Background: The molecular mechanisms regulating lymphangiogenesis remain unclear.
Results: Tetraspanin CD9 modulates molecular organization of integrins in LEC, thereby supporting several functions required for lymphangiogenesis.
Conclusion: Deletion of CD9 diminished lymphangiogenesis in mice and humans.
Significance: Given that CD9 mediates inflammation and tumor progression, CD9 might be a key component not only in tumor metastasis, but also in inflammation.
Keywords: Angiogenesis, Integrins, Lymphangiogenesis, Tetraspanins, Vascular Biology, CD81, CD9
Abstract
Tetraspanins have emerged as key players in malignancy and inflammatory diseases, yet little is known about their roles in angiogenesis, and nothing is known about their involvement in lymphangiogenesis. We found here that tetraspanins are abundantly expressed in human lymphatic endothelial cells (LEC). After intrathoracic tumor implantation, metastasis to lymph nodes was diminished and accompanied by decreased angiogenesis and lymphangiogenesis in tetraspanin CD9-KO mice. Moreover, lymphangiomas induced in CD9-KO mice were less pronounced with decreased lymphangiogenesis compared with those in wild-type mice. Although mouse LEC isolated from CD9-KO mice showed normal adhesion, lymphangiogenesis was markedly impaired in several assays (migration, proliferation, and cable formation) in vitro and in the lymphatic ring assay ex vivo. Consistent with these findings in mouse LEC, knocking down CD9 in human LEC also produced decreased migration, proliferation, and cable formation. Immunoprecipitation analysis demonstrated that deletion of CD9 in LEC diminished formation of functional complexes between VEGF receptor-3 and integrins (α5 and α9). Therefore, knocking down CD9 in LEC attenuated VEGF receptor-3 signaling, as well as downstream signaling such as Erk and p38 upon VEGF-C stimulation. Finally, double deletion of CD9/CD81 in mice caused abnormal development of lymphatic vasculature in the trachea and diaphragm, suggesting that CD9 and a closely related tetraspanin CD81 coordinately play an essential role in physiological lymphangiogenesis. In conclusion, tetraspanin CD9 modulates molecular organization of integrins in LEC, thereby supporting several functions required for lymphangiogenesis.
Introduction
Angiogenesis, the formation of new blood vessels from pre-existing vasculature, is an integral part of both normal developmental and numerous pathological processes, ranging from inflammation and wound healing to cancer (1). After decades of extensive study, anti-angiogenic drugs have recently entered the clinic, and with concomitant use of chemotherapy they can significantly prolong patient survival (2). Although both blood and lymphatic endothelial cells originate from common developmental precursors, their structures and functions are quite different (3). Unlike blood capillaries, lymphatic capillaries are formed by endothelial cells with discontinuous basal lamina and overlapping intercellular junctions, and they lack mural cells (4). Lymphatic vessels are essential for the maintenance of tissue fluid balance, immune surveillance, and absorption of fatty acids and are thus involved in pathological responses such as tumor metastasis and inflammation (5, 6). Nevertheless, the molecular mechanisms regulating lymphangiogenesis, the formation of new lymphatic vessels from pre-existing ones, have been explored far less than those of angiogenesis. For example, whereas tumor metastasis to regional lymph nodes is used clinically as a prognostic tool and a guide to treatment, the molecular mechanisms remain unclear (7). Because of the recent identification of lymphatic endothelial cell-specific markers, however, considerable progress has been made over the past several years (3). Vascular endothelial growth factor receptor (VEGFR)-32 KO mice die in utero because of abnormal development of the blood vasculature resulting in cardiovascular failure, whereas the loss of the VEGFR-3 ligand VEGF-C results in embryonic lethality because of a lack of lymphatic vessel formation (8, 9). Moreover, several papers have shown that blocking VEGFR-3 inhibits tumor lymphangiogenesis and metastasis in mice (10). Recently, additional growth factors have been reported to participate in lymphangiogenesis, such as PDGF, hepatocyte growth factor, and basic FGF (5). However, because many of these effects may be secondary to the induction of VEGF-C and VEGF-D, the VEGFR-3 axis may be regarded as crucial in lymphangiogenesis (3).
Numerous studies that use inhibitors of integrin functions and mice lacking specific integrins clearly implicate integrins in vasculogenesis and angiogenesis (11). Accumulating reports on integrins have also emerged in the field of lymphangiogenesis (3, 11). Integrin α9β1 is currently viewed as a major integrin associated with lymphangiogenesis because integrin α9-KO mice develop respiratory failure and postnatal death caused by chylothorax (12). It has also been shown that α9 integrin binds VEGF-C directly (13). Moreover, inhibition of α5β1 integrin reduced lymphangiogenesis in inflamed airways after Mycoplasma pulmonis infection and in corneal inflammation (14, 15). Recent papers also suggest that VEGFR-3 forms complexes with integrins, such as β1 and α5 in lymphatic endothelial cells (LEC) (16, 17).
Tetraspanins are also cell surface proteins that span the membrane four times and are abundantly expressed in various cells (16). A most unique feature of tetraspanins is their propensity to interact with one another and with various other transmembrane molecules, including integrins, thereby acting as molecular organizers that regulate the formation of functional clusters of proteins at tetraspanin-enriched microdomains. For example, the association of tetraspanins with growth factor receptors, including EGFR and c-Met, has been described previously (18, 19). By organizing various functional molecules, tetraspanins have been implicated in a large variety of biological processes including cell fusion, migration, proliferation, and morphogenesis, which affect fertilization, immune disease, and tumor metastasis (20). A number of clinicopathological studies have reported a link between the expression level of tetraspanins and metastasis and/or prognosis (21). Because of extensive study of the functional role of tetraspanins in tumor cells, increasing attention has been paid to their therapeutic application (22). For instance, an anti-CD151 blocking antibody prevents tumor cell dissemination by inhibiting intravasation without affecting primary tumor growth (23), whereas anti-CD9 monoclonal antibodies were found to inhibit the transendothelial migration of melanoma cells (24). Despite the abundant knowledge of the role of tetraspanins in tumor cells, little is known about their functions in angiogenesis (25, 26), and nothing is known about their involvement in lymphangiogenesis. This is the first report to demonstrate that CD9, the most abundant tetraspanin in LEC, promotes lymphangiogenesis in vitro, ex vivo, and in vivo, probably through post-adhesion events such as migration and morphogenesis.
EXPERIMENTAL PROCEDURES
Mice
CD9-KO and CD81-KO mice were provided by Dr. E. Mekada (Research Institute for Microbial Diseases, Osaka University, Osaka, Japan), and the generation of CD9/CD81-DKO mice was described previously (27). These mice were backcrossed more than eight generations into the C57BL/6J background in a barrier facility, and all of the animal procedures were performed in accordance with the Osaka University guidelines on animal care. Genotyping of all breeding pairs was confirmed by PCR analysis. 8–20-week-old CD9-KO mice and WT littermates matched for age and sex were used.
Cell Culture and siRNA Transfection
Human umbilical vein endothelial cells (HUVEC) and human dermal lymphatic endothelial cells (HDLEC) were cultured in an EGM-2 MV bullet kit (Lonza, Basel, Switzerland) (26). siRNA duplexes targeting mouse CD9 (JS-BBS3608-1), CD81 (CB-BBS4412-1), and CD151 (SHF27A-2034-C) were synthesized by and purchased from B-Bridge International (San Jose, CA). Cells cultured in a 6-well plate were transfected with a 10 nm mixture of either the siRNAs or control random RNAs (S20C-0600; B-Bridge International) with Lipofectamine RNAiMAX (Invitrogen).
