Background: Sphingosine 1-phosphate (S1P) signaling in vascular development is not well understood.
Results: S1P is present in zebrafish plasma. Combined knockdown of S1P receptors 1 and 2 (s1pr1 and s1pr2) in the zebrafish results in synergistic perturbation of vascular patterning and development.
Conclusion: Cooperative signaling between s1pr1 and s1pr2 regulates zebrafish vascular development.
Significance: S1P receptor regulation of vascular development is conserved in evolution.
Keywords: Angiogenesis, Development, Sphingolipid, Sphingosine 1-Phosphate, Vascular Biology, Zebrafish
Abstract
Sphingosine 1-phosphate (S1P) binds G-protein-coupled receptors (S1P1–5) to regulate a multitude of physiological effects, especially those in the vascular and immune systems. S1P receptors in the vascular system have been characterized primarily in mammals. Here, we report that the S1P receptors and metabolic enzymes are conserved in the genome of zebrafish Danio rerio. Bioinformatic analysis identified seven S1P receptor-like sequences in the zebrafish genome, including duplicated orthologs of receptors 3 and 5. Sphingolipidomic analysis detected erythrocyte and plasma S1P as well as high plasma ceramides and sphingosine. Morpholino-mediated knockdown of s1pr1 causes global and pericardial edema, loss of blood circulation, and vascular defects characterized by both reduced vascularization in intersegmental vessels, decreased proliferation of intersegmental and axial vessels, and hypersprouting in the caudal vein plexus. The s1pr2 gene was previously characterized as a regulator of cell migration and heart development, but its role in angiogenesis is not known. However, when expression of both s1pr1 and s1pr2 is suppressed, severely reduced vascular development of the intersegmental vessels was observed with doses of the s1pr1 morpholino that alone did not cause any discernible vascular defects, suggesting that s1pr1 and s1pr2 function cooperatively to regulate vascular development in zebrafish. Similarly, the S1P transporter, spns2, also cooperated with s1pr1. We propose that extracellular S1P acts through vascular S1P receptors to regulate vascular development.
Introduction
Sphingosine 1-phosphate (S1P)3 is a lipid mediator that binds to five G-protein-coupled receptors (S1P1–5) to regulate cell proliferation, survival, migration, and differentiation. Sphingosine, a metabolite of sphingomyelin metabolism, is phosphorylated by sphingosine kinases (SphK1 and -2) to form S1P, which in turn is metabolized by S1P phosphohydrolases (SPP1 and -2) or S1P lyase (1). The prototypic receptor, S1P1, was cloned as a phorbol 12-myristate 13-acetate-inducible immediate early transcript from human umbilical vein endothelial cells (2). Ultimately, S1P as a high affinity ligand for S1P1 (originally known as the endothelial differentiation gene or EDG-1) was described previously (3). S1P1, S1P2, and S1P3 are expressed in many tissues, particularly in the cardiovascular, nervous, and immune systems (4, 5). The expression patterns of S1P4 and S1P5, in contrast, are more restricted. S1P4 is mainly localized to the lymphoid, hematopoietic tissue, and lung (6), whereas S1P5 is mainly expressed in the brain and spleen (7, 8).
Functions of S1P have been revealed by genetic loss of function studies in mice (9, 10). Knock-out of S1pr1 in mice leads to intrauterine lethality between E12.5 to E14.5 due to severe hemorrhaging, which was presumed to result from a vascular maturation defect (9). Recent analysis of postnatal, conditional deletion of S1pr1 in endothelial cells results in hypersprouting of endothelial tip cells in the developing mouse retina independently of mural cell defects, suggesting its fundamental role in vascular development (11). In contrast, embryos lacking S1pr2 are viable; however, when challenged, they display decreased pathological neovascularization during the oxygen-induced retinopathy model (12). In addition, S1pr2 knock-out mice show defective vascular structures in the inner ear (stria vascularis), which leads to the degeneration of inner ear structures, deafness, and abnormal equilibrium phenotypes (13). Moreover, a combined knock-out of S1pr1 and S1pr2 results in bleeding, and lethality occurs about 2 days earlier (E10.5–12.5) than in S1pr1 single null embryos (10), thus indicating that S1pr2 functions as a supportive rather than essential receptor for regulating murine vascular development. Because of this early lethality, functions for embryonic angiogenesis beyond hemorrhaging have not been clearly delineated for such loss of function studies in mice.
S1P receptor signaling is widely utilized in vertebrates (14). In the zebrafish it was shown that a point mutation in the miles apart gene (mil), which encodes S1pr2, prevents cardiac precursor cells from migrating to the midline, thus resulting in cardia bifida (15). During zebrafish gastrulation, mil was shown to regulate cell motility and directionality of the prechordal plate progenitor cells (16). Additionally, zebrafish carrying a mutation in the S1P transporter, spinster 2 (spns2), phenocopy the mutant mil fish (17). Despite the cell migratory defects previously characterized in the mil and spns2 zebrafish, no vascular defects were described. In addition, a cardia bifida phenotype was not observed in the mouse.
Developing zebrafish embryos are sufficiently permeable to oxygen and therefore often tolerate defects in the cardiovascular system, which can facilitate detailed evaluation of phenotypes that are more difficult to delineate in the mouse. Here, we report that the S1P receptors and enzymes crucial for S1P metabolism are conserved in the zebrafish genome. We demonstrate that knockdown of s1pr1 via morpholinos causes edema, loss of blood circulation, and vascular defects. In addition, we show that knockdown of s1pr2 along with s1pr1 results in a much more dramatic vascular phenotype. Similarly, the S1P transporter, spns2, also cooperates with s1pr1. Therefore, s1pr1 is an important regulator of zebrafish vascular development and cooperates with s1pr2 and spns2 to exert its functions in the vascular system.
EXPERIMENTAL PROCEDURES
Quantitative RT-PCR
Staged wild-type embryos or adult tissue specimens were homogenized with TRIzol LS (Ambion), and total RNA was isolated (Qiagen). Total RNA (1 μg) was used to generate cDNA using reverse transcriptase and random hexamers (Roche Applied Science). Primer sequences were designed using Primer3 and are listed in Table 1. LightCycler 480 SYBR Green 1 Master Mix (Roche Applied Science) was used to analyze cDNA by quantitative RT-PCR using the Light Cycler 480II (Roche Applied Science). The PCR cycle conditions were 95 °C for 15 min followed by 40 cycles at 94 °C for 14 s, 54 °C for 30 s, and 72 °C for 30 s.
TABLE 1.
Primer sequences for qRT-PCR
The abbreviations used are as follows: F, forward; R, reverse.
| s1pr1 | F: CAC AAC AAT GGC AAG ACC TG |
| R: TAT GCT GTC GAT GCA GTT CC | |
| s1pr2 | F: CAT CAT CTT GCT GTC CAT CG |
| R: AGA TGA TGA AGA CGC CAA GG | |
| s1pr3a | F: ATG GAT GAC GAG CTT GAA CC |
| R: TTC TTG GTC TTC CCC ACT TG | |
| s1pr3b | F: CCA TTT GGA GGA ACC ACA AG |
| R: TCG GCT GGA GAT AAA TGG AG | |
| s1pr4 | F: TCA GCG TCT TCA TCA TCC TG |
| R: TGT TCC CGT CAA CAA GTC AC | |
| s1pr5a | F: ATG CGC AGA GCC ATT CTA AG |
| R: GCA TGC CAA ACT TCT TGA CC | |
| s1pr5b | F: TGC AGC GCC ACT ACA ACT AC |
| R: CAC CAC GTT CAG CAT GTA GG | |
| spp1 | F: ATA CCC CTG AGC CTG TTT CTG |
| R: TAG ATG CGG CTC AAA CAC AC | |
| spp2 | F: CAC ACG ACA GCA ACT CCA AC |
| R: AGA TTT CAT GGC CCA GAG TG | |
| sphk1 | F: TGG AGA CGG GCT ACT CTT TG |
| R: GTA ATG ATG GAC GGA AGC AG | |
| sphk2 | F: AAT TCT GCC TTG TGG CTC TG |
| R: GCA GAA AAC AGC AGT TGA GG | |
| spl | F: TCG GGG TAG TAT GGC TAA AGG |
| R: TCC AGA GCT TTG TTG AGC TG |
Maintenance of Zebrafish
Wild-type (AB/TUB) and transgenic zebrafish from the Zebrafish International Research Center (Eugene, OR) were maintained at 28.5 °C and staged as described previously (18). We used the following endothelial specific transgenic fluorescent reporter lines: Tg(fli:EGFP)y1 (cytosolic EGFP) (19), Tg(flk1:ras-mCherry)s896 (membrane-targeted mCherry) (20), and the nucleus-targeted EGFP reporter Tg(flk1:EGFP-NLS) (21). The Tg(gata1:dsRed) reporter line was used to examine red blood cell circulation (22).
