Background: Dystroglycan function is decreased in prostate and other cancers.
Results: Decreased functional glycosylation of α-dystroglycan is clinically correlated with reduced LARGE2 and restoring its expression rescues dystroglycan function, reducing the invasive and proliferative potential of prostate cancer cells.
Conclusion: LARGE2 regulates α-dystroglycan function in the prostatic epithelium.
Significance: Loss of LARGE2 expression may contribute to prostate cancer progression.
Keywords: Extracellular Matrix, Glycosylation, Glycosyltransferases, Pathology, Prostate Cancer, LARGE2 (GYLTL1B), Dystroglycan
Abstract
Dystroglycan (DG) is a cell surface receptor for extracellular matrix proteins and is involved in cell polarity, matrix organization, and mechanical stability of tissues. Previous studies documented loss of DG protein expression and glycosylation in a variety of cancer types, but the underlying mechanisms and the functional consequences with respect to cancer progression remain unclear. Here, we show that the level of expression of the βDG subunit as well as the glycosylation status of the αDG subunit inversely correlate with the Gleason scores of prostate cancers; furthermore, we show that the functional glycosylation of αDG is substantially reduced in prostate cancer metastases. Additionally, we demonstrate that LARGE2 (GYLTL1B), a gene not previously implicated in cancer, regulates functional αDG glycosylation in prostate cancer cell lines; knockdown of LARGE2 resulted in hypoglycosylation of αDG and loss of its ability to bind laminin-111 while overexpression restored ligand binding and diminished growth and migration of an aggressive prostate cancer cell line. Finally, our analysis of LARGE2 expression in human cancer specimens reveals that LARGE2 is significantly down-regulated in the context of prostate cancer, and that its reduction correlates with disease progression. Our results describe a novel molecular mechanism to account for the commonly observed hypoglycosylation of αDG in prostate cancer.
Introduction
Dystroglycan (DG)4 is an extracellular matrix receptor discovered as a component of the dystrophin glycoprotein complex in muscle (1, 2). DG is expressed in many tissue types including muscle, neuronal, adipose, and epithelial tissues. Transcribed from a single gene, DG is post-translationally cleaved into two non-covalently attached subunits (3, 4). The transmembrane βDG subunit binds to dystrophin or utrophin, whereas the extracellular αDG subunit interacts with laminin-G domain-containing matrix proteins including agrin, perlecan, and members of the laminin family (3, 5–8). DG thus serves as a link between the cytoskeleton and extracellular matrix.
αDG is heavily glycosylated with both O- and N-linked carbohydrates, and much attention has focused on an O-mannosyl glycan that is required for ligand binding. Production of this laminin-binding glycan involves at least six known and putative glycosyltransferases (POMT1, POMT2, POMGnT1, Fukutin, FKRP, and LARGE) (9–14). Mutations in these enzymes result in hypoglycosylation of αDG and prevent it from binding its ligands (15–18). Many of these glycosyltransferases have been implicated in diseases of muscle and neural tissue, in mice and in humans (12, 13, 19–21). Recently, the laminin-binding moiety of αDG was shown to be a unique phosphorylated O-mannosyl glycan (22). This modification is mediated by the xylosyl-glucuronyltransferase LARGE (15, 23). Notably, the overexpression of LARGE has been shown to functionally rescue αDG hypoglycosylation in cells from patients harboring mutations in other αDG glycosyltransferases (24). In addition, a mutation in LARGE is responsible for the myodystrophy mouse (Largemyd, MDC1D) phenotype, features of which include αDG hypoglycosylation (25). Although LARGE is expressed in many tissues the myodystrophy mouse phenotype is primarily evident in muscle and brain. A paralog of LARGE, LARGE2, has a narrower expression profile and is not expressed in muscle and brain. LARGE2 overexpression results in αDG hyperglycosylation and enhanced laminin binding, suggesting that LARGE and LARGE2 may be functionally redundant (26, 27).
DG is expressed in many epithelial tissues where it is thought to mediate cell-matrix interactions important for cell polarity and tissue morphogenesis, but recent studies have shown that DG is not required for the normal development and/or maintenance of some epithelial tissues, including prostate; and, in Drosophila, DG is required for the maintenance of epithelial cell polarity only during energetic stress (28–33). Loss of DG function has been implicated in various cancer cell phenotypes including increased growth in soft agar, invasive behavior in 2- and 3-dimensional culture systems, and xenograft tumor growth (34–36). It is proposed that DG is involved in these processes by participating in adhesive interactions with the extracellular matrix and/or by affecting intracellular signaling (35, 37). In a variety of adenocarcinomas, including prostate, expression of DG protein, αDG and/or βDG, is reduced and this is associated with increased tumor aggressiveness and loss of extracellular matrix integrity, e.g. (38, 39). So far, all evidence points toward post-transcriptional mechanisms, including proteolysis and hypoglycosylation to account for the observed loss of DG expression in cancer (35, 39–42). Herein we describe a novel mechanism accounting for the loss of functional αDG glycosylation in prostate cancer cell lines and prostate cancer specimens, reduced expression of LARGE2, a protein not previously implicated in cancer. Furthermore, we show that endogenous LARGE2, and not LARGE, is responsible for mediating the functional glycosylation of αDG in prostate cancer cells and normal prostatic epithelium.
EXPERIMENTAL PROCEDURES
Immunohistochemistry
Prostate tissue embedded in paraffin was sectioned at 7 μm, deparaffinized, and rehydrated. For the metastatic tissue microarray, samples were first baked at 70 °C for 64 h prior to antigen retrieval. Antigen retrieval for all samples was performed by a 20-min exposure to either citrate buffer (βDG) or proteinase K (αDG). Endogenous peroxidases were quenched with 3% hydrogen peroxide for 10 min. Sections were blocked in 10% horse serum and then incubated overnight with primary antibody, either IIH6 (1:100, Santa Cruz Biotechnology) or 8D5 (1:100, Novocastra). Samples were washed and incubated for 1 h at room temp with a biotinylated secondary antibody, which was then detected using the ABC reagent (Vector) and DAB plus (Dako) followed by counterstain with hematoxylin. Hematoxylin and eosin (H & E) staining was performed using standard procedures. Immunohistochemistry was performed by the UI Department of Pathology Core Lab. For evaluation of immunohistochemistry, pathologists (MMM and MBC) were blinded to sample identity. For metastases analysis, samples were scored as either positive or negative for membranous staining with the antibody.
Cell Culture
22Rv1 (American Type Culture Collection, ATCC), PC-3 (ATCC), LNCaP (M Cohen), PC-3E+, and TEM4–18 (PC-3 derivatives, Henry Lab) and GP2–293 packaging cells (BD Biosciences) were grown with 10% FBS (HyClone, Logan, Utah) and 1% non-essential amino acids. Cultures were maintained at 37 °C in 95% air and 5% CO2 and subcultured as needed.
shRNA Knockdown of LARGE2
To knockdown LARGE2, pLKO1 lentiviral vectors targeting human LARGE2 were purchased from Open Biosystems (TRC collection, RHS4533-NM_152312). Five different constructs were transfected into GP2–293 cells along with the lentiviral second generation plasmid VSV-G (kind gift from the Trono Lab) and culture supernatant from these cells was used to infect PC-3E+, DU145, and 22Rv1 cells for 8 h followed by puromycin selection (1.0 μg/ml). We were able to achieve 70–80% knockdown of LARGE2 using hairpin construct sequence TRCN0000035147 and TRCN0000035148.
Overexpression of LARGE2
Murine LARGE2 was excised from a mLARGE2 containing pLEX vector using NotI and cloned into the pQCXIP NotI site. Cells were selected and maintained with standard media containing 1.0 μg/ml puromycin.
Quantitative PCR
qPCR analysis was performed as described previously (43). For the analysis of bulk tumor samples, the TissueScan cDNA array (HRT103, Origene) was used. Relative expression values were calculated using the comparative Ct method (44).
Western Blot
Protein lysates were prepared from sub-confluent cultures following scrape harvesting in RIPA buffer (150 mm NaCl2, 1% Nonidet P-40, 10 mm deoxycholate-Na, 0.1% SDS, 50 mm Tris, pH 8.0; rocked at 4 °C for 1 h, boiled 5 min) with protease inhibitors. Protein samples (50 μg, Lowry protein analysis) were electrophoresed (4–20% Tris-HCl gels) and transferred (PVDF) prior to antibody incubation. Antibodies were used as follows: 1°-IIH6, glycosylation sensitive αDG, 1:500 overnight at 4 °C in low salt buffer (50 mm Tris, 100 mm NaCl2,0.1% Tween 20), 2°-donkeyαmouse IgM HRP, 1:2000 for 1 h at room temperature; 1°-sheep 5 core αDG, 1:100 overnight at 4 °C, 2°-donkeyαmouse IgG HRP, 1:2000 for 1 h at room temperature; 1°-βDG specific 8D5 (Novocastra/Leica) for 1:200 overnight at 4 °C, 2-donkey α-mouse IgG, 1:2000 for 1 h at room temperature; 1°-β-actin (Sigma), 1:5000 for 1 h at room temperature, 2°-goatαrabbit IgG HRP, 1:10,000 for 1 h at room temperature, was utilized as a marker for protein loading. All secondary antibodies were obtained from Jackson Immunoresearch.