Isolation of Mouse Blood Endothelial Cells and Lymphatic Endothelial Cells
Mouse blood endothelial cells (BEC) were isolated as described (26). Mouse LEC (mLEC) were derived from lymphangiomas induced by intraperitoneal injection of incomplete Freund's adjuvant, as previously reported with some modifications (28). Briefly, each mouse was injected intraperitoneally with 200 μl of an emulsion containing incomplete Freund's adjuvant (Sigma-Aldrich), followed by second injection of incomplete Freund's adjuvant after 11 days. After 21 days, the tumors were then explanted, mechanically disrupted, and incubated in PBS containing 0.1% collagenase type I (Worthington, Lakewood, NJ) at 37 °C for 45 min. Once confluence was reached, the cells were selected with anti-mouse LYVE-1-coated beads (R&D Systems, Minneapolis, MN), and its purity was confirmed by anti-Prox-1 Ab (more than 80%).
Immunoblotting and Immunoprecipitation
The cells were lysed in lysis buffer containing 1% Nonidet P-40, 20 mm Tris-HCl (pH 7.4), 150 mm NaCl, 2 mm EDTA, 2 mm PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mm orthovanadate, and 50 mm NaF. Cell lysates containing an equal amount of protein were electrophoresed as described previously (26). The membranes were probed with primary Abs followed by peroxidase-conjugated secondary Abs. Serum-starved HDLEC were put on collagen for VEGF-C signaling. Anti-phospho-Akt (Ser-473), anti-Akt, anti-phospho-p38 (Thr-180/Tyr-182), anti-p38, anti-phospho-Erk (Thr-202/Tyr-204), and anti-ERK were from Cell Signaling Technology (Beverly, MA); anti-phospho-VEGFR-3 (Tyr-1063/Tyr-1068) was from Cell Applications (San Diego, CA); anti-FAK was from BD Biosciences; anti-VEGFR-3 (9D9F9) was from Millipore; and anti-phospho-FAK (Tyr-397) was from Santa Cruz Biotechnology (Santa Cruz, CA). For immunoprecipitation, the cell lysates were precleared by incubation for 1 h at 4 °C with agarose beads conjugated with protein G-Sepharose 4 Fast Flow (GE Healthcare). Immune complexes were collected using appropriate mAbs prebound to protein G and subjected to SDS-PAGE followed by immunoblotting.
Proliferation and Adhesion
For mLEC seeded onto collagen-coated 96-well tissue culture plates at 3000 cells/well, proliferation was assessed with water-soluble tetrazolium salt (Cell Counting Kit-8; Dojindo, Kumamoto, Japan). Static cell adhesion was quantified as described (26) on substrates coated onto plastic for 12 h at 10 μg/ml fibronectin (FN) or diluted 1:30 from a stock solution of Matrigel (Mat) (BD Biosciences, Bedford, MA).
Cable Formation
To assess cable formation, endothelial cells (1 × 105 in 200 μl DMEM plus 5% FCS) were placed on Matrigel in triplicate wells of 48-well plates (26). The cables were photographed in at least three random fields and quantified with MetaMorph imaging software. The data are representative of three independent studies with similar results.
Motility Assays
Chemotactic migration was performed in polycarbonate filter wells (Transwell, 8-μm pores; Costar, Cambridge, MA) coated with FN. HUVEC, HDLEC, and mLEC were plated (5 × 104 cells in 100 μl of DMEM containing 1% FCS) in the upper chamber. The bottom chamber contained 20 ng/ml VEGF-A and 300 ng/ml VEGF-C as chemoattractants for HUVEC and HDLEC, respectively. After 6 h, the cells remaining on the upper surface of the membrane were wiped off, and cells migrating to the lower surface in triplicate wells were visualized with Diff-Quick stain and counted in each of four randomly chosen microscopic fields at ×20 objective with a Keyence BZ-9000 (Keyence, Osaka, Japan). Random migration was assessed in a glass-bottomed 35-mm dish (Matsunami, Osaka, Japan) that had been coated overnight with Mat as described (26) and blocked with 5 mg/ml cell culture grade BSA (ICN Pharmaceuticals, Santa Ana, CA). Motility images were acquired every minute for 1 h with an Olympus IX70 Inverted Microscope (Olympus, Tokyo, Japan). MetaMorph imaging software was used to calculate x and y centers, and the tracks and distances of random motility were determined.
Aortic Ring and Lymphatic Ring Assay
Thoracic aortas and thoracic ducts were isolated from WT and CD9-KO mice under a dissecting microscope, cut into 1-mm sections, and embedded in 24-well Matrigel-coated plates. Medium containing 20 ng/ml VEGF-A for aortas and 300 ng/ml VEGF-C (R&D Systems) for thoracic ducts was added to each well of gelled Matrigel (26, 29). The length of microvessel-like sprouting was measured with MetaMorph imaging software (version 7.5).
Tumor Implantation Assay
The induction of lymph node metastasis was performed by the orthotopic intrapulmonary implantation of Lewis lung carcinoma cells, as described previously (28). Briefly, tumor cells (3 × 103) were resuspended in 20 μl of PBS containing 10 μg of Matrigel to prevent the suspension from leaking out of the lung, and the cells were then injected into the lung parenchyma through the intercostal space into the lung. The microvascular density and lymphatic vascular density (LVD) were analyzed as described previously (30).
Whole Mount Staining
After anesthesia, the vasculature was perfused for 2 min with fixative (1% paraformaldehyde in PBS, pH 7.4) from a cannula inserted through the left ventricle into the aorta. Tracheas and diaphragms were immersed in fixative for 1 h at 4 °C (31, 32). The tissues were stained with the following primary antibodies: CD31 (rat anti-mouse, clone MEC13.3; BD Biosciences) and LYVE-1 (rabbit polyclonal antibodies; Upstate Biotechnology, Lake Placid, NY). Secondary antibodies were labeled with Alexa 488-conjugated goat anti-rabbit IgG, and Alexa 546-conjugated goat anti-rat IgG (Invitrogen). The specimens were viewed with a Leica TCS-SP5 (Wetzlar, Germany) confocal microscope using LAS AF software (version 2.3.6).
Transmission Electron Microscopy
Tissue samples were fixed at 4 °C in 2.5% glutaraldehyde in 0.1 m phosphate buffer (pH 7.2) and postfixed at room temperature in 2% osmium tetroxide in the same buffer. After dehydration in ethanol, the specimens were embedded in Epon 812 resin (TAAB, Berkshire, UK). The ultrathin sections (60 nm) were stained with uranyl acetate and lead citrate and observed under a transmission electron microscope (H-7100; Hitachi, Tokyo, Japan).
Statistical Analysis
In vitro assays were performed in quadruplicate cultures. The animal experiments were done with at least four mice for each group. All of the numerical results are expressed as the means ± S.E. The statistical differences were determined by Student's t test. p values <0.05 were considered statistically significant.