Morpholino Oligomer Injection
Morpholino oligomers (MOs) were purchased from Gene Tools (Philomath, OR). The sequences of all MOs used in this study are listed in Table 2. Two distinct MOs were designed for s1pr1 that target the 5′-UTR around the start codon to block mRNA translation (translation blockers, designated s1pr1 MO1 and MO2). Blast analysis indicated the MOs were specific for s1pr1 (no overlap with other sequences). A previously validated translation-blocking MO was used against s1pr2 (s1pr2 MO1) (23), and a second nonoverlapping MO (s1pr2 MO2) was designed to validate the double knockdown experiment. A previously validated p53 MO was used to ensure that the gene-specific phenotype described here was not the result of p53-dependent apoptosis (24). The spns2 MO was validated for specificity in previously published work (17). All morphants were compared with stage-matched embryos that were injected with a control morpholino. Each MO was titrated by injection into 1–4-cell fertilized embryos to determine a minimal dose for the reproducible phenotype. Microinjection of MOs was performed using a PLI-100 Pico-Injector (Harvard Apparatus).
TABLE 2.
Morpholino oligomer sequences
| s1pr1 MO1, 5′-AGTGTCTGGCGATTAGGTCATCCAT-3′ |
| s1pr1 MO2, 5′-GTTTGCACTGGCTTGGTAGTTTTAT-3′ |
| s1pr2 MO1, 5′-CCGCAAACAGACGGCAAGTAGTCAT-3′ |
| s1pr2 MO2, 5′-TCAGAGGGCAGCTTTTTTTTGAGCT-3′ |
| control MO, 5′-CCTCTTACCTCAGTTACAATTTATA-3′ |
| p53 MO, 5′-GCGCCATTGCTTTGCAAGAATTG-3′ |
| spns2 MO, 5′-GGAGGGAATATGTGATGCTTACTTC-3′ |
Cloning
The s1pr1 full-length clone was obtaining using cDNA generated from RNA of 24 h post-fertilization (hpf) wild-type zebrafish embryos. The open reading frame was PCR-amplified using the following forward and reverse primers, respectively: 5′-ATGGATGACCTAATCGCCAGACAC and 5′-TTAAGAAGAAGAGGTGATATT. Following ligation into the pCRII-TOPO vector (Invitrogen), the insert was subcloned into pCS2+. The insert was linearized with SacII, and capped mRNA was generated using the mMessage mMachine kit (Applied Biosystems). Two nonoverlapping in situ hybridization probes for s1pr1 were generated by PCR amplifying a 476- and 509-bp fragment of s1pr1 from the same cDNA. The following primers were used to generate the 476-bp fragment, forward 5′-GTTCATCATCGTGTGCTGCT and reverse 5′-ATACGGTGGTGCAGAAAAGG, and for the 509-bp fragment, forward 5′-TCAGCGTTATTCTCATGGCC and reverse 5′-GATATTCCCAGAAGACACTATGG. Following ligation into the pCRII-TOPO vector (Invitrogen), the vector was linearized, and SP6 or T7 RNA polymerase was used to synthesize the probes.
Whole-mount in Situ Hybridization
Whole-mount in situ hybridization was performed as described previously (25). Embryos were treated with 0.003% phenylthiourea (Sigma) to prevent pigmentation. Following fixation in 4% paraformaldehyde (Sigma), embryos were treated with 10 μg/ml proteinase K (Roche Applied Science). Hybridization was performed at 68 °C in 50% formamide buffer (Roche Applied Science) with digoxigenin-labeled RNA probes (Roche Applied Science).
Plasma Collection
Plasma was collected as described previously (26). Briefly, fish were anesthetized using ice water. A steel blade was used to make a diagonal incision between the anal fin and caudal fin. A low retention pipette tip attached to a P20 pipettor that had been coated with sodium heparin (Sigma) was used to collect the blood. Typically 5–15 μl of blood was obtained per fish, and samples from multiple fish (8) were pooled into a single Eppendorf tube. Samples were spun at 1000 rpm in an Eppendorf centrifuge (model 5424) for 15 min. The supernatant was removed into a clean Eppendorf tube. Packed blood cells and plasma were submitted to the analytical core facility at the Medical University of South Carolina for biochemical analysis of ceramide and sphingolipid levels by liquid chromatography mass spectrometry. For estimation of S1P concentrations, packed RBC volume, prepared as described above, was considered.
Preparation of Tissue Samples for Lipid Analysis
Brain, heart, liver, and tail tissue were dissected from adult zebrafish and snap-frozen in liquid nitrogen. The tissue samples were pulverized using a mortar and pestle; the resulting powder was dissolved in 150–300 μl of lysis buffer containing phosphatase and protease inhibitors. Following protein determination, the samples were submitted to the analytical core facility at the Medical University of South Carolina for ceramide and sphingolipid analysis.
Immunofluorescence Staining of Fixed Embryos
For confocal microscopy, embryos were fixed at the desired developmental stage and antibody stained to enhance the signal from the endogenous fluorescent transgene. Following overnight fixation in 4% paraformaldehyde (Sigma) at 4 °C, embryos were rinsed four times for 20 min each in PBS/Tween (Fisher) (0.1% Tween in 1× PBS), permeabilized for 25 min in cold acetone (Fisher), and then rinsed again four times for 20 min each in PBST. Embryos were blocked in 2% bovine serum albumin (Sigma) in PBST for 2 h and then incubated with the primary antibody diluted in blocking reagent overnight at 4 °C. Following four washes in PBST, the embryos were incubated in secondary antibody diluted in blocking reagent for 2 h at room temperature and then rinsed four times for 20 min each in PBST prior to imaging. The following primary antibodies were used at a concentration of 1:500: anti-green fluorescent protein mouse IgG2a, monoclonal 3E6 (Invitrogen), and rabbit DsRed polyclonal (Clontech). The following secondary antibodies were used at a concentration of 1:500: Alexa 488 goat anti-mouse IgG2a (Invitrogen) and Alexa 568 goat anti-rabbit IgG (Invitrogen).
Imaging Analysis
Fish were anesthetized using Tricaine (United States Biochemical) prior to imaging. Bright field images were taken using a Nikon SMZ1500 fluorescence microscope with an Insight Firewire 2 digital camera and SPOT advanced software. Fluorescent images were taken using a Zeiss Axio Observer.Z1 microscope and captured using a Zeiss AxioCam CCD camera. For confocal analysis, fixed and stained embryos were mounted in 1% low melt agarose (National Diagnostics) dissolved in water. Confocal images were taken with a Leica TCS SP5 microscope (Leipzig, Germany) using a water dipping ×40 lens with an NA of 0.8 and 1.3 digital zoom. Confocal images were also taken using an Olympus Fluoview Microscope with a ×60 lens and analyzed using Fluoview software. Images were processed with ImageJ (1.42q) Imaris (Bitplane Inc.) and Adobe PhotoshopCS4 software.