Flow Cytometry Analysis
Cells grown to 60–80% confluency were detached (Versene, Invitrogen) and washed prior to antibody incubation. The cells were then resuspended at 5 × 105 cells in 50 μl FACS buffer (PBS + 0.02% sodium azide + 5% BSA) + IIH6 antibody (1:100) for 45 min on ice. Next, cells were washed and incubated with secondary antibody (100 μl FACS buffer plus 1:100, donkey anti-mouse IgM Dylight 488, Jackson Immunoresearch) for 30 min on ice in the dark. Cells were washed again and resuspended in 400 μl of FACs buffer, and transferred to polystyrene FACs tubes (BD Biosciences). Samples were analyzed using the Becton Dickinson LSR II flow cytometer at The University of Iowa Flow Cytometry Core Facility.
Laminin-111 Binding
The methods were used as described above but cells were incubated with 50 μl of 100 nm laminin-111 (BD Biosciences) prior to incubation with anti-laminin-111 primary antibody (1:100, Sigma) and the subsequent detection with the DyLight488 secondary antibody. To inhibit laminin-111 binding, a subset of samples was first incubated with αDG IIH6 antibody at 1:100 in 100 μl of FACS+ buffer. All steps were done on ice.
Immunofluorescence
Tissues were processed and antigen retrieval performed as described above. IIH6 was incubated overnight at 1:100. Tissues were washed in blocking buffer and incubated with donkey anti-mouse IgG Cy3 (Jackson Immunoresearch) at 1:200 and counterstained with DAPI (1:5000, Sigma) for 1 h at room temperature.
Transwell Migration Assay
24 well, 8.0 μm transwell permeable inserts (Corning Life Sciences) were prepared as previously described (45). Briefly, transwells were coated with laminin-511 for 1 h at room temperature prior to washing with PBS. Luciferase-expressing cells were plated at 8.0 × 104 cells/well in serum-free DMEM/F12 and allowed to migrate down a chemoattractant gradient (DMEM/F12 supplemented with 10% FBS in lower chamber) for 18 h prior to trypsinization followed by quantification via bioluminescence. Data are presented as migration relative to vector control cells across an uncoated membrane.
Matrigel Invasion Assay
24 well, 8.0 μm BD Biocoat matrigel inserts (BD Biosciences) were thawed and equilibrated according to the manufacturer. Cells were plated in the top chamber at 8.0 × 104 cells/well in serum-free DMEM/F12. The lower chamber was filled with DMEM/F12 containing 10% serum to induce a chemoattractive response. Following 18 h of incubation, cells were trypsinized and quantified as described above. Data are presented as invasion relative to vector control cells.
Growth Assay
30,000 GS689.Li cells containing either empty vector or mLARGE2were plated in triplicate into 6-well dishes. On the indicated day, cells were trypsinized and counted using a Z2 series Coulter Counter (Beckman Coulter) with a cell diameter range of 8–24 μm. Three counts were averaged per well.
RESULTS
αDG Glycosylation and βDG Expression Are Reduced in Both Primary and Metastatic Prostate Cancer
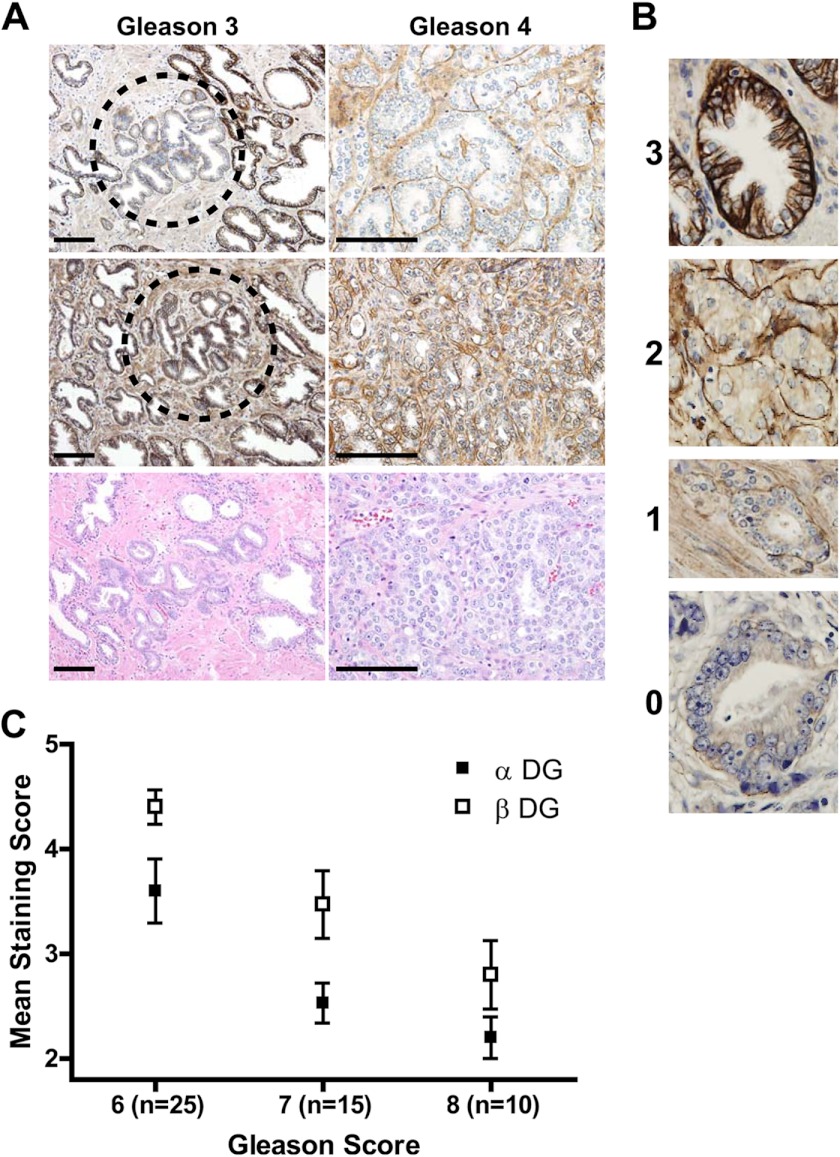
We previously demonstrated that βDG expression is reduced in prostate cancer, a finding which has been extended to loss of αDG immunostaining by other groups (38, 46, 47). These studies utilized glycosylation-sensitive antibodies IIH6 and VIA41, which detect the laminin-binding glycan on αDG (5). However, it remains unclear to what extent the loss of DG immunoreactivity reflects a reduction of the core protein expression or a loss of the epitopes on αDG recognized by IIH6 and VIA41. This remains a difficult question to answer due to the lack of an antiserum that reacts specifically with the core αDG protein in histochemical preparations. Therefore, we utilized a prostate cancer tissue microarray panel to assess both DG subunits on adjacent sections. Fig. 1A shows representative staining of sections from Gleason grade 3 and 4 patterns observed in tissue microarrays. Interestingly, we noted examples of some grade 3 glands (Fig. 1A) in which IIH6 staining (glycosylated DG) was reduced at the same time that 8D5 staining (βDG) was normal, i.e. levels were similar to the adjacent, healthy glands. This strongly suggested that the glycosylation of αDG is affected independently of core DG protein expression. To quantify DG staining intensity in these sections, we developed a quartile scoring system (Fig. 1B) grading each specimen with both a primary and secondary score, similar to the Gleason scoring system where a Gleason score (range: 2–10) is generated by summing the most prevalent and second-most prevalent Gleason grades (range: 1–5). The Gleason grades are generated based upon the histological architecture of the tumor as originally defined by Donald Gleason in 1974 and refined in 2005 by the International Society of Urological Pathology (48, 49). For both IIH6 and 8D5, staining intensity was reduced across cases with Gleason scores 6–8, with IIH6 staining more dramatically reduced than 8D5 staining (Fig. 1C). The observed reduction of both IIH6 (p = 0.0022) and 8D5 (p = 0.0003) staining relative to that in benign glands was significant, and both markers are independent predictors of Gleason score.
FIGURE 1.
αDG glycosylation and βDG expression are reduced in prostate adenocarcinoma. A, representative images of αDG and βDG immunostained human prostatic tissue showing loss of DG correlating with cancer progression. Scale bar, 100 μm. B, grading was determined using 0–3 grading scale where 0 = basal staining absent; 1 = discontinuous thin basal staining; 2 = thin basal staining with extension between cells or thin dark basal stain without basal-lateral staining; 3 = thick, dark basal staining with basal-lateral staining, characteristic of benign glands. C, mean DG staining of human prostate cancer tissue samples shows that DG expression is reduced across Gleason scores 6–8 (αDG p < 0.0022 and βDG p < 0.0003), while the magnitude of loss of αDG is greater than loss of βDG immunoreactivity.