RESULTS
Tetraspanins Are Abundantly Expressed in Lymphatic Endothelial Cells
To examine the expression of tetraspanins and integrins in lymphatic endothelial cells, we initially compared the expression of HDLEC with that in HUVEC. Although HUVEC showed substantial expression of integrins (α2, α3, and α5), as well as tetraspanins (CD9, CD81, CD151, and CD63), expression of CD81, CD151, and particularly CD9 in HDLEC was more abundant than that in HUVEC and that of the integrins (Fig. 1). These data suggest that tetraspanins are expressed not only in blood endothelial cells but also lymphatic endothelial cells.
FIGURE 1.
Expression of tetraspanins and integrins in HUVEC (A) and HDLEC (B). Expression of the indicated antigens (blue histograms) on the surface of HUVEC (A) and HDLEC (B) was determined by flow cytometry. Ten thousand cells were stained with CD9 (MM2/57), CD81 (JS64), CD151 (5C11), CD63 (MEM259), β1 (TS2/16), integrins α2 (BHA2.1), α3 (P1B5), α5 (P1D6), and α9 (2Q954) or podoplanin (NZ-1) at a concentration of 2 μg/ml and then labeled with FITC-conjugated goat anti-mouse, IgG (Vector Laboratories, Burlingame, CA). Normal mouse or rat IgG was used as a control. Stained cells were analyzed on a FACSCalibur (Becton Dickinson). Mean fluorescence intensity is shown in each histogram. Negative control peaks (green lines) were obtained with secondary antibody alone. Expression of podoplanin specific to LEC was observed in HDLEC, but not in HUVEC. Both HUVEC and HDLEC were analyzed between the third to fifth passages. The data are representative of three independent studies, which produced similar results.
Decreased Lymph Node Metastasis and Tumor Lymphangiogenesis in CD9-KO Mice
To test whether deletion of CD9 in mice affects lymphatic metastasis, we introduced a lymph node metastasis model. Although intrapulmonary growth of Lewis lung carcinoma cells in the CD9-KO mice appeared to be reduced, the difference in tumor weight did not reach significance (Fig. 2A). Consequently, the microvascular density of the implanted tumor in the CD9-KO mice was reduced (Fig. 2B). Of note, the peritumoral LVD of the implanted tumors in the CD9-KO mice was decreased relative to that in the WT mice (Fig. 2C). Consistent with this decreased lymphangiogenesis, the total weight of metastatic lymph nodes in CD9-KO mice was decreased (Fig. 2D).
FIGURE 2.
Decreased lymph node metastasis and lymphangiogenesis in CD9-KO mice. A, growth of lymph node metastases and primary tumors in the lungs was assessed (n = 5). Dotted lines show the margins of the metastatic lymph nodes. Arrowheads show primary tumors in the lung. B, CD31-positive microvascular density (MVD) of the primary tumors was decreased in the CD9-KO mice. C, peritumoral LVD stained with LYVE-1 (green). The peritumor area was defined as a 1-mm distance from the tumor periphery. Dotted lines show the margins of the implanted tumors in the lungs. The cell nuclei are counterstained with Hoechst (blue). D, metastatic lymph nodes were diminished in CD9-KO mice, although the sizes of isolated lymph nodes were indistinguishable before tumor implantation (data not shown). The data are representative of three independent studies with similar results. The bars represent the means ± S.E. *, p < 0.05 versus WT; ***, p < 0.005 versus WT. Scale bar, 1 cm for D and 100 μm for B and C.
Decreased Tumor Angiogenesis and Lymphangiogenesis in CD9-KO Mice
To further investigate tumor angiogenesis, as well as lymphangiogenesis, we subcutaneously injected Lewis lung carcinoma cells onto the back of each mouse. Local tumor growth in the CD9-KO mice was less pronounced than that in WT mice when determined by tumor volume and weight (Fig. 3A). Consistent with the results of the metastasis model, not only microvascular density but also LVD was decreased in the CD9-KO mice (Fig. 3, B and C). Collectively, these data indicate that deletion of CD9 diminishes pathological angiogenesis and lymphangiogenesis in mice, which might have caused decreased local tumor growth and lymph node metastasis, respectively.
FIGURE 3.
Decreased tumor growth and angiogenesis in CD9-KO mice. A, for primary tumor growth, mice were injected subcutaneously with Lewis lung carcinoma cells. Tumors were measured with Vernier calipers, and the volume was calculated (length × width2 × 0.52). After 20 days, tumors were collected and weighed (n = 10). Arrows show primary tumor growth in both sites at 20 days. B and C, cryosections of primary tumors were stained with anti-CD31 Ab (red) as a blood endothelial marker and with anti-LYVE-1 Ab (green) as a lymphatic endothelial marker. The data are representative of three independent studies with similar results. The bars represent the means ± S.E. *, p < 0.05 versus WT; **, p < 0.01 versus WT; ***, p < 0.005 versus WT. The scale bars represent 100 μm for B and C. MVD, microvascular density.
Decreased Expression of CD9 Diminishes Angiogenesis in Mice and Human Cells
Despite a few reports about the involvement of tetraspanins in angiogenesis (25, 26), a definitive role for CD9 in angiogenesis remained to be shown. To address this, we knocked down HUVEC with CD9 siRNA. Knockdown of CD9 (CD9-KD) altered neither expression of other tetraspanins (CD81 and CD151) and β1 integrin nor static cell adhesion on Mat and FN (Fig. 4A and data not shown). However, it diminished random motility, chemotaxis, and cable formation (Fig. 4, B–D). Furthermore, isolated mouse BEC from each group again showed expression of β1 integrin and CD81 at comparable levels (data not shown). Nevertheless, cable formation by CD9-KO mouse BEC was less remarkable (Fig. 4E). Moreover, aortic rings isolated from CD9-KO mice showed decreased microvascular sprouting (Fig. 4F). Together, these results indicate that decreased expression of CD9 diminishes angiogenesis in mice and human cells.
FIGURE 4.
CD9 promotes angiogenesis in blood vascular endothelial cells from mice and humans. A, after HUVEC were transfected with siRNAs against CD9 (CD9) or random RNAs (Control), knockdown of CD9 protein was confirmed by immunoblotting in parallel with integrin β1 (TS2/16), CD9 (MM2/57), CD81 (JS64), CD151 (11G5a), and β-actin (C4). B, to measure random migration, HUVEC were plated on coverslips coated with Mat. Cell movements recorded by time lapse video microscopy were quantified for 1 h with MetaMorph (n = 10). C, for chemotaxis, HUVEC were plated in upper Transwell chambers coated with FN. Cells migrating through the filter were counted in four randomly selected fields after 6 h. D and E, mouse BEC were isolated from collagenase-digested lung tissue (with anti-mouse CD31-coated beads) and enriched (with anti-mouse ICAM-2) to more than 90% purity (positively stained for von Willebrand factor). Both HUVEC and mBEC were seeded on Matrigel, and the total length of cables was quantitated after 24 h. F, shown are phase contrast images of branching vessel-like structures in WT and CD9-KO explants (day 4). The data are representative of three independent studies with similar results. The bars represent the means ± S.E. *, p < 0.05 versus WT; **, p < 0.01 versus WT; ***, p < 0.005 versus WT. Scale bar, 200 μm for C–E and 500 μm for F.