RESULTS
S1P Metabolic Enzymes and Receptor Family Are Conserved in the Zebrafish
Genomic database analysis identified the presence of seven S1P receptor sequences in the zebrafish. For two receptors, s1pr3 and s1pr5, the zebrafish genome contained duplicated copies of the mammalian orthologs (denoted s1pr3a, s1pr3b, s1pr5a, and s1pr5b) (Fig. 1A). The percent identity at the protein level between the human and zebrafish cognate receptors ranges from 48 to 71% (Fig. 1B).
FIGURE 1.
S1P receptors and enzymes required for its biosynthesis are conserved in the zebrafish. A, seven S1P receptors were identified in the zebrafish with duplication of s1pr3 and s1pr5. B, percent identity at the protein level between the zebrafish S1P receptors and the corresponding human receptors. C, percent identity at the protein level between the enzymes responsible for biosynthesis of S1P in the zebrafish and human.
Additionally, the DNA sequences of enzymes responsible for the biosynthesis and breakdown of S1P were found to be conserved in the zebrafish. We identified two sphingosine 1-phosphate phosphatases (spp1 and spp2), two sphingosine kinases (sphk1 and sphk2), and one sphingosine lyase (spl). The percent identity between the human and zebrafish enzymes ranges from 48 to 64% at the protein level (Fig. 1C).
Bioinformatics analysis indicates synteny between the zebrafish sphk2 gene and the human SPHK2 gene. Likewise, synteny is also observed between the Xenopus sphk1 gene and the zebrafish sphk1 as well as between the zebrafish spp genes and the human spp genes.
Quantitative RT-PCR analysis was conducted to examine relative expression levels of S1PRs during embryogenesis. Transcripts for s1pr1 were first detected during somitogenesis (Fig. 2A). In sharp contrast, s1pr2 is maternally expressed; those transcripts are depleted during the cleavage phase, and the gene is subsequently expressed during gastrulation (Fig. 2A). Transcripts for s1pr3 were first detected at 24 hpf (Fig. 2A). Transcripts encoding the other zebrafish S1P receptors examined (s1pr3b, s1pr4, s1pr5a, and s1pr5b) were not detectable by quantitative RT-PCR at the early developmental time points analyzed.
FIGURE 2.
Expression levels quantified by quantitative RT-PCR of the S1P receptors and enzymes required for its biosynthesis. A, s1pr1, s1pr2, and s1pr3 are expressed during early embryonic development in the zebrafish. Expression levels are normalized to 24 hpf. B, s1p1, s1pr2, s1pr3a, s1pr3b, s1pr4, and s1pr5a are expressed in the intestine, heart, brain kidney, and eye of adult zebrafish. Expression levels are normalized to the intestine. C, spp1, spp2, spl, sphk1, and sphk2 are expressed during early embryonic development in the zebrafish. Expression levels are normalized to 24 hpf. D, spp1, spl, and sphk2 are expressed in the intestine, heart, brain, kidney, and eye of the adult zebrafish. Expression levels are normalized to the intestine. E, sorting of 24 hpf Tg(fli:EGFP)y1 embryos for GFP-positive endothelial cells and Tg(Gata1:dsRed) embryos for dsRed positive blood cells followed by qRT-PCR analysis revealed that s1pr1, s1pr2, and s1pr3 are enriched in zebrafish endothelial cells, and spp1, spl, and sphk2 are enriched in both zebrafish endothelial and blood cells. Expression levels are normalized to the whole embryo.
Relative expression levels of the zebrafish s1prs were examined in select tissues (intestine, heart, brain, kidney, and eye) from adult zebrafish. Enrichment of s1pr1 was seen in the brain, as in mammalian species, whereas s1pr2, s1pr3a, s1pr3b, and s1pr5a were ubiquitously expressed in the adult tissue types examined (Fig. 2B). Expression of s1pr4 was enriched in the kidney, the site of zebrafish hematopoiesis, which is consistent with its expression in mammalian lymphoid and hematopoietic tissues (6). Expression of s1pr5b was not detected in the tissues examined.
Relative expression levels of the key enzymes important for S1P biosynthesis were also examined by quantitative RT-PCR. The two zebrafish spp genes displayed inverse expression patterns (Fig. 2C). Although detectable at low levels during early developmental stages, spp1 was most highly expressed from somitogenesis to 3 days post-fertilization (dpf). In contrast, abundant maternal transcripts for spp2 were found, although its expression levels dropped precipitously by the cleavage stage. Low levels of spl transcripts were detectable at the early time points studied, although expression levels increased by 3 days post-fertilization (Fig. 2C). Expression of sphk1 was initially detected during somitogenesis and began to decline by 3 days post-fertilization, whereas sphk2 was maternally expressed. The sphk2 transcript levels decreased slightly during the cleavage stage and then were maintained at a relatively similar level for the remainder of the developmental time points analyzed (Fig. 2C). In the adult zebrafish, only spp1, spl, and sphk2 were detectable. Expression levels were highest in the intestine for all three genes, and spp1 was also highly expressed in the kidney (Fig. 2D).
We sorted GFP-positive endothelial cells or dsRed-positive erythroid cells from 24 hpf Tg(fli:EGFP)y1 and Tg(gata1:dsRed) zebrafish embryos, respectively. We found that transcripts for s1pr1, s1pr2, and s1pr3a were enriched in endothelial cells (Fig. 2E). Upon examining the S1P enzymes that were highly expressed at 24 hpf, we found that spp1, spl, and sphk2 were enriched in both endothelial and erythroid cells (Fig. 2E).
Zebrafish Sphingolipid Levels Are Enriched in Blood Plasma
Plasma and RBC samples were isolated from the adult zebrafish and analyzed by liquid chromatography/mass spectrometry. In comparison with mouse plasma, zebrafish plasma displayed significantly higher levels of ceramides, in particular long chain fatty acid-containing species (Fig. 3A and Table 3). This was not seen in packed blood cells, which are primarily RBCs (Fig. 3B and Table 4). Importantly, zebrafish plasma and packed blood cells contained significant levels of S1P (∼90 and ∼140 nm, respectively) (Fig. 3, C and D and Tables 3 and 4). This was not as high as the S1P levels in mouse plasma and packed blood cells (∼800 and 2400 nm, respectively). Interestingly, zebrafish plasma contained higher dihydrosphingosine, and sphingosine than mouse plasma (Fig. 3C and Table 3). In contrast, mouse RBCs have higher levels of ceramide, dihydrosphingosine, sphingosine, and S1P as compared with the zebrafish (Fig. 3, B and D and Table 4). As significantly higher levels of S1P (∼90 nm) were found in zebrafish plasma than the affinity of S1P to its receptors, these data suggest that plasma-derived S1P is sufficiently abundant to activate its vascular receptors.
FIGURE 3.
Ceramide, sphingosine, and S1P levels in plasma, RBC, and tissue samples obtained from zebrafish and mice. A, plasma ceramide levels in samples obtained from zebrafish (black) and mice (gray). B, RBC ceramide levels in samples obtained from zebrafish (black) and mice (gray). C, plasma dihydrosphingosine, sphingosine, and sphingosine 1-phosphate levels in samples obtained from zebrafish (black) and mice (gray). D, RBC dihydrosphingosine, sphingosine, and sphingosine 1-phosphate levels in samples obtained from zebrafish (black) and mice (gray). E, ceramide levels in adult zebrafish brain (black), heart (dark gray), liver (light gray), and tail tissue samples (white). F, dihydrosphingosine, sphingosine, and sphingosine 1-phosphate levels in adult zebrafish brain (black), heart (dark gray), liver (light gray), and tail tissue samples (white).
TABLE 3.