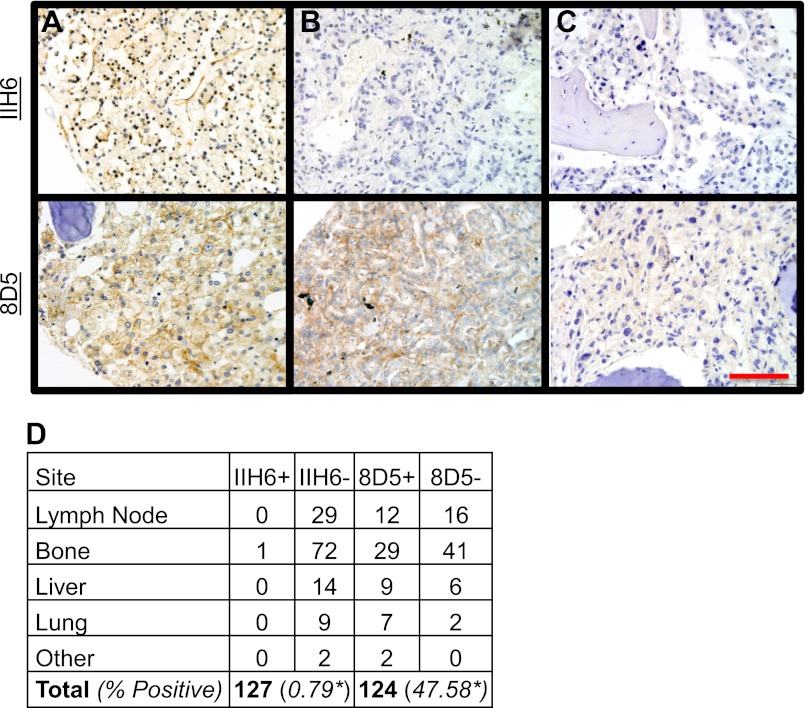
We next assessed DG status in a panel of prostate cancer metastases to determine if the expression and glycosylation patterns differ from those observed in primary tumors. Because prostate cancer metastases do not retain the glandular architecture seen in the prostate, samples were graded according to a positive/negative system; a signal was evaluated as positive if membranous staining was present on the tumor cell(s). Strikingly, IIH6 staining was present in less than 1% (1/127) of cases, whereas 8D5 reactivity was present in 47.6% (59/124) of cases (Fig. 2). This indicates that during metastatic progression, αDG glycosylation is more frequently perturbed than is expression of the core protein. Staining for both IIH6 and 8D5 was clearly evident in the positive control (healthy kidney glomeruli) (supplemental Fig. S1).
FIGURE 2.
αDG functional glycosylation is nearly absent in prostate adenocarcinoma metastases. Representative images of IIH6 staining (top row) and serially sectioned analysis of 8D5 (bottom row) showing retention of glycosylation (A), loss of glycosylation only (B), or loss of protein expression (C). Analysis of DG in metastatic tumor shows a significant difference between expression and glycosylation (p < 0.0001) (D). Bar, 100 μm.
Having shown that DG glycosylation is more frequently lost in metastatic disease, we attempted to determine if IIH6 staining is an independent prognostic indicator of disease recurrence in a cohort of 135 prostate cancer patients seen at University of Iowa Hospitals and Clinics during the years 1994–2000. Patient characteristics are listed in supplemental Table S1. Sections from prostatectomy specimens were stained with IIH6 and scored by the method described above. We first demonstrated that DG glycosylation was inversely correlated with Gleason score (p = 0.0367) consistent with our finding from the tissue microarray. We then assessed whether DG glycosylation status correlates with either mortality or biochemical recurrence. With only 9 patients in this cohort having died from prostate cancer, despite having a mean follow-up time of 12.3 years, we were unable to draw any conclusion regarding DG status and mortality. However, we did detect a modest, though not statistically significant, association between DG hypoglycosylation and biochemical recurrence, using both univariate and multivariate regression analysis (supplemental Fig. S2).
Functional Glycosylation of αDG across Prostate Cancer Cell Lines Is Heterogeneous
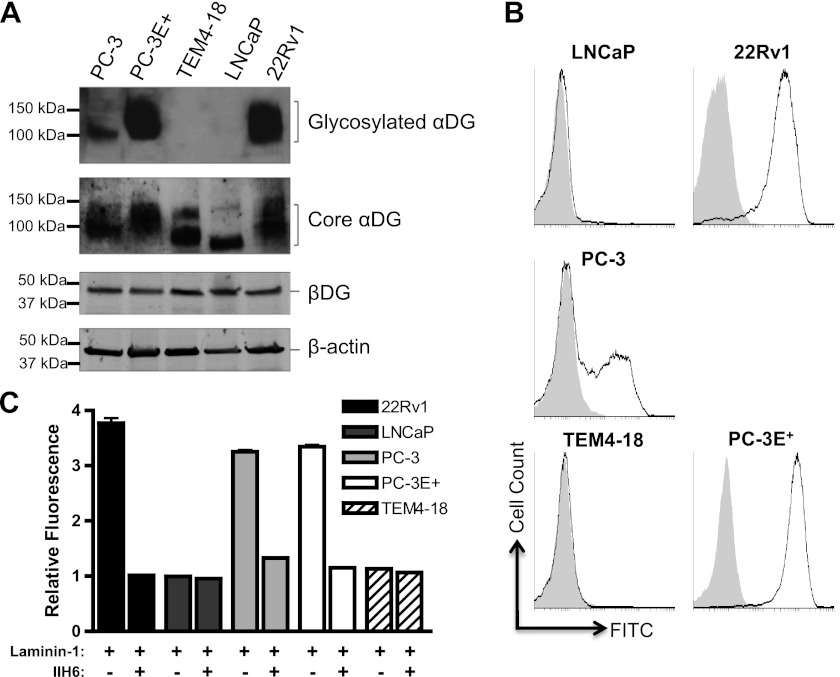
To determine the status of DG expression in prostate cancer cell lines, we examined DG expression by Western blot analysis using an antiserum that detects the αDG core protein. αDG protein was expressed in all prostate cancer cell lines examined (Fig. 3A). The observed molecular weight of the bands was within the range of ∼80 to 150 kDa, with 22Rv1 and PC-3 expressing higher molecular weight species than LNCaP and TEM4–18. This heterogeneous staining pattern is consistent with the fact that DG has a number of carbohydrate modifications, only some of which are involved in ligand binding and recognized by IIH6 (2, 5, 15, 22, 23). The expression of βDG protein (8D5 immunoreactivity) was equivalent across the cell lines (Fig. 3A). These data indicate that both αDG and βDG are expressed in the prostate cancer cell lines examined.
FIGURE 3.
αDG functional glycosylation is heterogeneous in prostate cancer. A, both αDG core protein (80–100kDa, Sheep 5) and the transmembrane βDG (43kDa, 8D5) protein were expressed in all prostate cancer cell lines. Glycosylated αDG is apparent in PC-3, PC-3E+, and 22Rv1 cell lysates. β-actin was used as a loading control. B, analysis of cell surface αDG shows that 22Rv1, PC-3, and PC-3E+ cell lines are positive for IIH6 staining while LNCaP and TEM4–18 cell lines are negative by flow cytometry analysis. C, 22Rv1, PC-3, and PC-3E+ cells are positive for DG-dependent laminin-111 staining by flow cytometry following incubation with exogenous laminin-111 (100 nm). LNCaP and TEM4–18 cells are negative. Prior Incubation with the antibody IIH6 inhibits laminin-111 binding. An isotype control was used for all FACS experiments (gray) (n = 2).
Because ligand binding requires proper glycosylation of cell-surface localized αDG, we carried out flow cytometry analysis with the IIH6 antibody; this revealed that αDG glycosylation is heterogeneous across the prostate cancer cell lines (Fig. 3B). Among the cell lines examined, 22Rv1 and PC-3E+ (E-cadherin-positive (50)) were positive for IIH6 staining, whereas PC-3 (obtained from the ATCC) was comprised of distinct IIH6-positive (IIH6+) and IIH6-negative (IIH6−) subpopulations. The TEM4–18 and LNCaP cell lines were IIH6−, consistent with the Western blot data analyzing glycosylated DG. Interestingly, cells of the TEM4–18 line, an aggressive subpopulation of the PC-3 line that were isolated following migration through an endothelial cell monolayer, are IIH6− (50). In contrast, cells of the PC-3E+ line, a less aggressive subpopulation also derived from the PC-3 line, are IIH6+. Thus, in PC-3 cells, loss of DG glycosylation is associated with a more aggressive phenotype.
