rubia K. Fronto-striatal hypo-activation during interference inhibition and attention allocation in a group of grown up children with ADHD with persistent hyperactive/inattentive behaviours in adulthood. Hum Brain Mapp. 2011;193:17–27. [Google Scholar]
- Stevens MC, Pearlson GD, Kiehl KA. An FMRI auditory oddball study of combined-subtype attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164:1737–1749. doi: 10.1176/appi.ajp.2007.06050876. [DOI] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Stigler KA, McDonald BC, Anand A, Saykin AJ, McDougle CJ. Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Res. 2011;1380:146–161. doi: 10.1016/j.brainres.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan B, Daly EM, McAlonan G, Loth E, Toal F, O'Brien F, et al. Brain morphometry volume in autistic spectrum disorder: a magnetic resonance imaging study of adults. Psychol Med. 2009;39:337–346. doi: 10.1017/S0033291708003383. [DOI] [PubMed] [Google Scholar]
- Toal F, Daly EM, Page L, Deeley Q, Hallahan B, Bloemen O. Clinical and anatomical heterogeneity in autistic spectrum disorder: a structural MRI study. Psychol Med. 2010;40:1171–1181. doi: 10.1017/S0033291709991541. [DOI] [PubMed] [Google Scholar]
- Deeley Q, Daly E, Surguladze S, Tunstall N, Mezey G, Beer D, et al. Facial emotion processing in criminal psychopathy - Preliminary functional magnetic resonance imaging study. Br J Psychiatry. 2006;189:533–539. doi: 10.1192/bjp.bp.106.021410. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Smith RG, Piven J. Cortical Gray and White Brain Tissue Volume in Adolescents and Adults with Autism. Biol Psychiatry. 2006;59:1–6. doi: 10.1016/j.biopsych.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism. Archives Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD Brain Wired Differently? A Review on Structural and Functional Connectivity in Attention Deficit Hyperactivity Disorder. Hum Brain Mapp. 2010;31:904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci and Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: event-related fMRI evidence. Am J Psychiatry. 2006;163:1033–1043. doi: 10.1176/ajp.2006.163.6.1033. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Vertie S, Oosterlaan J, Roeyers H, Sergeant JA. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism. J Child Psychol Psychiatry. 2004;45:836–854. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD and the effects of stimulant medication: Voxel-based meta-analysis. Am J Psychiatry. 2011;168:1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Murphy SE. Using Functional Neuroimaging to Investigate the Mechanisms of Action of Selective Serotonin Reuptake Inhibitors (SSRIs) Curr Pharm Des. 2010;16:1990–1997. doi: 10.2174/138161210791293051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.