Plasma ceramide, sphingosine, and sphingosine-1-phosphate levels
| Mouse plasma | Zebrafish plasma | |
|---|---|---|
| average (nm) ± S.D. | average (nm) ± S.D. | |
| C14-Cer | 4.4 ± 0.2 | 24.1 ± 2.8 |
| C16-Cer | 46 ± 8.4 | 165.3 ± 18.3 |
| C18-Cer | 17.8 ± 1.6 | 17.6 ± 0.5 |
| C18:1-Cer | 1.1 ± 0.8 | 3.9 ± 1.3 |
| C20-Cer | 34.2 ± 5.2 | 404.1 ± 120.1 |
| C20:1-Cer | 1 ± 0.0 | 22.8 ± 7.5 |
| C20:4-Cer | 1.2 ± 0.6 | 2.0 ± 0.6 |
| C22-Cer | 108.2 ± 13.3 | 472.1 ± 66.0 |
| C22:1-Cer | 11.2 ± 2.2 | 93.2 ± 22.0 |
| C24-Cer | 504.3 ± 108.9 | 2660.5 ± 361.5 |
| C24:1-Cer | 489.3 ± 84.6 | 3156.2 ± 558.0 |
| C26-Cer | 12.6 ± 1.0 | 1883.6 ± 375.5 |
| C26:1-Cer | 3.7 ± 0.0 | 1509.2 ± 291.5 |
| Dihydro-C16-Cer | 6.2 ± 2.1 | 78.3 ± 17.9 |
| Dihydrosphingosine | 13.0 ± 5.0 | 31.6 ± 3.7 |
| Dihydro-S1P | 27.9 ± 4.9 | 138.0 ± 25.6 |
| Sphingosine | 253.6 ± 37.6 | 9.4 ± 1.5 |
| S1P | 789.4 ± 142.0 | 88.4 ± 6.1 |
TABLE 4.
RBC ceramide, sphingosine, and sphingosine-1-phosphate levels
| Mouse RBC | Zebrafish RBC | |
|---|---|---|
| average (nm) ± S.D. | average (nm) ± S.D. | |
| C14-Cer | 10.7 ± 0.7 | 69.8 ± 21.1 |
| C16-Cer | 582.1 ± 109.5 | 318.3 ± 31.7 |
| C18-Cer | 164.1 ± 0.2 | 24.4 ± 2.5 |
| C18:1-Cer | 12.3 ± 3.2 | 2.1 ± 2.2 |
| C20-Cer | 154.3 ± 1.6 | 234.6 ± 31.2 |
| C20:1-Cer | 6.8 ± 2.5 | 22.1 ± 11.5 |
| C20:4-Cer | 6.2 ± 1.0 | 30.1 ± 5.2 |
| C22-Cer | 1037.3 ± 21.4 | 239.7 ± 16.2 |
| C22:1-Cer | 83.6 ± 7.3 | 134.6 ± 27.8 |
| C24-Cer | 4459.3 ± 370.6 | 626.0 ± 81.8 |
| C24:1-Cer | 2084.1 ± 65.7 | 1657.1 ± 151.1 |
| C26-Cer | 706.1 ± 177.4 | 410.4 ± 115.7 |
| C26:1-Cer | 97.7 ± 20.1 | 368.8 ± 42.5 |
| Dihydro-C16-Cer | 149.7 ± 4.8 | 53.1 ± 8.0 |
| Dihydrosphingosine | 239.7 ± 111.5 | 19.9 ± 2.0 |
| Dihydro-S1P | 245.9 ± 33.6 | 61.9 ± 4.5 |
| Sphingosine | 1588.4 ± 388.7 | 11.6 ± 3.2 |
| S1P | 2424.6 ± 142.0 | 142.8 ± 16.3 |
Sphingolipid levels were also analyzed in zebrafish brain, heart, liver, and tail tissue (Fig. 3, E–F, and Table 5). Among these tissues, S1P levels were highest in the zebrafish brain (33.9 pmol/mg). These values coincide with previously published data showing enrichment of S1P levels in the mouse brain as compared with other tissues (27). S1P levels in the heart, liver, and tail were 5.8, 4.5, and 5.7 pmol/mg, respectively. These levels are similar to S1P levels measured in mouse thymus (27) and intestine (28) and would correlate with concentrations well below what is found in blood. These data support our finding that S1P is enriched in the zebrafish blood.
TABLE 5.
Adult zebrafish tissue ceramide, sphingosine, and sphingosine-1-phosphate levels
| Brain | Heart | Liver | Tail | |
|---|---|---|---|---|
| (pmol/mg) ± S.D. | (pmol/mg) ± S.D. | (pmol/mg) ± S.D. | (pmol/mg) ± S.D. | |
| C14-Cer | 3.2 ± 0.1 | 7.2 ± 2.2 | 6.8 ± 5.1 | 4.2 ± 1.1 |
| C16-Cer | 20.4 ± 5.7 | 44.4 ± 10.1 | 34.9 ± 21.4 | 45.7 ± 10.8 |
| C18-Cer | 70.0 ± 7.1 | 5.7 ± 1.6 | 5.8 ± 3.2 | 3.0 ± 0.7 |
| C18:1-Cer | 0.4 ± 0.1 | 0.3 ± 0.3 | 0.7 ± 0.4 | 0.1 ± 0.0 |
| C20-Cer | 49.3 ± 3.5 | 10.0 ± 0.4 | 27.4 ± 18.9 | 9.6 ± 2.3 |
| C20:1-Cer | 0.8 ± 0.3 | 0.6 ± 0.1 | 2.3 ± 1.4 | 0.7 ± 0.1 |
| C20:4-Cer | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| C22-Cer | 276.9 ± 77.6 | 28.1 ± 1.9 | 26.2 ± 16.5 | 12.5 ± 2.7 |
| C22:1-Cer | 41.9 ± 9.5 | 26.0 ± 7.8 | 18.5 ± 11.3 | 10.6 ± 3.7 |
| C24-Cer | 380.4 ± 112.1 | 69.9 ± 15.3 | 47.2 ± 17.3 | 20.8 ± 4.6 |
| C24:1-Cer | 449.6 ± 102.8 | 97.0 ± 20.5 | 80.8 ± 48.9 | 35.0 ± 8.9 |
| C26-Cer | 62.4 ± 15.4 | 29.1 ± 9.0 | 26.0 ± 9.4 | 4.7 ± 1.2 |
| C26:1-Cer | 86.1 ± 17.9 | 34.9 ± 10.5 | 23.2 ± 9.6 | 8.0 ± 1.8 |
| Dihydro-C16-Cer | 4.2 ± 0.4 | 4.1 ± 3.1 | 2.4 ± 1.9 | 1.5 ± 0.3 |
| Dihydrosphingosine | 16.6 ± 1.8 | 23.4 ± 18.2 | 3.8 ± 0.7 | 2.8 ± 0.4 |
| Dihydro-S1P | 35.7 ± 6.5 | 2.5 ± 1.4 | 0.4 ± 0.3 | 1.8 ± 0.5 |
| Sphingosine | 48.0 ± 4.4 | 29.7 ± 5.3 | 53.8 ± 34.1 | 6.4 ± 1.5 |
| S1P | 33.9 ± 4.6 | 5.8 ± 2.6 | 4.5 ± 2.5 | 5.7 ± 1.3 |
Knockdown of s1pr1 Results in Pericardial Edema, Loss of Blood Circulation, and Vascular Defects
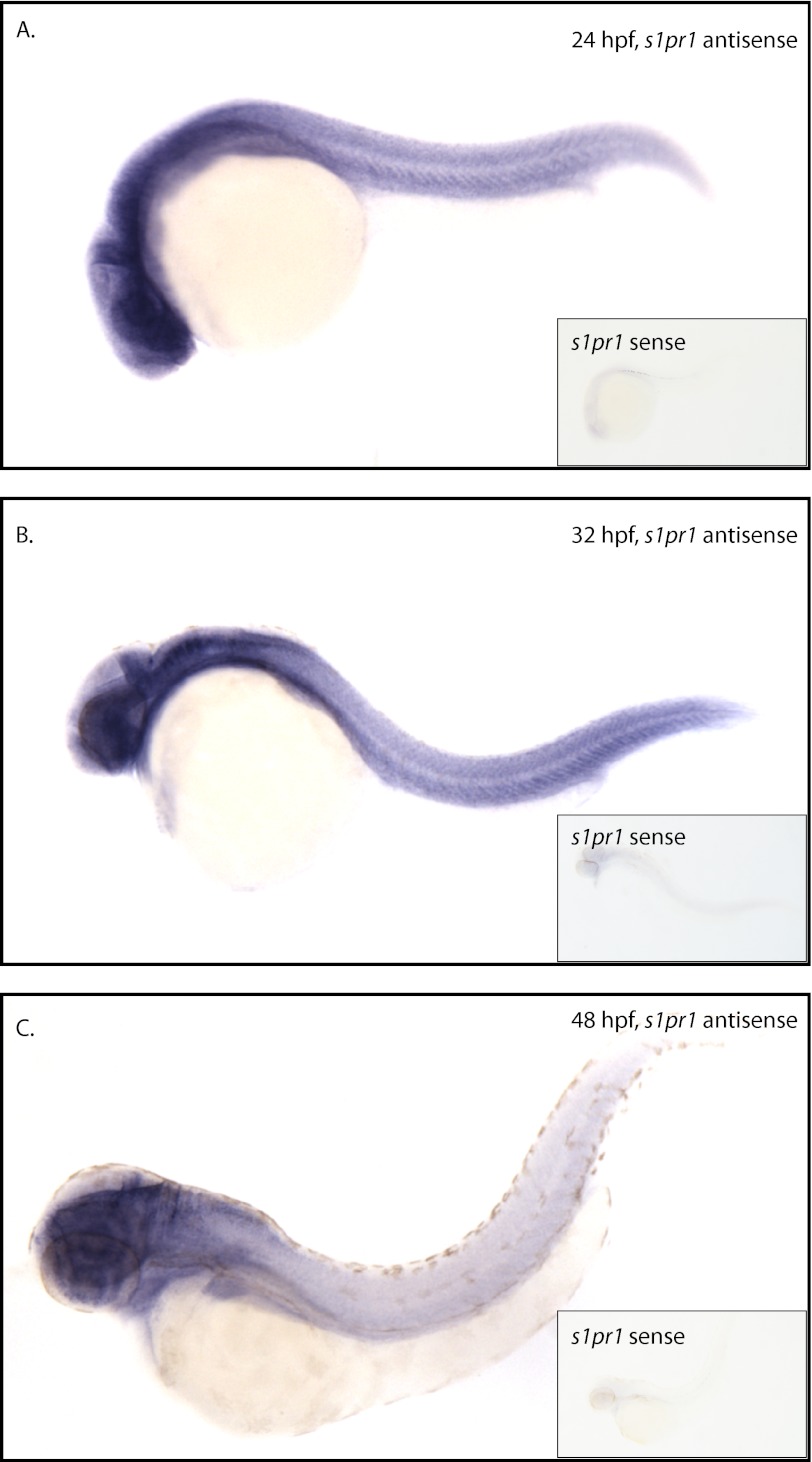
Given that vascular phenotypes are caused by deletion of S1pr1 in mice, detectable levels of S1P were measured in zebrafish plasma, and s1pr1 is enriched in zebrafish endothelial cells, we next examined the effect of s1pr1 knockdown on zebrafish embryogenesis and vascular development. By in situ hybridization, s1pr1 was strongly detected in the brain, neural tube, dorsal aorta, and posterior cardinal vein at 24 hpf (Fig. 4A) and 32 hpf (Fig. 4B). By 48 hpf, expression was localized mainly to the brain as the tail was only weakly stained (Fig. 4C).