We next assessed whether the glycosylation status of αDG correlates with laminin binding ability using a flow cytometry-based ligand binding assay. IIH6 + 22Rv1, PC-3, and PC-3E+ cells were able to bind exogenous laminin-111 at the cell surface whereas the IIH6- LNCaP and TEM4–18 cells lines were not (Fig. 3C). This laminin binding was specific to DG as it was inhibited by prior incubation with IIH6. Some laminin immunoreactivity was also detected in cells in the absence of exogenous laminin-111, likely representing endogenous laminin bound during tissue culture. Overall, these results are consistent with those of IIH6 immunoreactivity, indicating that functional αDG glycosylation in metastatic prostate cancer cell lines is variable.
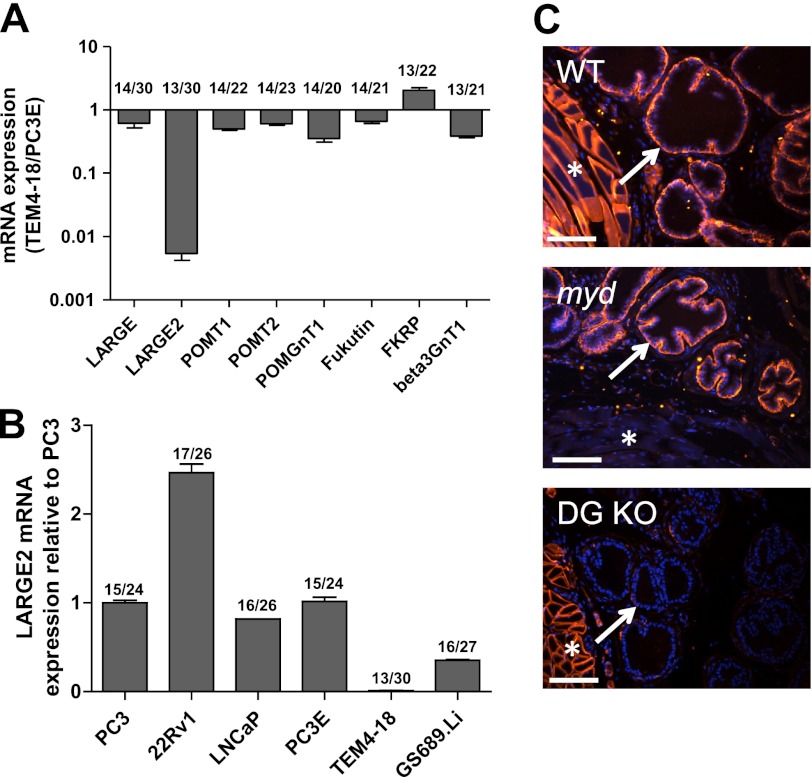
Functional Glycosylation of αDG Correlates with Expression of the LARGE2 mRNA
To elucidate the molecular mechanism responsible for the loss of functional αDG glycosylation in prostate cancer cells, we compared mRNA expression levels of the six known and putative αDG glycosyltransferases in the PC-3 derived cell lines PC-3E+ (IIH6+) and TEM4–18 (IIH6−). Given that the glycosyltransferase β3GnT1 has also been implicated in LARGE function, it was also included in the glycosyltransferase panel (35). Among these enzymes, only LARGE2 exhibited a dramatic reduction in expression: mRNA levels were 100-fold lower in TEM4–18 relative to PC-3E+ cells (Fig. 4A). We next compared levels of the LARGE2 mRNA across the prostate cancer cell line panel to those in PC-3 cells (Fig. 4B). Notably, they generally correlated with the IIH6 immunoreactivity of each cell line. The exception was the LNCAP cell line, suggesting that an alternate mechanism is responsible for the reduction of αDG glycosylation in LNCaP cells.
FIGURE 4.
LARGE2 mRNA expression correlates with hypoglycosylation of αDG. A, mRNA analysis by qRT-PCR shows dramatically reduced LARGE2 expression, compared with other genes known to influence αDG glycosylation, in TEM4–18 cells compared with PC-3E+ cells. B, LARGE2 mRNA is detectable in the prostate cancer cell line panel and is greatly reduced in TEM4–18 and GS689.Li cells. All LARGE2 samples validated with two probe sets on two separate RNA isolations. Numbers on top of bars are Ct values: GAPDH/gene of interest. C, immunofluorescence staining shows αDG glycosylation (IIH6) is present in a WT mouse prostate and the myodystrophy mouse (Largemyd, MDC1D) prostate (arrows). Staining is not present in a prostate-specific DG knock-out mouse. Muscle staining (asterisks) indicates tissue-specific deletion. Bar, 50 μm.
LARGE2 Functionally Glycosylates αDG in the Prostate
LARGE is required for the proper glycosylation of αDG in skeletal muscle and neural tissue. However, prior studies indicated that in human prostate tissue, the expression of LARGE2 mRNA was significantly greater than that of LARGE (26). Therefore, we next evaluated αDG glycosylation status of prostate tissue from the myodystrophy (myd) mouse (Largemyd, MDC1D), which harbors a loss-of-function mutation in LARGE. Immunofluorescence staining with the IIH6 antibody in a wild-type mouse revealed positive staining in the muscle surrounding the urethra and on the basal side of the prostate epithelium (Fig. 4C). As expected, functional αDG glycosylation was reduced in the muscle tissue of the myd mouse. However it was detectable in the prostate epithelium indicating that LARGE is not required for αDG glycosylation in the prostate. Tissue from a prostate-specific DG knock-out mouse (31) is shown as a control; as expected, IIH6 staining is maintained in the muscle but lost in the prostate gland.
To assess whether endogenous LARGE2 functionally glycosylates αDG, we generated PC-3E+ (IIH6+) cells that express LARGE2 targeted shRNAs. qRT-PCR analysis revealed that two separate shRNAs were capable of producing >70% reduction of LARGE2 mRNA expression (supplemental Fig. S3A) with no significant effect on LARGE mRNA expression (supplemental Fig. S3B). Staining with IIH6 revealed that αDG glycosylation was reduced by 77% with expression of shRNA #147 and 83% with expression of shRNA #148 (Fig. 5A, comparison is to a vector-transfect control). To confirm that ligand binding was reduced in the knockdown cells, we assessed laminin-111 binding by flow cytometry. As expected, laminin binding in the shLARGE2 #147 was significantly reduced relative to the control line (Fig. 5B); moreover, it was nearly absent in the shLARGE2 #148 cell line. Prior incubation with the IIH6 antibody inhibited laminin-1 binding in all cases demonstrating that binding was specific to DG. We further validated the requirement for LARGE2 by knocking-down the protein in two additional IIH6+ cell lines, 22Rv1 and DU145. Flow cytometry revealed that IIH6 immunoreactivity was significantly reduced (supplemental Fig. S3, C and D). Thus, LARGE2 is required for the functional glycosylation of αDG in these cell lines.
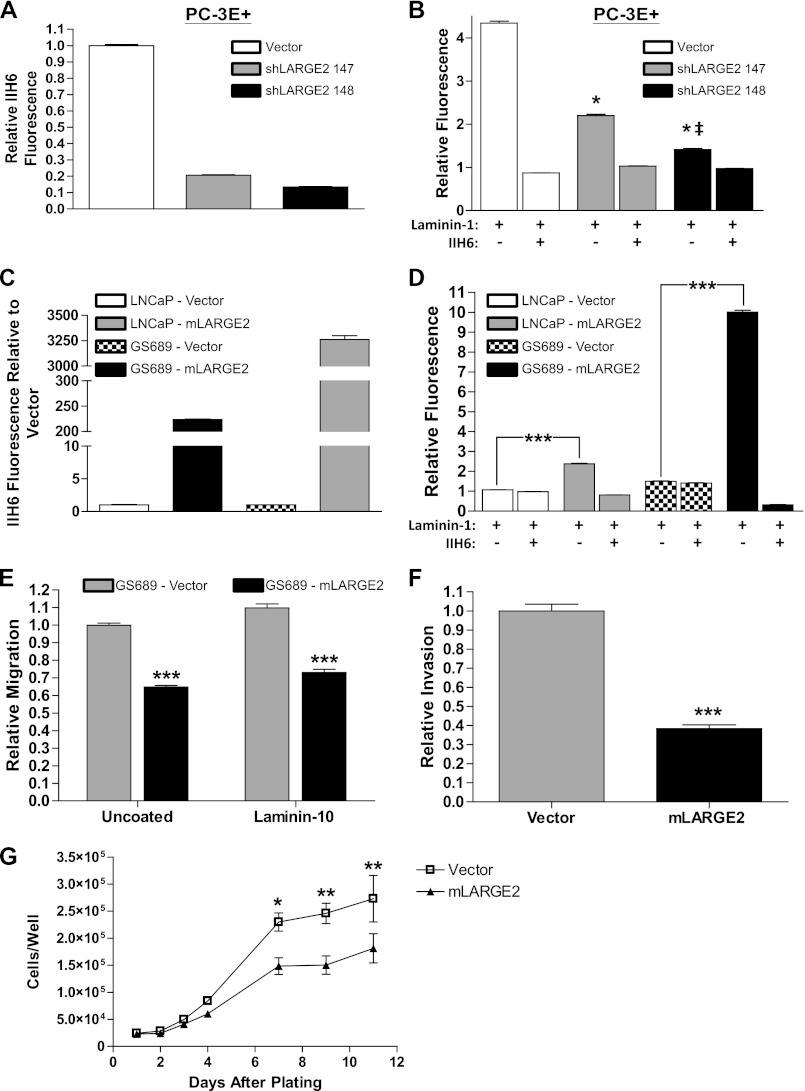
FIGURE 5.