Diminished Lymphangiogenesis in CD9-KO Mice in Vitro, ex Vivo, and in Vivo
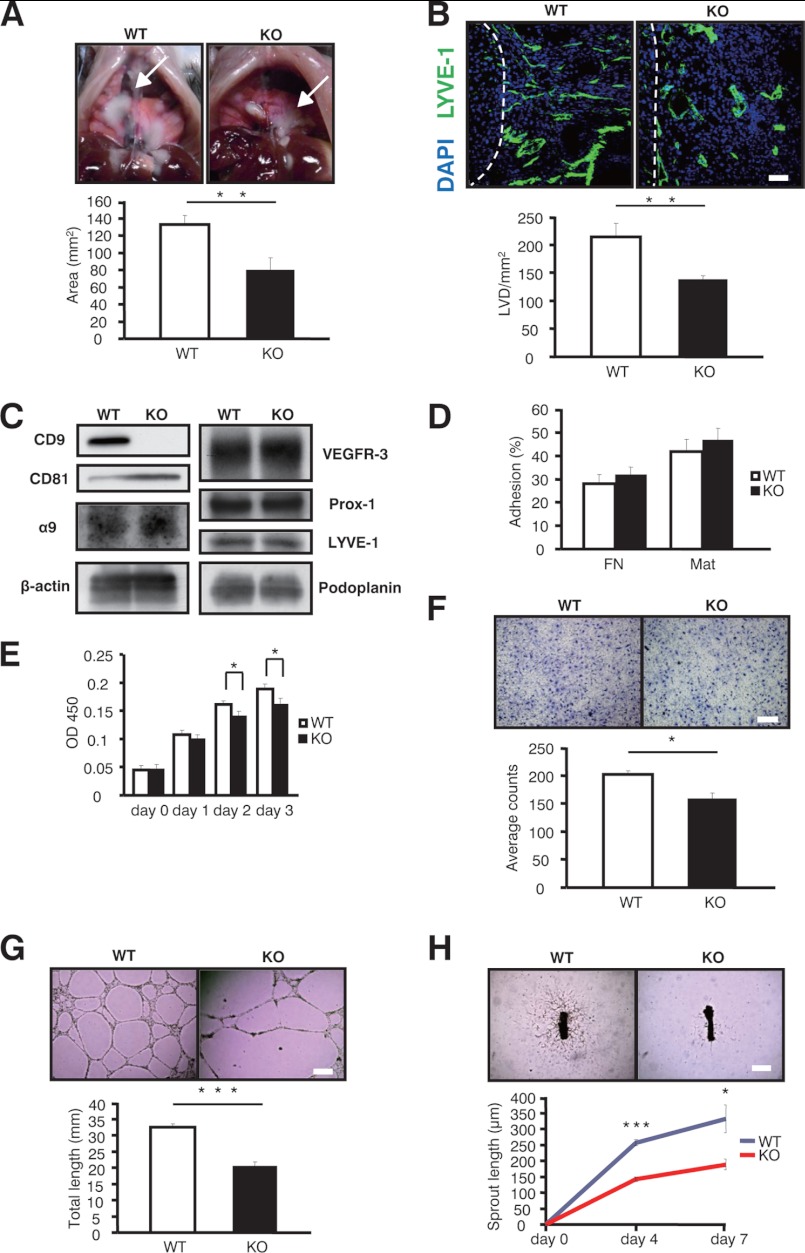
To further test the role of CD9 in inflammation-induced lymphangiogenesis, we induced lymphangiomas in mice (33). Lymphangiomas in CD9-KO mice were decreased compared with those in WT mice (Fig. 5A). Consistent with this, the LVD in the lymphangiomas from CD9-KO mice was decreased (Fig. 5B), indicating that CD9 could also be involved in inflammation-induced lymphangiogenesis. Moreover, isolated mLEC from these lymphangiomas expressed integrins (α5, α9, and β1) and lymphatic-specific molecules (VEGFR-3, Prox-1, LYVE-1, and podoplanin) at similar levels, except for a slight increase in CD81 in the CD9-KO cells (Fig. 5C, and not shown). Although mLEC from CD9-KO mice did show comparable ability to adhere (Fig. 5D), they exhibited decreased proliferation (Fig. 5E), impaired chemotactic ability toward VEGF-C (Fig. 5F), and reduced cable formation (Fig. 5G). Of note, lymphatic sprouting of thoracic ducts isolated from CD9-KO mice was decreased upon VEGF-C stimulation (Fig. 5H). Together, deletion of CD9 in LEC diminished lymphangiogenesis in vitro, ex vivo, and in vivo.
FIGURE 5.
CD9 in mice promotes lymphangiogenesis in vitro, ex vivo, and in vivo. A, shown are representative images of lymphangiomas in WT and CD9-KO mice (arrows). B, LVD defined with LYVE-1 Ab (green) was also decreased in the CD9-KO mice. The cell nuclei were counterstained with Hoechst (blue). Dotted lines show the margins of the lymphangiomas and the livers. C, isolated mLEC were confirmed by immunoblotting with the indicated antibodies: CD9 (KMC8), CD81 (Eat-2), integrin α9 (R&D Systems), VEGFR-3 (R&D Systems), Prox-1 (AngioBio), LYVE-1 (abcam), and podoplanin (8.1.1; eBioscience). D, static cell adhesion was performed on FN and Matrigel. E, isolated mLEC were stimulated with VEGF-C for the proliferation assay (300 ng/ml) (n = 3). F, for chemotaxis, mLEC were plated on upper Transwell chambers coated with FN. The bottom chambers contained VEGF-C (300 ng/ml). G, for cable formation, mLEC were seeded on Matrigel, and the total length was quantitated after 24 h. The cables were photographed in at least three random fields and quantified with MetaMorph imaging software. H, sprouting from thoracic rings was quantitated (n = 6). The data are representative of four independent studies with similar results. The bars represent the means ± S.E. *, p < 0.05 versus WT; **, p < 0.01 versus WT; ***, p < 0.005 versus WT. Scale bar, 200 μm for B and F and 500 μm for G and H.
Knockdown of CD9 from HDLEC Impairs Lymphangiogenesis through Post-adhesion Events
To further determine the functional role of CD9 in lymphangiogenesis, HDLEC were knocked down with CD9 siRNA. Treatment with siRNA reduced the level of CD9 protein in HDLEC, as determined by blotting (Fig. 6A). Although knockdown of CD9 from HDLEC did not alter expression of membrane molecules as indicated, it did slightly up-regulate the expression of CD81 (Fig. 6A). Although knockdown of CD9 did not alter static cell adhesion (Fig. 6B), it impaired several cell functions relevant to lymphangiogenesis. First, upon VEGF-C stimulation of CD9-KD cells, proliferation was decreased in a time-dependent manner (Fig. 6C). Second, chemotaxis toward VEGF-C was reduced in CD9-KD cells compared with control cells (Fig. 6D). Third, morphogenesis of CD9-KD cells was significantly attenuated (Fig. 6E). Overall, these data further indicate that decreased expression of CD9 in HDLEC impairs pivotal functions required for lymphangiogenesis in vitro.
FIGURE 6.