FIGURE 4.
In situ hybridization reveals that s1pr1 is ubiquitously expressed in the zebrafish. At 24 hpf (A) and 32 hpf (B), in situ hybridization for s1pr1 reveals enriched expression in the brain, neural tube, and axial vessels of the trunk, compared with the sense control (inset). C, by 48 hpf, s1pr1 transcripts are highly enriched in the brain.
A translation-blocking morpholino, s1pr1 MO1, was designed to target the ATG translational initiation start site of s1pr1. In comparison with stage-matched zebrafish that were injected with a control morpholino (Fig. 5A), injection of 4 ng of s1pr1 MO1 resulted in a phenotype of severe general and pericardial edema by 48 hpf (Fig. 5B; 548 affected/579 injected; n = 8 independent experiments). The morphant fish also displayed improperly looped hearts that beat slowly. The severity of the phenotype was dependent on the dose of the morpholino injected, but 4 ng generated a consistent phenotype. Embryos derived from Tg(Gata1:dsRed) fish injected with a control morpholino displayed normal red blood cell development by 48 hpf (Fig. 5C). By examining s1pr1 morphant embryos, we noticed that the red blood cells failed to enter circulation (Fig. 5D; 548 affected/579 injected; n = 8 independent experiments). Examination of Tg(fli:EGFP)y1 embryos that had been injected with a control morpholino shows complete formation of the primary angiogenic network in essentially all injected embryos (Fig. 5E). In contrast, similar analysis of fish injected with 4 ng of s1pr1 MO1 revealed that many of the intersegmental vessels (ISVs) failed to sprout beyond the horizontal myoseptum to produce the dorsal longitudinal anastomotic vessel (DLAV) (Fig. 5F; 319 affected/361 injected; n = 6 independent experiments, yellow asterisks indicate stunted ISVs). Thus, by 48 hpf the DLAV was discontinuous (Fig. 5F). These phenotypes were not the result of a developmental delay, as s1pr1 morphant fish did not recover by 72 hpf (supplemental Fig. 1). To determine whether any of the phenotypes displayed by the s1pr1 morphants were the result of off-target induced p53-dependent apoptosis, zebrafish were co-injected with 4 ng of a morpholino that has previously been validated at this dose to deplete p53 (24, 29) along with 4 ng of the s1pr1 MO1. Co-injection of the s1pr1 morphants with the p53 MO did not lessen the severity of the edema and circulatory phenotypes or the vascular phenotype (supplemental Fig. 2, A–D). Thus, the phenotype characterized for s1pr1 knockdown is not the result of a p53-dependent off-target effect and is likely due to specific knockdown of the s1pr1 receptor.
FIGURE 5.
Knockdown of s1pr1 causes edema, circulatory, and vascular phenotypes. Bright field images of control morphant (A) and s1pr1 morphant (B) are shown. The s1pr1 morphants have edema of the yolk and pericardium (548 affected/579 injected; n = 8 independent experiments) at 48 hpf. In Tg(gata1:dsRed) fish injected with control MO (C), there was normal blood cell circulation, whereas following injection of s1pr1 MO1 4 ng (D), there was failure of the red blood cells to migrate from the caudal hematopoietic tissue (548 affected/579 injected; n = 8 independent experiments) at 48 hpf. In Tg(fli:EGFP)y1 fish injected with control MO (E), there was normal vascular development, whereas embryos injected with s1pr1 MO1 4 ng (F) revealed that many injected fish displayed some ISVs that failed to sprout beyond the horizontal myoseptum to produce the DLAV (319 affected/361 injected; n = 6 independent experiments) at 48 hpf. Yellow asterisks indicate stunted ISVs. To determine whether the knockdown of s1pr1 affected endothelial cell abundance, Tg(flk1:ras-mCherry)s896 and Tg(flk1:EGFP-NLS) double transgenic fish were injected with control MO 4 ng (G) or s1p1 MO1 4 ng (H) and imaged at 32 hpf. Quantitation of nuclei number shows more nuclei in the angiogenic vessels (ISVs and DLAV) of control-injected fish (n = 14) compared with the s1pr1 morphant embryos (n = 15), p < 0.0001 (I) and more nuclei in the vasculogenic axial vessels (DA and PCV) in the control-injected fish compared with the s1pr1 morphant embryos p = 0.0242 (J). Nuclei were quantified in four consecutive ISVs or in axial vessels from a representative field of view.