LARGE2 functionally glycosylates αDG reducing invasive and proliferative potential. A, PC-3E+ cells transfected with a LARGE2 targeted shRNA #147 or #148 reduced IIH6 staining by 77 and 86%, respectively by FACS analysis when compared with vector (pLKO) control. B, PC-3E+ pLKO control cells bind exogenous laminin-111. Incubation with IIH6 prior to laminin-111 inhibited binding. Laminin antibody only was used as a background control. PC-3E+ shLARGE2 #147 cells have reduced laminin-111 binding when compared with vector control (*, p < 0.001). PC-3E+ shLARGE2 #148 cells were further inhibited and significantly different from both vector (*, p < 0.001) and shLARGE2 #147 (‡, p < 0.001). C, LNCaP and GS689.Li cells transfected with mLARGE2 or vector control show a significant shift in IIH6 immunoreactivity via flow cytometry. D, LARGE2 expression in IIH6- cell lines induces a significant increase in laminin binding capacity that is specific to DG as shown via inhibition with IIH6 antibody. E, GS689.Li cells show retarded migration through a Boyden chamber both in the absence and presence of a laminin-511 substrate. F, rescue of αDG glycosylation by mLARGE2 also inhibits migration through a Matrigel substrate by >60%. G, mLARGE2 expression causes a significant reduction in growth of GS689.Li cells under standard growth conditions (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
To examine whether reduced LARGE2 expression accounts for αDG hypoglycosylation in other epithelial cancers, we assessed the glycosylation status of αDG and the expression levels of glycosyltransferase mRNA in breast, colon, and pancreatic cancer cell lines. αDG glycosylation was heterogeneous across the cell lines in the panel (supplemental Fig. S4A), and levels of the LARGE2 mRNA correlated with αDG glycosylation status. Of these cancer lines, the breast cancer line ZR75–1 and the colon cancer line HT-29, in which αDG was highly IIH6+, expressed the highest levels of LARGE2, though less than the reference PC-3 cell line; the pancreatic cancer lines BxPC3 and PANC-1 expressed LARGE2 at very low levels. Similarly, the IIH6- MDA.MB231 breast cancer cells expressed very low levels of LARGE2 mRNA (supplemental Fig. S4B). Notably, expression of the LARGE mRNA was also correlated with αDG glycosylation status in the HT-29 and ZR75–1 lines, consistent with prior studies in breast cancer cells (41); expression was very low in the BxPC3, PANC-1, and MDA-MB-231 (Fig. S4C). Therefore, the utilization of LARGE or LARGE2 for αDG functional glycosylation is likely to depend on tissue type.
LARGE2 Overexpression Restores DG Function and Diminishes Invasion and Cell Proliferation Potential
Overexpression of LARGE2 has been shown to functionally glycosylate αDG (27). To assess whether we could revert the hypoglycosylation phenotype in the IIH6− cell lines TEM4–18 and LNCaP, we stably expressed murine LARGE2 in each. Flow cytometry revealed that LARGE2 overexpression indeed led to a prominent, homogenous increase in IIH6 immunoreactivity within the LNCaP cells (Fig. 5C). The TEM4–18 cells exhibited two distinct subpopulations of IIH6 high and low staining cells with a reduction of IIH6+ population during serial passage (data not shown) suggesting that rescue of LARGE2 may not be stable in these cells (supplemental Fig. S3E). Therefore we overexpressed mLARGE2 in a cell line similar to TEM4–18, GS689.Li, that was derived from two in vivo passages in mice as previously described (50). Overexpression of mLARGE2 in these cells produced a significant increase in IIH6 reactivity (Fig. 5C). Furthermore, expression of exogenous mLARGE2 produced a significant increase in the laminin binding capacity in both the LNCaP and GS689 lines (p < 0.0001, Fig. 5D) showing that the rescue of IIH6 reactivity coincides with functional DG glycosylation.
Earlier studies have demonstrated that increasing DG glycosylation reduces migration, orthotopic growth, and invasion of cancer cells (35, 51). We first assessed the effect of LARGE2 knockdown in our less metastatic PC-3E+ cells, but we were unable to show a significant difference in transwell migration, gap closure rate, or single cell motility (data not shown); therefore, we sought to assess the functional consequences of rescuing DG glycosylation in the GS689.Li cell line. We performed the transwell migration assay and found that DG glycosylation inhibited directional migration through both uncoated and laminin-10 (laminin-511)-coated wells (Fig. 5E). Furthermore, utilizing a Matrigel invasion assay, we found a >60% reduction in invasive potential with re-expression of mLARGE2 (Fig. 5F). Having defined a clear effect on migratory and invasive potential, we next performed a growth assay under standard tissue culture conditions and showed a significant difference in the growth characteristics after 6 days in culture of the mLARGE2-expressing cells when compared with vector control (Fig. 5G). These results illustrate a clear effect on multiple characteristics that could impact on metastatic tumor growth in vivo.
LARGE2 Expression Diminishes during Prostate Cancer Progression
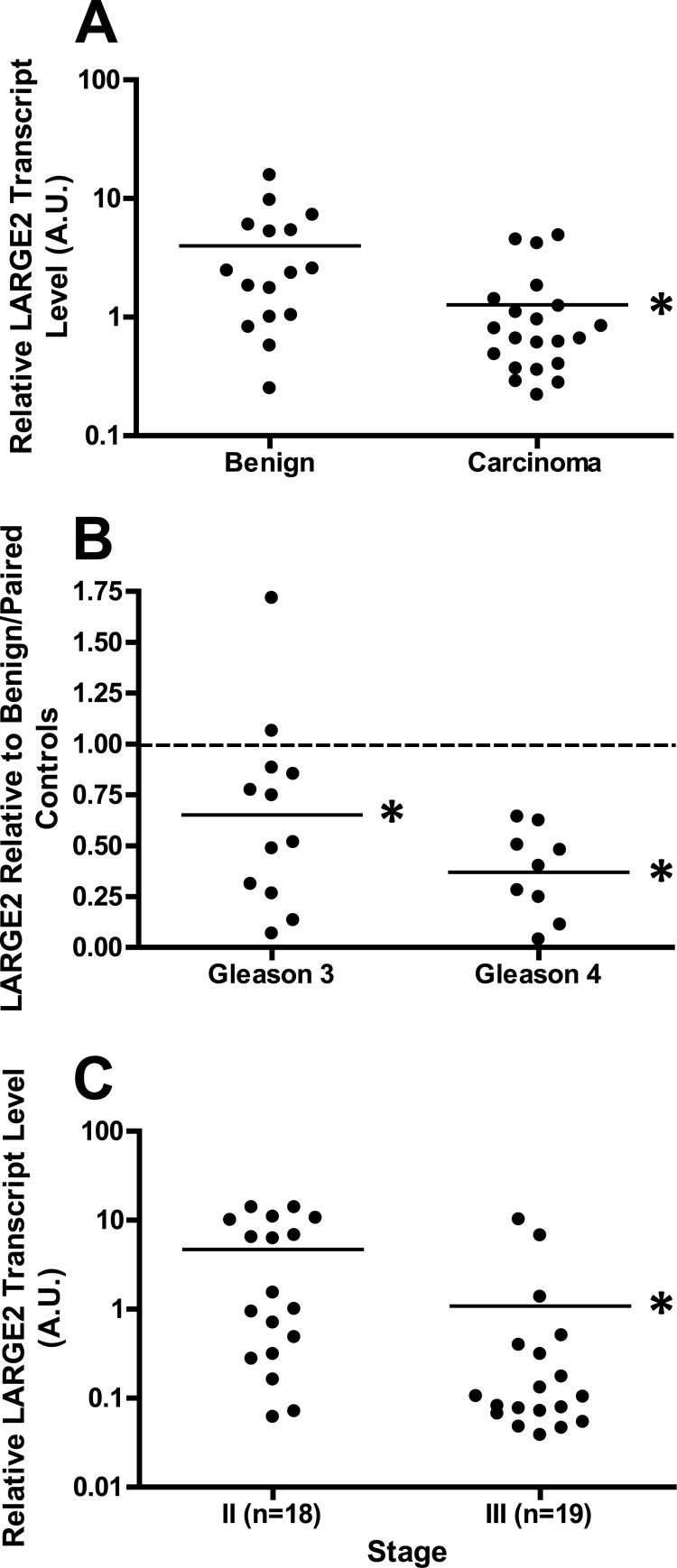
We next assessed whether LARGE2 expression status is associated with hypoglycosylation of αDG in prostate cancer patients. We first used qRT-PCR on samples of laser-capture microdissected tumors and patient-matched benign tissue. Normalization of values for all samples to a random benign control revealed that LARGE2 expression is significantly decreased (benign = 3.999 ± 1.036 a.u., tumor = 1.271 ± 0.3105 a.u., p = 0.0079) in tumors compared with healthy prostate epithelium (Fig. 6A). In order to mitigate patient-to-patient variation, we next compared LARGE2 expression in each tumor to its patient-matched, benign control. LARGE2 expression was reduced in both Gleason grade 3 and 4 samples relative to benign tissue (p = 0.0105 and p = 0.0020, respectively). Moreover, although comparison of the data for Gleason grade 3 and Gleason grade 4 samples did not reveal a statistically significant difference between the two (p = 0.0809), it did suggest a trend toward reduction of LARGE2 levels with advancing disease grade (Fig. 6B). Finally, to confirm that LARGE2 expression inversely correlates with tumor progression, we analyzed LARGE2 on a tissue cDNA microarray organized according to disease stage. Pathological prostate cancer staging is determined by the extent of disease involvement in the patient with stage III being significant as it is the lowest pathological stage where there is evidence of extraprostatic extension of the tumor (52). Analysis of the values normalized to Stage II disease suggested that as the disease progresses from a localized (stage II; 4.690 ± 1.221 a.u.) to an invasive (stage III; 1.084 ± 0.6151 a.u.) phenotype, LARGE2 is significantly reduced (p = 0.0112, Fig. 6C). This is consistent with reduced LARGE2 being an underlying cause of the loss of laminin-binding glycans on αDG in prostate cancer.