CD9 promotes lymphangiogenesis in lymphatic endothelial cells from humans. A, after HDLEC were transfected with siRNAs against CD9 (CD9) or random RNAs (Control), knockdown of CD9 protein was confirmed by immunoblotting in parallel with integrin (β1, α5), CD9 (MM2/57), CD81 (JS64), CD151 (11G5a), VEGFR-3 (C20), LYVE-1 (RELIATech), podoplanin (NZ-1), and β-actin (C4). B, adhesion of HDLEC was quantified (n = 3). C, proliferation was stimulated with VEGF-C (300 ng/ml) (n = 3). D, for chemotactic migration, HDLEC were plated in upper Transwell chambers coated with FN. E, for the two-dimensional assay, HDLEC were seeded on Matrigel, and total cable length was quantitated after 24 h. The cables were photographed in at least three random fields and quantified with MetaMorph imaging software. The data are representative of three independent studies with similar results. The bars represent the means ± S.E. *, p < 0.05 versus WT; ***, p < 0.005 versus WT. Scale bar, 200 μm for D and E.
Deletion of CD9 Impairs Functional Complexes between Integrins and VEGFR-3 in LEC
Stimulation of β1 integrin induced tyrosine phosphorylation of VEGFR-3 and FN and not only transactivated VEGFR-3 but also enhanced the phosphorylation of VEGFR-3 induced by VEGF-C (16, 17). Given that β1 integrin forms complexes with VEGFR-3 in LEC, we hypothesized that deletion of CD9 might affect these functional complexes in LEC. To test this, we initially examined the association among these molecules in LEC by immunoprecipitation. As reported, β1 integrin did associate with VEGFR-3. At the same time, CD9 associated with integrins (α5 and α9), which was also confirmed by reciprocal immunoprecipitation (Fig. 7A). Next, to study how CD9 deletion affects these functional complexes associated with β1 integrin, we performed immunoprecipitation analysis with biotinylated LEC. As shown in Fig. 7B, several co-immunoprecipitates were diminished after CD9 deletion. The diminished molecules in the co-immunoprecipitates were identified as VEGFR-3 and integrins (α5 and α9), whereas integrins α6 and CD151 were not altered among the co-immunoprecipitates. Four other bands that were not identified (designated w, x, y, and z) were also diminished in the co-immunoprecipitates. Taken together, these data suggest that deletion of CD9 impairs formation of functional complexes between integrins and VEGFR-3 in LEC.
FIGURE 7.
Deletion of CD9 impairs functional complexes between integrins and VEGFR-3 in LEC. A, to confirm the association between integrin β1, α5, CD9, and VEGFR-3, HDLEC lysed in 1% Brij-97 buffer were immunoprecipitated with the indicated antibody. B, after CD9 knockdown, immunoprecipitation with integrin β1 was compared between control siRNA (Control) and CD9-KD HDLEC (CD9). Note that co-precipitates with integrin β1 were down-regulated after CD9-KD. The antibodies used here were as follows: β1 (TS2/16), α5 (P1D6), α6(GOH3), α9 (2Q954), CD9 (MM2/57), CD151 (5C11), and VEGFR-3 (9D9F9). Whole cell lysates (WCL) were blotted with β-actin as a loading control. The data are representative of three independent studies with similar results. IP, immunoprecipitation; IB, immunoblotting.
Knockdown of CD9 Diminishes VEGFR-3 Signaling in Lymphatic Endothelial Cells
The VEGF-C/VEGFR-3 axis constitutes the key signal transduction for proliferation, migration, and morphogenesis in LEC (34). Given that CD9 facilitates formation of functional complexes between integrins and VEGFR-3, we speculated that deletion of CD9 in LEC could impair VEGF-C-dependent signaling pathways. Upon stimulation with VEGF-C, up-regulation of phosphorylated VEGFR-3 was attenuated in CD9-KD LEC (Fig. 8). Moreover, as downstream signaling pathways of VEGFR-3, activated (phosphorylated) focal adhesion kinase (FAK), ERK, and p38 signaling were also diminished, whereas activation of Akt kinase was minimally altered (Fig. 8). Knockdown of CD9 impairs VEGFR-3 and its downstream signaling in LEC.
FIGURE 8.
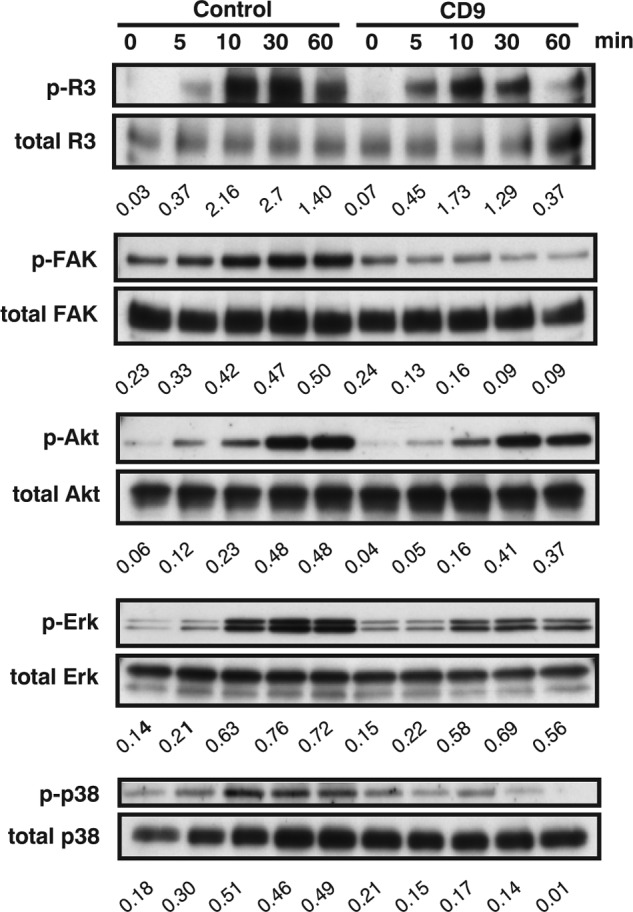
Deletion of CD9 impairs VEGF-C-induced signaling in LEC. Serum-starved HDLEC were stimulated with human VEGF-C (200 μg/ml). Equal amounts of lysate were probed with Abs to activated VEGFR-3 (p-VEGFR-3), activated FAK (p-FAK), activated Akt (p-Akt), activated Erk (p-Erk), activated p38 (p-p38), and β-actin (to control for loading). The numbers under blots represent ratios of phosphorylated to total protein from densitometry analysis. The data are representative of three independent studies from different cell preparations.
Tetraspanins CD9 and CD81 Function Cooperatively in Physiological Angiogenesis and Lymphangiogenesis
Considering that CD9 and CD81 were co-localized in the LEC (data not shown) and that each tetraspanin was reciprocally up-regulated, we hypothesized that these closely related tetraspanins might compensate for each other, thereby maintaining the integrity of the LEC. To confirm this functional redundancy, we first determined the effect of knocking down CD9 and CD81 in HDLEC. As expected, knockdown of either CD9 or CD81 did up-regulate the other without affecting CD151 expression, whereas CD151 knockdown did not affect the expression of CD9 and CD81 (Fig. 9A). Importantly, knockdown of CD81 also inhibited cable formation at levels similar to that of CD9 knockdown. Furthermore, double knockdown of CD9 and CD81 additively suppressed cable formation compared with knockdown of either one alone, whereas knockdown of CD151 did not (Fig. 9B).
FIGURE 9.