To determine whether the knockdown of s1pr1 affected endothelial cell abundance, fertilized eggs derived from Tg(flk1:ras-mCherry)s896 and Tg(flk1:EGFP-NLS) double transgenic fish were injected with 4 ng of the control MO (Fig. 5G) or 4 ng of the s1pr1 MO1 (Fig. 5H) and imaged at 32 hpf, a time point by which the primary angiogenic vasculature, namely the ISVs and DLAV, should have formed. Control morphants had significantly more endothelial cells in both the angiogenic (ISV and DLAV) vessels (Fig. 5I) and the vasculogenic axial vessels (dorsal aorta and posterior cardinal vein) (Fig. 5J) as compared with the s1pr1 morphants, suggesting that s1p1 could regulate cell abundance within both angiogenic and vasculogenic vessels by affecting endothelial cell specification, proliferation, and/or survival.
Several antibodies to mammalian S1pr1 did not cross-react with the zebrafish protein (data not shown). The S1P receptors consist of two exons, the second of which encodes the entire open reading frame for the receptor, thus making the use of splice-blocker morpholinos problematic. Therefore, to confirm specificity of the phenotype, a second translation-blocking morpholino (s1pr1 MO2) was generated to target a distinct sequence upstream of the ATG transcription start site. Injection of 8 ng of s1pr1 MO2 phenocopied the edema, cardiac, and blood phenotypes seen in the embryos injected with 4 ng of s1pr1 MO1 (supplemental Fig. 3, A and B) and resulted in embryos with mild vascular defects consisting of improper lateral connections of the ISVs (supplemental Fig. 3C, yellow asterisks). Co-injection of both translation-blocking morpholinos at sub-threshold doses (that do not generate a phenotype when injected alone; supplemental Fig. 3, D–G) yielded embryos that also displayed the edema, cardiac, and blood phenotypes seen in the embryos injected with 4 ng of MO1 (supplemental Fig. 3, H and I) suggesting that these two morpholinos have the same target and generate similar phenotypes.
In addition to the ISVs, development of the caudal vein plexus (CaVP) is a well studied model for developmental angiogenesis in the zebrafish (30). As compared with control-injected zebrafish (Fig. 6A), fish injected with 4 ng of s1pr1 MO1 (Fig. 6B) displayed a significant decrease in length, width, and total area of the CaVP. Strikingly, injection of 100 pg of s1pr1 mRNA (Fig. 6C) resulted in the opposite effect by increasing width and total area of the CaVP (Fig. 6D). Compared with control-injected fish (Fig. 6E), s1pr1 morphants phenocopied the mouse retinal defect as injection of s1pr1 MO1 resulted in significantly increased filopodial abundance (Fig. 6F), whereas injection of mRNA resulted in decreased filopodial abundance (Fig. 6, G and H). These findings suggest that s1pr1 is important for critical developmental events such as vascular sprouting, patterning, endothelial cell abundance, and barrier function.
FIGURE 6.
Knockdown of s1pr1 promotes hypersprouting of the zebrafish CaVP whereas overexpression of s1pr1 causes decreased sprouting. CaVP in control morphants (A), s1rp1 morphants (B), and following overexpression of 100 pg of s1pr1 mRNA at ×10 magnification at 32 hpf (C). D, fish injected with s1pr1 MO1 displayed a significant decrease in length, width, and total area of the CaVP, whereas fish injected with s1pr1 mRNA displayed an increase in width and total area of the CaVP. CaVP in control morphants (E), s1pr1 morphants (F), and following overexpression of s1p1 mRNA (G) at ×60 magnification. H, injection of s1pr1 MO1 resulted in a significantly increased number of filopodia per field, whereas injection of mRNA resulted in decreased filopodial abundance.
Knockdown of s1pr1 and s1pr2 Exacerbates the Phenotype seen Following Knockdown of s1pr1 Alone
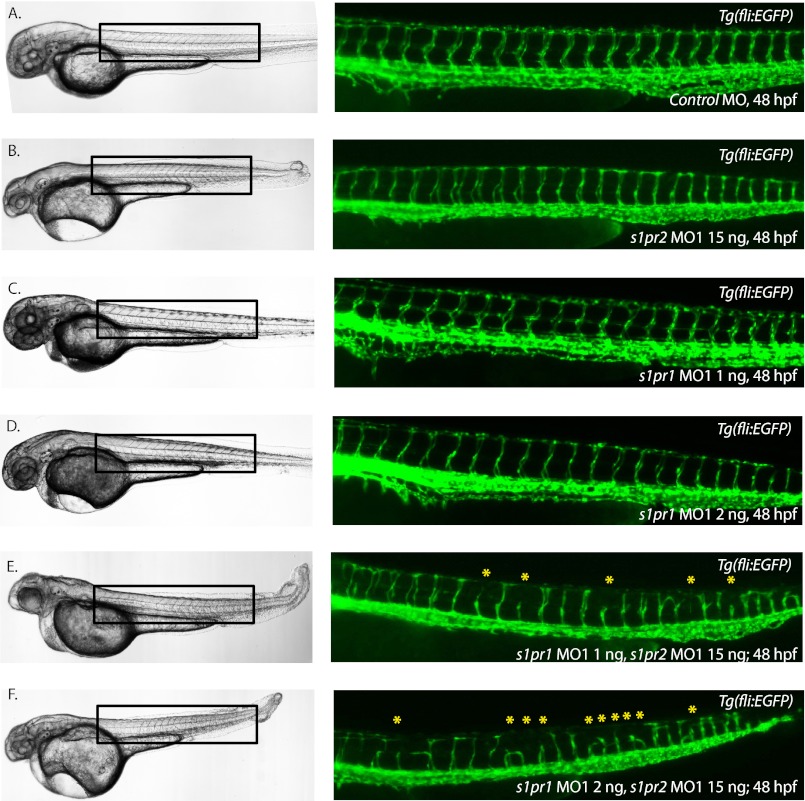
The s1pr2 gene is important for regulating the migration of cardiac precursor cells to the midline, as a point mutation in the gene yields cardiac bifida (15). Vascular defects were not described for these mil mutants, such that s1pr2 is not known to regulate angiogenesis. ISV sprouting is normal in embryos injected with the control MO (Fig. 7A), 15 ng of s1pr2 MO1 (a dose that recapitulates a cardiac and tail-blistering phenotype as described previously (17)) (Fig. 7B), or low doses of s1pr1 MO1 at 1 or 2 ng, respectively (Fig. 7, C and D). However, when expression of both s1pr1 and s1pr2 is blocked by co-injection of both morpholinos, severely reduced vascular development of the intersegmental vessels was observed with doses of the s1pr1 morpholino that alone did not cause any discernible vascular defects (Fig. 7, E and F). Co-injection of the s1pr1 and s1pr2 double morphants with the p53 MO did not lessen the severity of the phenotypes (supplemental Fig. 2, E–H). To confirm that the results obtained with these two morpholinos were specific, s1pr1 MO1 was injected with a second translation-blocking morpholino against s1pr2 (s1pr2 MO2). Results obtained with s1pr1 MO1 and s1pr2 MO2 phenocopied those observed with s1pr1 MO1 and s1pr2 MO1 (supplemental Fig. 4).
FIGURE 7.