FIGURE 6.
LARGE2 expression is diminished during prostate cancer progression. A, microdissected samples of prostate tumor and benign tissue cDNA were obtained and analyzed via qRT-PCR. All samples were normalized to a random, benign control. Carcinoma samples show a markedly reduced level of LARGE2 expression when compared with benign epithelium (benign = 3.999 ± 1.036 a.u., tumor = 1.271 ± 0.3105 a.u., p = 0.0079). B, microdissected samples were all normalized to the patient-matched benign sample with the normalized value of the benign, 1.0, marked by the dashed line. Both Gleason grade 3 and Gleason grade 4 samples show a significant decrease in expression levels (p = 0.0105 and p = 0.0020) when compared with benign. C, analysis of the tissue cDNA array normalized to benign samples. There is a significant decrease of LARGE2 (p = 0.0112) when stage II (disease is completely confined to the prostate) is compared with stage III (extension beyond prostatic capsule or seminal vesicle involvement).
DISCUSSION
Loss of DG expression is a remarkably consistent feature in many cancer types, as documented by multiple groups, including breast, prostate, colon, cervical, renal adenocarcinomas, squamous cell carcinomas, and neural tumors, e.g. (38, 39, 42, 53–56). These studies have implicated proteolysis of α- and βDG, and/or hypoglycosylation of αDG detected via IIH6 or VIA41 monoclonal antibodies. Whether there is a relationship between hypoglycosylation of αDG and proteolysis of α- and/or βDG is unclear at present. Here we identify a new mechanism by which the functional properties of αDG become abrogated in prostate cancer-loss of expression of LARGE2, a putative glycosyltransferase that mediates the formation of laminin-binding glycans on αDG. LARGE2 has not been previously implicated in cancer, and very little is known about its function. Based on its sequence similarity to LARGE, it is inferred that it has similar function. As mentioned above, LARGE has recently been demonstrated to have a unique dual xylosyl-glucuronyl-transferase activity that extends this disaccharide chain that is essential for extracellular matrix ligand binding from phospho-mannose residues in the mucin-like domain of αDG. Our studies suggest that endogenous LARGE2 has this activity in prostate epithelial cells, and prostate cancer cell lines; where LARGE expression is markedly absent in these cells. This outcome could be predicted from consideration of the tissue-specific expression of LARGE and LARGE2, which shows relatively high expression of LARGE2 in the prostate compared with muscle and neural tissue that show much higher expression of LARGE (26). Consistent with the tissue-specific expression pattern of LARGE2, we also find a correlation between low LARGE2 expression and αDG hypoglycosylation in pancreatic cancer cell lines. It remains to be determined whether LARGE2 elaborates glycan structures identical to LARGE and whether client proteins other than αDG exist for LARGE2.
The complexity of αDG functional glycosylation raises a number of other possible causes underlying the hypoglycosylation observed in cancer. Previous studies showed that epigenetic silencing of LARGE results in hypoglycosylation of αDG in breast cancer (10). We do not yet know whether LARGE2 is epigenetically silenced in prostate cancer, but this represents one of several possible mechanisms by which its expression may be reduced. In our studies, we found that the LNCaP cell line, LARGE2 mRNA expression is equivalent to other prostate cancer cell lines that are IIH6+, and we did not detect mutations in the coding sequence of LARGE2 in this cell line. Nonetheless, overexpression of LARGE2 in LNCaP cells restored IIH6 reactivity. Overexpression of LARGE has been shown to override deficiencies in earlier steps of αDG glycosylation, which suggests that one of these may be defective in LNCaP cells (24). A previous study in prostate cancer showed that β3-N-acetylglucosaminyltransferase (β3GnT1) cooperated with LARGE to indirectly promote glycosylation of αDG (35). In that study, reduced expression of β3GnT1 was noted in IIH6− PC-3 cell derivatives and was suggested as an underlying cause of αDG hypoglycosylation. However, here we did not detect a conspicuous loss of β3GnT1 in IIH6− PC-3 cells, whereas LARGE2 was dramatically reduced. In the previous study, LARGE2 mRNA was not detected in prostate cancer cell lines and the basis for this discrepancy with our results is unclear. We cannot rule out a role for β3GnT1 in glycosylation of αDG, however our data indicates that it is unlikely to cooperate with LARGE in prostate epithelial tissue and cancer based on multiple lines of evidence: 1) as mentioned, prior work indicates that LARGE2 is preferentially expressed in the normal prostate compared with LARGE (26); 2) we show here that in a mouse that lacks a functional LARGE gene, that αDG is still glycosylated in normal prostate tissue; 3) using multiple probe sets, we find very little expression of LARGE in IIH6+ prostate cancer cell lines compared with much higher levels of LARGE2; 4) knockdown of LARGE2 in IIH6+ prostate cancer cells results in hypoglycosylation of αDG indicating a role for endogenous LARGE2 in this process. It remains possible that β3GnT1 cooperates with LARGE2 to promote glycosylation of αDG, but this awaits further investigation.
As mentioned above, loss of DG function is associated with cancer cell phenotypes that may promote disease progression such as loss of cell polarity, decreased adhesion to extracellular matrix, increased invasive potential and altered intracellular signaling. Prostate cancer cell lines exhibit heterogeneous functional glycosylation of αDG. Our previous work identified a subpopulation of PC-3 prostate cancer cells, designated TEM4–18, which was isolated on the basis of its enhanced ability to invade an endothelial monolayer (50). These cells also show a Zeb1-dependent epithelial-to-mesenchymal transition and behave more aggressively in metastatic colonization models. Interestingly, in TEM4–18 cells, IIH6 staining was absent, while these cells did still express the core αDG protein and βDG. Using another metastatic derivative of PC-3 cells (GS689.Li), which lack LARGE2 expression and demonstrate hypoglycosylation of αDG, we showed that restoration of LARGE2 expression rescues functional glycosylation of αDG and results in diminished ability of these cells to migrate in response to chemotactic stimulus, invade through Matrigel and reduces cell proliferation. However, we were unable to show that knockdown of LARGE2 promotes invasive phenotypes. These data are consistent with the interpretation that loss of LARGE2 is necessary, but not sufficient, to promote cellular phenotypes associated with prostate cancer progression and metastasis.
Consistent with the idea that αDG hypoglycosylation is associated with a more aggressive disease phenotype, hypoglycosylation of αDG has, in some small retrospective studies, demonstrated potential prognostic value in predicting disease recurrence or survival (54, 57–60). Prior work in prostate cancer demonstrated that αDG hypoglycosylation is associated with increasing Gleason score (46, 47). Here we have independently confirmed these findings in two separate patient cohorts using prostatectomy specimens. Expanding upon this, we report here for the first time that αDG glycosylation is virtually undetectable in prostate cancer metastases, while βDG immunostaining is more frequently retained. Because of the extensive loss of IIH6 in metastatic disease, we attempted to determine if loss of αDG glycosylation could have independent prognostic value in prostatectomy specimens in a retrospective patient cohort. Unfortunately, because of lower than expected mortality in our cohort, we were unable to determine if IIH6 status predicted survival. However, there was a trend toward an association between loss of αDG glycosylation and biochemical recurrence, but, this did not reach statistical significance. Moving forward, analysis of a much larger number of patients will be necessary to evaluate the prognostic value of αDG glycosylation in prostate cancer. In addition, our finding that LARGE2 expression is diminished during prostate cancer progression suggests that it, too, may be investigated as a potential prognostic biomarker in this disease.