CD9 and CD81 coordinately promote lymphangiogenesis in HDLEC. A, after HDLEC were transfected with siRNAs against CD9, CD81, and CD151, knockdown of these proteins was confirmed by immunoblotting in parallel with integrin β1 and β-actin. None of siRNAs modulated the expression of β1 integrin. β-Actin was blotted to control for loading. The numbers under the blots indicate ratios from densitometry analyses. The ratios were calculated by dividing densitometry values for actin by those for β1, CD9, CD81, and CD151. B, for the cable formation assay, HDLEC were seeded on Mat, and the total length of cellular cables was quantitated after 24 h. The scale bars represent 200 μm. The bars represent the means ± S.E. ***, p < 0.005 versus WT. The data are representative of three independent studies with similar results.
To define the overlapping functions of CD9 and CD81 in mice, we performed whole mount staining of tracheal vasculature isolated from WT, CD9-KO, CD81-KO, and CD9/CD81-double knock-out (DKO) mice. No gross morphologic differences in blood vessels and lymphatic vessels were observed in either of the KO mice, whereas the DKO mice displayed compromised lymphatic networks in the trachea, where the lymphatic vessels appeared to be disconnected and thinner than those of WT mice (Fig. 10A). Likewise, the morphology and abundance of the blood vascular network were also compromised in the DKO mice, although to a much lesser extent. To exclude the possibility that the emphysematous lung phenotype in DKO mice might influence these vascular phenotypes (27), we further examined the diaphragm. Lymphatics in the diaphragm were markedly decreased in DKO mice (Fig. 10B). To delineate the ultrastructural change, we added electron microscopic analysis. Of interest, protrusions and pinocytes in tracheal LEC were dramatically reduced, and anchoring filaments, which support the structure and function of lymphatic vessels, were disrupted in DKO mice, indicating that the LEC in DKO mice might also be functionally impaired (Fig. 10C). Moreover, the basement membrane was much thinner, and pinocytes were decreased in tracheal BECs from DKO mice (Fig. 10D). Together, tetraspanins CD9 and CD81 cooperatively play an essential role in physiological angiogenesis and lymphangiogenesis.
FIGURE 10.
Impaired physiological lymphangiogenesis as well as angiogenesis in CD9/CD81-DKO mice. A, lymphatic vessels and blood vessels in trachea isolated from tetraspanin-KO mice. CD9, CD9-KO mice; CD81, CD81-KO mice; KO, CD9/CD81-DKO mice. Confocal microscopic images of immunoreactivities of tracheal whole mounts stained for CD31 (red, blood vessels) and LYVE-1 (green, lymphatic vessels). B, diaphragms were stained with LYVE-1. C, shown are pictures of LEC in tracheas from WT and DKO mice by electron microscopy. Arrowheads, protrusions from mLEC; arrows, anchoring filaments. D, shown are pictures of BEC from WT and DKO mice by electron microscopy. Arrows, basement membranes; arrowheads, pinocytes. Magnification: upper panel, ×20,000; lower panel, ×20,000. The bars represent the means ± S.E. ***, p < 0.005 versus WT. Scale bar, 200 μm for A and B.
DISCUSSION
Here, we demonstrate for the first time that: 1) tetraspanins, including CD9, are abundantly expressed in lymphatic endothelial cells; 2) CD9 promotes lymphangiogenesis through post-adhesion events such as migration, morphogenesis, and proliferation; 3) CD9 promotes VEGFR-3 signaling, presumably by organizing functional complexes between integrins and VEGFR-3; 4) diminished lymphangiogenesis occurs in pathological conditions in tumor and inflammation models; and 5) tetraspanins CD9 and CD81 play complimentary roles in physiological angiogenesis and lymphangiogenesis.
Diminished Tumor Lymphangiogenesis in CD9-KO Mice
Tumor cells have been thought to enter the lymphatic vessels by invading pre-existing lymphatic vessels in the tumor periphery or by eliciting lymphangiogenesis through production of growth factors (6). We revealed that tetraspanin CD9 in LEC participates in lymphatic metastasis, probably through promoting tumor lymphangiogenesis. Considering that tetraspanin CD9 in tumor sites plays key roles in tumor progression, our finding that CD9 in host sites supports not only angiogenesis but also lymphangiogenesis is important. However, our results should be interpreted cautiously. First, it remains to be determined whether nonendothelial cells might contribute to tumor progression, because tumor microenvironments contain many different types of cells, including platelets, macrophages, and fibroblasts, most of which express tetraspanins to varying degrees (35). Indeed, macrophages actively participate in lymphangiogenesis through secretion of lymphangiogenesis-promoting growth factors, such as VEGF-C (36), and CD11b+ macrophages physically contribute to lymphangiogenesis under pathologic conditions (37). Second, tumor metastasis involves multiple complicated cascades. For example, because tetraspanins in endothelial cells could promote tumor-endothelial interactions through adhesion and integrin clustering (24), deletion of CD9 may have hindered the interaction between endothelial cells and tumor cells in our study. It will be intriguing to examine the interaction between tumor cells and lymphatic endothelial cells in future investigations.
CD9 Supports Lymphangiogenesis through Post-adhesion Events
Tetraspanin CD9 supports lymphangiogenesis, most likely through modulating essential endothelial functions, which include proliferation, migration, chemotaxis, and morphogenesis but not static cell adhesion. Consistent with our results, tetraspanins have been considered not to affect initial ligand binding by laminin-binding integrins (38). A common feature of these assays would be strengthening of adhesion, which gives rise to tension forces (39). For example, this decreased adhesion strengthening also contributed to impaired angiogenesis in CD151-KO mice (26). Of importance, these functional defects among in vitro studies largely recapitulate defects observed in vivo. Although CD9 deletion also decreased proliferation of LEC (Fig. 6C), it is less likely that this decreased proliferation contributes to most of the functional defects, because it takes less than 24 h to complete in vitro assays such as migration and morphogenesis.
Impairment of several functions was further supported by signaling analysis. For example, activation of p38 and Erk, crucial for VEGF-C induced proliferation of LEC, was diminished (40). In addition, activation of FAK, a pivotal downstream effecter of integrin signaling, was also diminished. Although Akt has also been viewed as associated with downstream signaling under VEGF-C-induced stimulation (40), activation of Akt was not detectable. Further investigation will be needed to clarify how deletion of CD9 affects each signaling pathway between VEGFR-3 and integrins in LEC.
Tetraspanin CD9 Forms Complexes with Integrins and VEGFR-3
Thus far, research on VEGFR-3 signaling has been focused on the interaction between ligands and receptors, including VEGFR-2 and VEGFR-3 (34). Although the interplay between integrins and growth factor receptors has been extensively studied, the precise mechanisms regulating their cross-talk remain largely unknown (41). Herein, we provide novel evidence that tetraspanins, a unique protein family, play pivotal roles in lymphangiogenesis by a mechanism in which CD9 may facilitate the interplay between VEGFR-3 and its partners (integrin α5 and α9) at the tetraspanin-enriched microdomains. Although numerous molecules have been reported to associate with tetraspanins, CD9/EWI-F, CD151/α3β1, and CD81/CD19 have been regarded as primary partners (20). Hence, new partners such as VEGFR-3 and integrin (α5 and α9), which we found under conditions in which mild detergents were present, could be secondary interacting partners through tetraspanins or primary partners.