Knockdown of s1pr1 and s1pr2 exacerbates the vascular phenotype seen following knockdown of s1pr1 alone. Tg(fli:EGFP)y1 embryos injected with control MO (A), s1pr2 MO1 15 ng (B), s1pr1 MO1 1 ng (C), or s1pr1 MO1 2 ng (D) display normal ISV sprouting at 48 hpf. Tg(fli:EGFP)y1 embryos injected with s1pr1 MO1 1 ng, and s1pr2 MO1 15 ng display a more severe vascular defect (182 affected/205 injected; n = 5 individual experiments) than embryos injected with either MO alone at 48 hpf (E). Tg(fli:EGFP)y1 embryos injected with s1pr1 MO1 2 ng and s1pr2 MO1 15 ng display a more severe vascular defect (207 affected/224 injected; n = 4 individual experiments) than embryos injected with either MO alone at 48 hpf (F).
S1P Transporter spns2 Cooperates with s1pr1 to Regulate Angiogenesis
The spns2 gene functions as an S1P transporter in S1P secretion and regulates myocardial precursor migration (17). The phenotype of zebrafish with a point mutation in spns2 is identical to the phenotype exemplified by mil mutant fish; both display cardia bifida and epithelial tail blisters. Based on these findings, we tested whether spns2 played a cooperative role with s1pr1 in regulating vascular sprouting. ISV sprouting is normal in embryos injected with the control MO (Fig. 8A) or 2 ng of spns2 MO (a sub-threshold dose of the previously validated morpholino) (Fig. 8B). Knockdown of both s1pr1 and spns2 (at doses that by themselves did not affect ISV sprouting) resulted in stunted development of the ISVs (Fig. 8, C and D). Therefore, the interaction between s1pr1 and spns2 is required for proper embryonic vascular patterning in the zebrafish.
FIGURE 8.
S1P transporter spns2 also functions in the s1pr1-regulated pathway controlling vascular patterning. Tg(fli:EGFP)y1 embryos injected with control MO (A) and spns2 MO 2 ng (B) display normal ISV sprouting at 48 hpf. Tg(fli:EGFP)y1 embryos injected with s1pr1 MO1 1 ng and spns2 MO 2 ng display a more severe vascular defect (94 affected/149 injected; n = 3 individual experiments) than embryos injected with either MO alone at 48 hpf (C). Tg(fli:EGFP)y1 embryos injected with s1pr1 MO1 2 ng and spns2 MO 2 ng display a more severe vascular defect (160 affected/183 injected; n = 3 individual experiments) than embryos injected with either MO alone at 48 hpf (D).
DISCUSSION
Here, we show conservation of the key S1P receptors and enzymes important for S1P biosynthesis in the zebrafish. Database analysis revealed zebrafish nucleotide sequences similar to all five mammalian S1P receptors. Additionally, two homologs were identified for s1pr3 and s1pr5, likely due to the whole genome duplication of the teleost fish lineage (31–33). Early embryonic development is dependent upon maternal gene transcripts that are generated during oogenesis. Some transcripts of the S1P receptors and enzymes important for S1P metabolism are maternally expressed (s1pr2, spp1, spp2, spl, and sphk2), suggesting that these function during early embryonic development. There is a decrease in transcript levels for these genes during the maternal-zygotic transition and a subsequent increase as the zygotic genes are activated.
Additionally, expression of the s1pr genes in adult fish tissue confirms at least generally conserved expression patterns with mice. Enrichment of s1pr1 was seen in the brain, as in mammals, and expression of s1pr4 was enriched in the kidney, the site of zebrafish hematopoiesis, which is consistent with its expression in mammalian lymphoid and hematopoietic tissues (6). The other receptors, s1pr2, s1pr3a, s1pr3b, and s1pr5a, were ubiquitously expressed in the adult tissue types examined. In the adult zebrafish, high expression levels of spp1, spl, and sphk2 were detectable in the intestine, a result consistent with findings that dietary sphingolipid hydrolysis and metabolism occur in the intestine of mice (34).
The ligand specificity of zebrafish s1pr1 has been shown to be similar to mammalian S1pr1. Im et al. (35) showed that 100 pm S1P was sufficient to inhibit intracellular cAMP production in s1pr1-transfected cells (IC50 = 1 nm). High levels of lysophosphatidic acid (as high as 10 μm) were unable to have the same effect. Structurally related agonists of S1P (such as dihydro-S1P) inhibit cAMP accumulation; however, other lysophospholipids had no effect.
S1P levels measured from adult zebrafish plasma samples (∼90 nm) display sufficient levels to activate endothelial cells as bioactive S1P in the plasma is ∼10 nm, which is near the Kd value of the ligand for the receptor (36). Interestingly, zebrafish plasma contains higher levels of ceramide, dihydrosphingosine, and sphingosine but lower levels of dihydro-S1P and S1P in comparison with mouse plasma. These findings suggest that in the zebrafish, high sphingosine-1-phosphate phosphatase activity may aid in the conversion of S1P to its precursors sphingosine and ultimately ceramide, whereas in the mouse the activity of sphingosine kinase may dominate to convert ceramide and sphingosine into S1P. In mammals a steep vascular S1P gradient, formed due to significantly higher levels of S1P in the plasma and lymph as compared with the tissue interstitium (37), promotes the egress of lymphocytes and hematopoietic stem cells (38, 39). In the zebrafish, the role of the S1P gradient has not been examined, but our data indicate that significant levels of S1P are enriched in blood cells (RBCs) and plasma. This could result in the activation of S1P receptors in vascular and hematopoietic systems.
A recent paper examining s1pr1 knockdown by morpholinos in zebrafish also showed edema (pericardial and general) and the circulation phenotype that we describe here (40). Although the authors did not characterize the ISV-sprouting phenotype, this might be due to the lower dose of morpholino that they used for their injections. The authors did describe thinner ISVs and defects in the duct of Cuvier and subintestinal vein basket, which are vascular phenotypes that we also observed in our experiments (see supplemental Fig. 1).
In mice it has been shown that deletion of S1pr1 results in embryonic lethality because of its role within endothelial cells in regulating the coverage of blood vessels by vascular smooth muscle cells (9, 41). To understand whether this phenotype is conserved in other species, and to evaluate in more detail potential functions in embryonic angiogenesis, we examined knockdown of s1pr1 in the zebrafish. Here, we characterized vascular defects in two vascular beds, the ISVs and CaVP. Following morpholino knockdown of s1pr1, we saw stunted vascular sprouting of the ISVs resulting in incomplete formation of the DLAV. In contrast, we showed that morpholino knockdown of s1pr1 causes excessive filopodial sprouting in the CaVP, whereas injection of s1pr1 mRNA has the opposite effect and decreases filopodial sprouting as compared with control-injected fish. These two findings likely indicate heterogeneity in the function of s1pr1 in these two vascular beds. The ISV defects occur in sprouts from the axial artery, whereas the defects in the CaVP occur in a vein, thus suggesting that s1pr1 exerts different effects on arteries and veins.
Our finding of excessive sprouting angiogenesis following knockdown of s1pr1 in the zebrafish CaVP complements recent work (11, 42) showing that conditional knock-out of S1pr1 in murine endothelial cells results in alterations in the primary vascular plexus of the retina. More specifically, these mice display increased vascular density, excessive numbers of filopodia-containing tip cells, and increased branch points in the retinal vessels as compared with wild-type mice (11). Similarly, when we examine sprouting angiogenesis in the zebrafish CaVP following s1pr1 morpholino knockdown, we also saw increased filipodia sprouting in this vascular bed as compared with control morphants. In contrast, overexpression of S1pr1 results in suppression of tip cell formation in the developing mouse retina, a finding that is paralleled following injection of s1pr1 mRNA into zebrafish embryos. Additionally, S1pr1 was shown to be essential for flow-dependent endothelial shear stress sensing in endothelial cells in vitro and in the mouse aorta in vivo. As the zebrafish can allow for the examination of flow-dependent signaling in real time, it may be feasible to use the zebrafish as a tool for better understanding the mechanism of flow-mediated S1P1 activation.