Acknowledgments
We thank Robert Vessella (University of Washington) for providing the metastatic prostate tissue array; and the Biospecimen Core of the Pacific Northwest Prostate Cancer SPORE (CA097186) for providing prostate cancer cDNAs and members of the Henry Laboratory and Christine Blaumueller for comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01CA130916 and P30 CA086862 and Contract HHSN261201000032C.

This article contains supplemental Table S1 and Figs. S1–S4.
- DG
- dystroglycan
- β3GnT1
- β3-N-acetylglucosaminyltransferase.
REFERENCES
- 1. Ervasti J. M., Ohlendieck K., Kahl S. D., Gaver M. G., Campbell K. P. (1990) Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 345, 315–319 [DOI] [PubMed] [Google Scholar]
- 2. Ervasti J. M., Campbell K. P. (1991) Membrane organization of the dystrophin-glycoprotein complex. Cell 66, 1121–1131 [DOI] [PubMed] [Google Scholar]
- 3. Ibraghimov-Beskrovnaya O., Ervasti J. M., Leveille C. J., Slaughter C. A., Sernett S. W., Campbell K. P. (1992) Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355, 696–702 [DOI] [PubMed] [Google Scholar]
- 4. Ibraghimov-Beskrovnaya O., Milatovich A., Ozcelik T., Yang B., Koepnick K., Francke U., Campbell K. P. (1993) Human dystroglycan: skeletal muscle cDNA, genomic structure, origin of tissue specific isoforms and chromosomal localization. Hum Mol. Genet 2, 1651–1657 [DOI] [PubMed] [Google Scholar]
- 5. Ervasti J. M., Campbell K. P. (1993) A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell Biol. 122, 809–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bowe M. A., Deyst K. A., Leszyk J. D., Fallon J. R. (1994) Identification and purification of an agrin receptor from Torpedo postsynaptic membranes: a heteromeric complex related to the dystroglycans. Neuron 12, 1173–1180 [DOI] [PubMed] [Google Scholar]
- 7. Campanelli J. T., Roberds S. L., Campbell K. P., Scheller R. H. (1994) A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell 77, 663–674 [DOI] [PubMed] [Google Scholar]
- 8. Gee S. H., Montanaro F., Lindenbaum M. H., Carbonetto S. (1994) Dystroglycan-α, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell 77, 675–686 [DOI] [PubMed] [Google Scholar]
- 9. Manya H., Chiba A., Yoshida A., Wang X., Chiba Y., Jigami Y., Margolis R. U., Endo T. (2004) Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity. Proc. Natl. Acad. Sci. U.S.A. 101, 500–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takahashi T., Honda R., Nishikawa Y. (2000) Cloning of the human cDNA which can complement the defect of the yeast mannosyltransferase I-deficient mutant alg 1. Glycobiology 10, 321–327 [DOI] [PubMed] [Google Scholar]
- 11. Aravind L., Koonin E. V. (1999) The fukutin protein family–predicted enzymes modifying cell-surface molecules. Curr. Biol. 9, R836–837 [DOI] [PubMed] [Google Scholar]
- 12. Brockington M., Blake D. J., Prandini P., Brown S. C., Torelli S., Benson M. A., Ponting C. P., Estournet B., Romero N. B., Mercuri E., Voit T., Sewry C. A., Guicheney P., Muntoni F. (2001) Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin α2 deficiency and abnormal glycosylation of α-dystroglycan. Am. J. Hum. Genet. 69, 1198–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grewal P. K., Holzfeind P. J., Bittner R. E., Hewitt J. E. (2001) Mutant glycosyltransferase and altered glycosylation of α-dystroglycan in the myodystrophy mouse. Nat. Genet. 28, 151–154 [DOI] [PubMed] [Google Scholar]
- 14. Hayashi Y. K., Ogawa M., Tagawa K., Noguchi S., Ishihara T., Nonaka I., Arahata K. (2001) Selective deficiency of α-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology 57, 115–121 [DOI] [PubMed] [Google Scholar]
- 15. Hara Y., Kanagawa M., Kunz S., Yoshida-Moriguchi T., Satz J. S., Kobayashi Y. M., Zhu Z., Burden S. J., Oldstone M. B., Campbell K. P. (2011) Like-acetylglucosaminyltransferase (LARGE)-dependent modification of dystroglycan at Thr-317/319 is required for laminin binding and arenavirus infection. Proc. Natl. Acad. Sci. U.S.A. 108, 17426–17431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanagawa M., Michele D. E., Satz J. S., Barresi R., Kusano H., Sasaki T., Timpl R., Henry M. D., Campbell K. P. (2005) Disruption of perlecan binding and matrix assembly by post-translational or genetic disruption of dystroglycan function. FEBS Lett. 579, 4792–4796 [DOI] [PubMed] [Google Scholar]
- 17. Kanagawa M., Saito F., Kunz S., Yoshida-Moriguchi T., Barresi R., Kobayashi Y. M., Muschler J., Dumanski J. P., Michele D. E., Oldstone M. B., Campbell K. P. (2004) Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell 117, 953–964 [DOI] [PubMed] [Google Scholar]
- 18. Michele D. E., Barresi R., Kanagawa M., Saito F., Cohn R. D., Satz J. S., Dollar J., Nishino I., Kelley R. I., Somer H., Straub V., Mathews K. D., Moore S. A., Campbell K. P. (2002) Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418, 417–422 [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi K., Nakahori Y., Miyake M., Matsumura K., Kondo-Iida E., Nomura Y., Segawa M., Yoshioka M., Saito K., Osawa M., Hamano K., Sakakihara Y., Nonaka I., Nakagome Y., Kanazawa I., Nakamura Y., Tokunaga K., Toda T. (1998) An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature 394, 388–392 [DOI] [PubMed] [Google Scholar]
- 20. Yoshida A., Kobayashi K., Manya H., Taniguchi K., Kano H., Mizuno M., Inazu T., Mitsuhashi H., Takahashi S., Takeuchi M., Herrmann R., Straub V., Talim B., Voit T., Topaloglu H., Toda T., Endo T. (2001) Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev. Cell 1, 717–724 [DOI] [PubMed] [Google Scholar]
- 21. Beltran-Valero de Bernabé D., Voit T., Longman C., Steinbrecher A., Straub V., Yuva Y., Herrmann R., Sperner J., Korenke C., Diesen C., Dobyns W. B., Brunner H. G., van Bokhoven H., Brockington M., Muntoni F. (2004) Mutations in the FKRP gene can cause muscle-eye-brain disease and Walker-Warburg syndrome. J. Med. Genet. 41, e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoshida-Moriguchi T., Yu L., Stalnaker S. H., Davis S., Kunz S., Madson M., Oldstone M. B., Schachter H., Wells L., Campbell K. P. (2010) O-mannosyl phosphorylation of α-dystroglycan is required for laminin binding. Science 327, 88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inamori K., Yoshida-Moriguchi T., Hara Y., Anderson M. E., Yu L., Campbell K. P. (2012) Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science 335, 93–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barresi R., Michele D. E., Kanagawa M., Harper H. A., Dovico S. A., Satz J. S., Moore S. A., Zhang W., Schachter H., Dumanski J. P., Cohn R. D., Nishino I., Campbell K. P. (2004) LARGE can functionally bypass α-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat. Med. 10, 696–703 [DOI] [PubMed] [Google Scholar]
- 25. Grewal P. K., Hewitt J. E. (2002) Mutation of Large, which encodes a putative glycosyltransferase, in an animal model of muscular dystrophy. Biochim. Biophys. Acta. 1573, 216–224 [DOI] [PubMed] [Google Scholar]
- 26. Grewal P. K., McLaughlan J. M., Moore C. J., Browning C. A., Hewitt J. E. (2005) Characterization of the LARGE family of putative glycosyltransferases associated with dystroglycanopathies. Glycobiology 15, 912–923 [DOI] [PubMed] [Google Scholar]
- 27. Fujimura K., Sawaki H., Sakai T., Hiruma T., Nakanishi N., Sato T., Ohkura T., Narimatsu H. (2005) LARGE2 facilitates the maturation of α-dystroglycan more effectively than LARGE. Biochem. Biophys. Res. Commun. 329, 1162–1171 [DOI] [PubMed] [Google Scholar]
- 28. Durbeej M., Larsson E., Ibraghimov-Beskrovnaya O., Roberds S. L., Campbell K. P., Ekblom P. (1995) Non-muscle α-dystroglycan is involved in epithelial development. J. Cell Biol. 130, 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deng W. M., Schneider M., Frock R., Castillejo-Lopez C., Gaman E. A., Baumgartner S., Ruohola-Baker H. (2003) Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development 130, 173–184 [DOI] [PubMed] [Google Scholar]