Although tetraspanins form a complex with laminin-binding integrins like α3β1 and α6β1, thereby functioning on laminin substrates, deletion of CD9 in LEC affected migration on FN and morphogenesis in collagen. Given that the known ligands of α9β1 are considered to be tenascin, FN, and collagen (42), this might partly explain why CD9 functions on various extracellular matrixes except laminin.
Double Deletion of CD9 and CD81 Impairs Lymphangiogenesis under Physiological Conditions
Here we provide unanticipated evidence that DKO mice show diminished physiological lymphangiogenesis, and CD9 and CD81 also cooperatively function in LEC and in the vasculature of DKO mice. Although dozens of molecules have been listed that are essential to normal lymphatic development (3), tetraspanins could represent a novel type among these molecules in that they could facilitate the interactions of some of the players at an appropriate point at the tetraspanin-enriched microdomain. We have previously shown that CD9 and CD81 coordinately prevent the fusion of macrophages and that DKO mice develop an emphysematous lung phenotype (27, 43). Hence, our present data suggest that both CD9 and CD81 additively function not only in macrophages, but also in LEC. Of interest, LEC were found to selectively express proteins involved in intracellular protein transport, vesicle formation, and fusion, such as the syntaxins, and vesicle-associated membrane proteins compared with BEC (44). Given that CD9 and CD81 actively participate in membrane trafficking and fusion, taken together with the in vitro and in vivo results (Fig. 10), a key role of these tetraspanins in lymphangiogenesis would not be surprising. Further study, however, will be required to determine whether CD9 affects vasculogenesis or developmental lymphangiogenesis.
Diminished Inflammation-induced Lymphangiogenesis in CD9-KO Mice
Because lymphatic vessels act as a conduit not only for tumor cells, but also for immune cells, increasing attention has been paid to the role of lymphangiogenesis in inflammation. Considering that enhanced lymphangiogenesis through VEGFR-3 significantly reduces inflammation (45), impaired lymphangiogenesis in DKO mice could partly explain augmented inflammation in the lungs (27). Taking into consideration that tetraspanins CD9 and CD81, both of which are expressed in various cells including macrophages and lymphocytes, play key roles in many aspects of inflammation, augmenting the expression of CD9 and CD81 could be a novel strategy for anti-inflammatory therapy.
In conclusion, tetraspanin CD9, an abundant protein in LEC, could be a novel component in the interaction between VEGFR-3 and integrins. Deletion of CD9 diminishes physiological and pathological (tumor-induced and inflammation-induced) lymphangiogenesis. Given that CD9 participates not only in angiogenesis but also lymphangiogenesis and that tetraspanins mediate inflammation, immune response, and tumor progression, they might be key components not only in tumor metastasis but also in inflammation.
Acknowledgments
We thank Dr. Hirakawa (Hamamatsu University in Japan) for helpful discussions and critical reading of the manuscript and Dr. E. Mekada (Osaka University in Japan) for generously providing CD9-KO mice and CD81-KO mice.
This work was supported by grants from the Osaka Foundation for Promotion of Clinical Immunology (to Y. T.); Grant-in-Aid for Scientific Research (C) 20590922 (to I. K.); a Grant-in-Aid for Scientific Research from the Ministry of Health, Labour and Welfare and a grant from “Kansai Biomedical Cluster” project in Saito (to I. T.); and the Funding Program for Next Generation World-Leading Researchers (NEXT Program) and Special Coordination Funds for Promoting Science and Technology (to A. K.).
- VEGFR
- VEGF receptor
- LEC
- lymphatic endothelial cell(s)
- HUVEC
- human umbilical vein endothelial cell(s)
- HDLEC
- human dermal lymphatic endothelial cell(s)
- BEC
- blood endothelial cell(s)
- mLEC
- mouse LEC
- Ab
- antibody
- FN
- fibronectin
- Mat
- Matrigel
- LVD
- lymphatic vascular density
- FAK
- focal adhesion kinase
- DKO
- double knock-out.
REFERENCES
- 1. Adams R. H., Alitalo K. (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8, 464–478 [DOI] [PubMed] [Google Scholar]
- 2. Grothey A., Galanis E. (2009) Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat. Rev. Clin. Oncol. 6, 507–518 [DOI] [PubMed] [Google Scholar]
- 3. Tammela T., Alitalo K. (2010) Lymphangiogenesis. Molecular mechanisms and future promise. Cell 140, 460–476 [DOI] [PubMed] [Google Scholar]
- 4. Baluk P., Fuxe J., Hashizume H., Romano T., Lashnits E., Butz S., Vestweber D., Corada M., Molendini C., Dejana E., McDonald D. M. (2007) Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alitalo K., Tammela T., Petrova T. V. (2005) Lymphangiogenesis in development and human disease. Nature 438, 946–953 [DOI] [PubMed] [Google Scholar]
- 6. Tobler N. E., Detmar M. (2006) Tumor and lymph node lymphangiogenesis. Impact on cancer metastasis. J. Leukocyte Biol. 80, 691–696 [DOI] [PubMed] [Google Scholar]
- 7. Sundar S. S., Ganesan T. S. (2007) Role of lymphangiogenesis in cancer. J. Clin. Oncol. 25, 4298–4307 [DOI] [PubMed] [Google Scholar]
- 8. Dumont D. J., Jussila L., Taipale J., Lymboussaki A., Mustonen T., Pajusola K., Breitman M., Alitalo K. (1998) Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 282, 946–949 [DOI] [PubMed] [Google Scholar]
- 9. Karkkainen M. J., Haiko P., Sainio K., Partanen J., Taipale J., Petrova T. V., Jeltsch M., Jackson D. G., Talikka M., Rauvala H., Betsholtz C., Alitalo K. (2004) Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 [DOI] [PubMed] [Google Scholar]
- 10. He Y., Kozaki K., Karpanen T., Koshikawa K., Yla-Herttuala S., Takahashi T., Alitalo K. (2002) Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 94, 819–825 [DOI] [PubMed] [Google Scholar]
- 11. Avraamides C. J., Garmy-Susini B., Varner J. A. (2008) Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer. 8, 604–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang X. Z., Wu J. F., Ferrando R., Lee J. H., Wang Y. L., Farese R. V., Jr., Sheppard D. (2000) Fatal bilateral chylothorax in mice lacking the integrin α9β1. Mol. Cell. Biol. 20, 5208–5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vlahakis N. E., Young B. A., Atakilit A., Sheppard D. (2005) The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin α9β1. J. Biol. Chem. 280, 4544–4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okazaki T., Ni A., Ayeni O. A., Baluk P., Yao L. C., Vossmeyer D., Zischinsky G., Zahn G., Knolle J., Christner C., McDonald D. M. (2009) α5β1 integrin blockade inhibits lymphangiogenesis in airway inflammation. Am. J. Pathol. 174, 2378–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dietrich T., Onderka J., Bock F., Kruse F. E., Vossmeyer D., Stragies R., Zahn G., Cursiefen C. (2007) Inhibition of inflammatory lymphangiogenesis by integrin α5 blockade. Am. J. Pathol. 171, 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J. F., Zhang X. F., Groopman J. E. (2001) Stimulation of β1 integrin induces tyrosine phosphorylation of vascular endothelial growth factor receptor-3 and modulates cell migration. J. Biol. Chem. 276, 41950–41957 [DOI] [PubMed] [Google Scholar]
- 17. Zhang X., Groopman J. E., Wang J. F. (2005) Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin α5β1. J. Cell. Physiol. 202, 205–214 [DOI] [PubMed] [Google Scholar]
- 18. Odintsova E., Voortman J., Gilbert E., Berditchevski F. (2003) Tetraspanin CD82 regulates compartmentalisation and ligand-induced dimerization of EGFR. J. Cell Sci. 116, 4557–4566 [DOI] [PubMed] [Google Scholar]
- 19. Klosek S. K., Nakashiro K., Hara S., Shintani S., Hasegawa H., Hamakawa H. (2005) CD151 forms a functional complex with c-Met in human salivary gland cancer cells. Biochem. Biophys. Res. Commun. 336, 408–416 [DOI] [PubMed] [Google Scholar]
- 20. Hemler M. E. (2005) Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 6, 801–811 [DOI] [PubMed] [Google Scholar]
- 21. Romanska H. M., Berditchevski F. (2011) Tetraspanins in human epithelial malignancies. J Pathol. 223, 4–14 [DOI] [PubMed] [Google Scholar]
- 22. Hemler M. E. (2008) Targeting of tetraspanin proteins. Potential benefits and strategies. Nat. Rev. Drug Discov. 7, 747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zijlstra A., Lewis J., Degryse B., Stuhlmann H., Quigley J. P. (2008) The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell 13, 221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Longo N., Yáñez-Mó M., Mittelbrunn M., de la Rosa G, Muñoz M. L., Sánchez-Madrid F., Sánchez-Mateos P. (2001) Regulatory role of tetraspanin CD9 in tumor-endothelial cell interaction during transendothelial invasion of melanoma cells. Blood 98, 3717–3726 [DOI] [PubMed] [Google Scholar]
- 25. Junge H. J., Yang S., Burton J. B., Paes K., Shu X., French D. M., Costa M., Rice D. S., Ye W. (2009) TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/β-catenin signaling. Cell 139, 299–311 [DOI] [PubMed] [Google Scholar]
- 26. Takeda Y., Kazarov A. R., Butterfield C. E., Hopkins B. D., Benjamin L. E., Kaipainen A., Hemler M. E. (2007) Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood 109, 1524–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takeda Y., He P., Tachibana I., Zhou B., Miyado K., Kaneko H., Suzuki M., Minami S., Iwasaki T., Goya S., Kijima T., Kumagai T., Yoshida M., Osaki T., Komori T., Mekada E., Kawase I. (2008) Double deficiency of tetraspanins CD9 and CD81 alters cell motility and protease production of macrophages and causes chronic obstructive pulmonary disease-like phenotype in mice. J. Biol. Chem. 283, 26089–26097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakamura E. S., Koizumi K., Kobayashi M., Saiki I. (2004) Inhibition of lymphangiogenesis-related properties of murine lymphatic endothelial cells and lymph node metastasis of lung cancer by the matrix metalloproteinase inhibitor MMI270. Cancer Sci. 95, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bruyère F., Melen-Lamalle L., Blacher S., Roland G., Thiry M., Moons L., Frankenne F., Carmeliet P., Alitalo K., Libert C., Sleeman J. P., Foidart J. M., Noël A. (2008) Modeling lymphangiogenesis in a three-dimensional culture system. Nat. Methods 5, 431–437 [DOI] [PubMed] [Google Scholar]
- 30. Padera T. P., Kadambi A., di Tomaso E., Carreira C. M., Brown E. B., Boucher Y., Choi N. C., Mathisen D., Wain J., Mark E. J., Munn L. L., Jain R. K. (2002) Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 296, 1883–1886 [DOI] [PubMed] [Google Scholar]
- 31. Baluk P., Tammela T., Ator E., Lyubynska N., Achen M. G., Hicklin D. J., Jeltsch M., Petrova T. V., Pytowski B., Stacker S. A., Ylä-Herttuala S., Jackson D. G., Alitalo K., McDonald D. M. (2005) Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Invest. 115, 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang S., Lee S. P., Kim K. E., Kim H. Z., Mémet S., Koh G. Y. (2009) Toll-like receptor 4 in lymphatic endothelial cells contributes to LPS-induced lymphangiogenesis by chemotactic recruitment of macrophages. Blood 113, 2605–2613 [DOI] [PubMed] [Google Scholar]
- 33. Danussi C., Spessotto P., Petrucco A., Wassermann B., Sabatelli P., Montesi M., Doliana R., Bressan G. M., Colombatti A. (2008) Emilin1 deficiency causes structural and functional defects of lymphatic vasculature. Mol. Cell. Biol. 28, 4026–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lohela M., Bry M., Tammela T., Alitalo K. (2009) VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 21, 154–165 [DOI] [PubMed] [Google Scholar]
- 35. Mantovani A., Allavena P., Sica A., Balkwill F. (2008) Cancer-related inflammation. Nature 454, 436–444 [DOI] [PubMed] [Google Scholar]
- 36. Skobe M., Hamberg L. M., Hawighorst T., Schirner M., Wolf G. L., Alitalo K., Detmar M. (2001) Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am. J. Pathol. 159, 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maruyama K., Ii M., Cursiefen C., Jackson D. G., Keino H., Tomita M., Van Rooijen N., Takenaka H., D'Amore P. A., Stein-Streilein J., Losordo D. W., Streilein J. W. (2005) Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 115, 2363–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berditchevski F. (2001) Complexes of tetraspanins with integrins. More than meets the eye. J. Cell Sci. 114, 4143–4151 [DOI] [PubMed] [Google Scholar]
- 39. Lammerding J., Kazarov A. R., Huang H., Lee R. T., Hemler M. E. (2003) Tetraspanin CD151 regulates α6β1 integrin adhesion strengthening. Proc. Natl. Acad. Sci. U.S.A. 100, 7616–7621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mäkinen T., Veikkola T., Mustjoki S., Karpanen T., Catimel B., Nice E. C., Wise L., Mercer A., Kowalski H., Kerjaschki D., Stacker S. A., Achen M. G., Alitalo K. (2001) Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 20, 4762–4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Somanath P. R., Ciocea A., Byzova T. V. (2009) Integrin and growth factor receptor alliance in angiogenesis. Cell Biochem. Biophys. 53, 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yokosaki Y., Matsuura N., Higashiyama S., Murakami I., Obara M., Yamakido M., Shigeto N., Chen J., Sheppard D. (1998) Identification of the ligand binding site for the integrin α9 β1 in the third fibronectin type III repeat of tenascin-C. J. Biol. Chem. 273, 11423–11428 [DOI] [PubMed] [Google Scholar]
- 43. Takeda Y., Tachibana I., Miyado K., Kobayashi M., Miyazaki T., Funakoshi T., Kimura H., Yamane H., Saito Y., Goto H., Yoneda T., Yoshida M., Kumagai T., Osaki T., Hayashi S., Kawase I., Mekada E. (2003) Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J. Cell Biol. 161, 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Podgrabinska S., Braun P., Velasco P., Kloos B., Pepper M. S., Skobe M. (2002) Molecular characterization of lymphatic endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 99, 16069–16074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huggenberger R., Ullmann S., Proulx S. T., Pytowski B., Alitalo K., Detmar M. (2010) Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J. Exp. Med. 207, 2255–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]