- 30. Weir M. L., Oppizzi M. L., Henry M. D., Onishi A., Campbell K. P., Bissell M. J., Muschler J. L. (2006) Dystroglycan loss disrupts polarity and {β}-casein induction in mammary epithelial cells by perturbing laminin anchoring. J. Cell Sci. 119, 4047–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Esser A. K., Cohen M. B., Henry M. D. (2010) Dystroglycan is not required for maintenance of the luminal epithelial basement membrane or cell polarity in the mouse prostate. Prostate 70, 777–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jarad G., Pippin J. W., Shankland S. J., Kreidberg J. A., Miner J. H. (2011) Dystroglycan does not contribute significantly to kidney development or function, in health or after injury. Am. J. Physiol. Renal Physiol. 300, F811–F820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mirouse V., Christoforou C. P., Fritsch C., St Johnston D., Ray R. P. (2009) Dystroglycan and perlecan provide a basal cue required for epithelial polarity during energetic stress. Dev. Cell 16, 83–92 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Muschler J., Levy D., Boudreau R., Henry M., Campbell K., Bissell M. J. (2002) A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 62, 7102–7109 [PubMed] [Google Scholar]
- 35. Bao X., Kobayashi M., Hatakeyama S., Angata K., Gullberg D., Nakayama J., Fukuda M. N., Fukuda M. (2009) Tumor suppressor function of laminin-binding α-dystroglycan requires a distinct β3-N-acetylglucosaminyltransferase. Proc. Natl. Acad. Sci. U.S.A. 106, 12109–12114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sgambato A., Camerini A., Faraglia B., Pavoni E., Montanari M., Spada D., Losasso C., Brancaccio A., Cittadini A. (2004) Increased expression of dystroglycan inhibits the growth and tumorigenicity of human mammary epithelial cells. Cancer Biol. Ther. 3, 967–975 [DOI] [PubMed] [Google Scholar]
- 37. Higginson J. R., Winder S. J. (2005) Dystroglycan: a multifunctional adaptor protein. Biochem. Soc. Trans. 33, 1254–1255 [DOI] [PubMed] [Google Scholar]
- 38. Henry M. D., Cohen M. B., Campbell K. P. (2001) Reduced expression of dystroglycan in breast and prostate cancer. Hum. Pathol. 32, 791–795 [DOI] [PubMed] [Google Scholar]
- 39. Sgambato A., Migaldi M., Montanari M., Camerini A., Brancaccio A., Rossi G., Cangiano R., Losasso C., Capelli G., Trentini G. P., Cittadini A. (2003) Dystroglycan expression is frequently reduced in human breast and colon cancers and is associated with tumor progression. Am. J. Pathol. 162, 849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singh J., Itahana Y., Knight-Krajewski S., Kanagawa M., Campbell K. P., Bissell M. J., Muschler J. (2004) Proteolytic enzymes and altered glycosylation modulate dystroglycan function in carcinoma cells. Cancer Res. 64, 6152–6159 [DOI] [PubMed] [Google Scholar]
- 41. de Bernabé D. B., Inamori K., Yoshida-Moriguchi T., Weydert C. J., Harper H. A., Willer T., Henry M. D., Campbell K. P. (2009) Loss of α-dystroglycan laminin binding in epithelium-derived cancers is caused by silencing of LARGE. J. Biol. Chem. 284, 11279–11284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shang Z. J., Ethunandan M., Górecki D. C., Brennan P. A. (2008) Aberrant expression of β-dystroglycan may be due to processing by matrix metalloproteinases-2 and -9 in oral squamous cell carcinoma. Oral oncology 44, 1139–1146 [DOI] [PubMed] [Google Scholar]
- 43. Svensson R. U., Barnes J. M., Rokhlin O. W., Cohen M. B., Henry M. D. (2007) Chemotherapeutic agents up-regulate the cytomegalovirus promoter: implications for bioluminescence imaging of tumor response to therapy. Cancer Res. 67, 10445–10454 [DOI] [PubMed] [Google Scholar]
- 44. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Drake J. M., Barnes J. M., Madsen J. M., Domann F. E., Stipp C. S., Henry M. D. (2010) ZEB1 coordinately regulates laminin-332 and {β}4 integrin expression altering the invasive phenotype of prostate cancer cells. J. Biol. Chem. 285, 33940–33948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sgambato A., De Paola B., Migaldi M., Di Salvatore M., Rettino A., Rossi G., Faraglia B., Boninsegna A., Maiorana A., Cittadini A. (2007) Dystroglycan expression is reduced during prostate tumorigenesis and is regulated by androgens in prostate cancer cells. J. Cell. Physiol. 213, 528–539 [DOI] [PubMed] [Google Scholar]
- 47. Shimojo H., Kobayashi M., Kamigaito T., Shimojo Y., Fukuda M., Nakayama J. (2011) Reduced glycosylation of α-dystroglycans on carcinoma cells contributes to formation of highly infiltrative histological patterns in prostate cancer. Prostate 71, 1151–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Epstein J. I., Allsbrook W. C., Jr., Amin M. B., Egevad L. L. (2005) The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 29, 1228–1242 [DOI] [PubMed] [Google Scholar]
- 49. Gleason D. F., Mellinger G. T. (1974) Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J. Urol. 111, 58–64 [DOI] [PubMed] [Google Scholar]
- 50. Drake J. M., Strohbehn G., Bair T. B., Moreland J. G., Henry M. D. (2009) ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol. Biol. Cell 20, 2207–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yoneyama T., Angata K., Bao X., Courtneidge S., Chanda S. K., Fukuda M. (2012) Fer kinase regulates cell migration through α-dystroglycan glycosylation. Mol. Biol. Cell 23, 771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Edge S. B., and American Joint Committee on Cancer (2010) AJCC Cancer Staging Manual, 7th ed., Springer, New York: [DOI] [PubMed] [Google Scholar]
- 53. Sgambato A., Tarquini E., Resci F., De Paola B., Faraglia B., Camerini A., Rettino A., Migaldi M., Cittadini A., Zannoni G. F. (2006) Aberrant expression of α-dystroglycan in cervical and vulvar cancer. Gynecol. Oncol. 103, 397–404 [DOI] [PubMed] [Google Scholar]
- 54. Sgambato A., Camerini A., Amoroso D., Genovese G., De Luca F., Cecchi M., Migaldi M., Rettino A., Valsuani C., Tartarelli G., Donati S., Siclari O., Rossi G., Cittadini A. (2007) Expression of dystroglycan correlates with tumor grade and predicts survival in renal cell carcinoma. Cancer Biol. Ther. 6, 1840–1846 [DOI] [PubMed] [Google Scholar]
- 55. Calogero A., Pavoni E., Gramaglia T., D'Amati G., Ragona G., Brancaccio A., Petrucci T. C. (2006) Altered expression of α-dystroglycan subunit in human gliomas. Cancer Biol. Ther. 5, 441–448 [DOI] [PubMed] [Google Scholar]
- 56. Martin L. T., Glass M., Dosunmu E., Martin P. T. (2007) Altered expression of natively glycosylated α-dystroglycan in pediatric solid tumors. Hum. Pathol. 38, 1657–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shen J. G., Xu C. Y., Li X., Dong M. J., Jiang Z. N., Wang J., Wang L. B. (2012) Dystroglycan is associated with tumor progression and patient survival in gastric cancer. Pathol. Oncol. Res. 18, 79–84 [DOI] [PubMed] [Google Scholar]
- 58. Sgambato A., Caredda E., Leocata P., Rossi G., Boninsegna A., Vitale A., Grandi T., Cittadini A., Migaldi M. (2010) Expression of α-dystroglycan correlates with tumour grade and predicts survival in oral squamous cell carcinoma. Pathology 42, 248–254 [DOI] [PubMed] [Google Scholar]
- 59. Jiang X., Rieder S., Giese N. A., Friess H., Michalski C. W., Kleeff J. (2011) Reduced α-dystroglycan expression correlates with shortened patient survival in pancreatic cancer. J. Surg. Res. 171, 120–126 [DOI] [PubMed] [Google Scholar]
- 60. Moon Y. W., Rha S. Y., Zhang X., Jeung H. C., Yang W. I., Kwon O., Jeong J. H., Cheon S. H., Yoo N. C., Chung H. C. (2009) Increments of α-dystroglycan expression in liver metastasis correlate with poor survival in gastric cancer. J. Surg. Oncol. 100, 459–465 [DOI] [PubMed] [Google Scholar]