We believe that our knockdown phenotypes reflect specific targeting of s1pr1 for several reasons. First, the phenotypes are reproducible in essentially 100% of the embryos and are highly consistent using two distinct morpholinos. Importantly, co-injection of two s1pr1 morpholinos at concentrations that are individually sub-threshold recapitulates the phenotype of each when used above the threshold, arguing that they target the same transcript. Finally, the phenotypes are independent of p53, suggesting that they cannot be attributed to p53-dependent off-targeting artifacts.
To unmask collaborative functions between s1pr1 and s1pr2, we knocked down expression of both receptors and found a substantially more severe vascular phenotype than was seen in embryos following knockdown of only s1pr1. These results corroborate murine data showing that S1pr1 and S1pr2 double knock-out mice display a more severe vascular phenotype than do S1pr1 single knock-out mice. Formation of an immature vascular network in the S1pr1 and S1pr2 double knock-out mice results in embryonic lethality that is on average 2 days earlier (E10.5–12.5) than in the S1pr1 single null embryos (E12.5–14.5) (10). Because of this early hemorrhaging and subsequent lethality, the role of S1pr1 and S1pr2 in embryonic angiogenesis had not been clearly examined in mice. As knockdown of both receptors in the zebrafish did not result in early lethality but rather a distinct vascular phenotype, we showed that s1pr1 and s1pr2 have redundant or cooperative functions for the development of a stable and mature vascular system during embryonic development.
The S1P transporter spns2 functions in the same pathway as mil because both regulate myocardial precursor cell migration. As a consequence, zebrafish expressing mutant spns2 phenocopy the mil phenotypes of cardia bifida and epithelial tail blistering. Knockdown of both s1pr1 and spns2 (at doses that themselves did not affect ISV sprouting) resulted in stunted development of the ISVs, showing a remarkably similar phenotype as full knockdown of s1pr1. Therefore, the interaction between s1pr1 and spns2 is required for proper vascular patterning in the zebrafish.
In conclusion, we show that the key receptors and enzymes important for sphingosine metabolism and signaling are conserved in the zebrafish. We also show the presence of significant levels of S1P in the zebrafish plasma that are sufficient to activate its receptors on endothelial cells. Morpholino knockdown of s1pr1 results in pericardial edema, lack of blood circulation, and distinct vascular defects in the arterial and venous beds, namely failure of arterial-derived ISVs to sprout completely and excessive sprouting of the venous-derived CaVP. Combined knockdown of s1pr1 and s1pr2 causes a stronger vascular phenotype in the ISVs indicating that s1pr1 and s1pr2 function cooperatively to regulate vascular formation in the zebrafish. Thus, sufficient S1P is present in the plasma to signal via multiple endothelial S1P receptors to regulate vascular development in vertebrates.
Acknowledgments
Sphingolipid measurements were supported by National Institutes of Health Grant P30 CA138313 from the Lipidomics Shared Resource, Hollings Cancer Center, Medical University of South Carolina and by National Institutes of Health COBRE Grant P20 RR017677 from the Lipidomics Core, South Carolina Lipidomics and Pathobiology.
This work was supported, in whole or in part, by National Institutes of Health Grants T32HL083824 (to K. M.), HL092263-01A1 (to J. T.-V.), HL056182, and HL111400 (to T. E.), and HL67330, HL89934, and HL70694 (to T. H.) from NHLBI and Grant C06 RR018823 from Office of the Director of the National Institutes of Health through NCRR.

This article contains supplemental Figs. 1–4.
- S1P
- sphingosine 1-phosphate
- MO
- morpholino oligomer
- ISV
- intersegmental vessel
- CaVP
- caudal vein plexus
- DLAV
- dorsal longitudinal anastomotic vessel
- hpf
- hours post-fertilization
- EGFP
- enhanced GFP.
REFERENCES
- 1. Le Stunff H., Milstien S., Spiegel S. (2004) Generation and metabolism of bioactive sphingosine 1-phosphate. J. Cell. Biochem. 92, 882–899 [DOI] [PubMed] [Google Scholar]
- 2. Hla T., Maciag T. (1990) An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J. Biol. Chem. 265, 9308–9313 [PubMed] [Google Scholar]
- 3. Lee M. J., Van Brocklyn J. R., Thangada S., Liu C. H., Hand A. R., Menzeleev R., Spiegel S., Hla T. (1998) Sphingosine 1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279, 1552–1555 [DOI] [PubMed] [Google Scholar]
- 4. Ishii I., Friedman B., Ye X., Kawamura S., McGiffert C., Contos J. J., Kingsbury M. A., Zhang G., Brown J. H., Chun J. (2001) Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J. Biol. Chem. 276, 33697–33704 [DOI] [PubMed] [Google Scholar]
- 5. Liu C. H., Hla T. (1997) The mouse gene for the inducible G-protein-coupled receptor edg-1. Genomics 43, 15–24 [DOI] [PubMed] [Google Scholar]
- 6. Gräler M. H., Bernhardt G., Lipp M. (1998) EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics 53, 164–169 [DOI] [PubMed] [Google Scholar]
- 7. Im D. S., Heise C. E., Ancellin N., O'Dowd B. F., Shei G. J., Heavens R. P., Rigby M. R., Hla T., Mandala S., McAllister G., George S. R., Lynch K. R. (2000) Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. J. Biol. Chem. 275, 14281–14286 [DOI] [PubMed] [Google Scholar]
- 8. Malek R. L., Toman R. E., Edsall L. C., Wong S., Chiu J., Letterle C. A., Van Brocklyn J. R., Milstien S., Spiegel S., Lee N. H. (2001) Nrg-1 belongs to the endothelial differentiation gene family of G protein-coupled sphingosine 1-phosphate receptors. J. Biol. Chem. 276, 5692–5699 [DOI] [PubMed] [Google Scholar]
- 9. Liu Y., Wada R., Yamashita T., Mi Y., Deng C. X., Hobson J. P., Rosenfeldt H. M., Nava V. E., Chae S. S., Lee M. J., Liu C. H., Hla T., Spiegel S., Proia R. L. (2000) Edg-1, the G protein-coupled receptor for sphingosine 1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kono M., Mi Y., Liu Y., Sasaki T., Allende M. L., Wu Y. P., Yamashita T., Proia R. L. (2004) The sphingosine 1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 279, 29367–29373 [DOI] [PubMed] [Google Scholar]
- 11. Jung B., Obinata H., Galvani S., Mendelson K., Ding B. S., Skoura A., Kinzel B., Brinkmann V., Rafii S., Evans T., Hla T. (2012) Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 23, 600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skoura A., Sanchez T., Claffey K., Mandala S. M., Proia R. L., Hla T. (2007) Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J. Clin. Invest. 117, 2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kono M., Belyantseva I. A., Skoura A., Frolenkov G. I., Starost M. F., Dreier J. L., Lidington D., Bolz S. S., Friedman T. B., Hla T., Proia R. L. (2007) Deafness and stria vascularis defects in S1P2 receptor-null mice. J. Biol. Chem. 282, 10690–10696 [DOI] [PubMed] [Google Scholar]
- 14. Hla T. (2005) Genomic insights into mediator lipidomics. Prostaglandins Other Lipid Mediat. 77, 197–209 [DOI] [PubMed] [Google Scholar]
- 15. Kupperman E., An S., Osborne N., Waldron S., Stainier D. Y. (2000) A sphingosine 1-phosphate receptor regulates cell migration during vertebrate heart development. Nature 406, 192–195 [DOI] [PubMed] [Google Scholar]
- 16. Kai M., Heisenberg C. P., Tada M. (2008) Sphingosine 1-phosphate receptors regulate individual cell behaviours underlying the directed migration of prechordal plate progenitor cells during zebrafish gastrulation. Development 135, 3043–3051 [DOI] [PubMed] [Google Scholar]
- 17. Kawahara A., Nishi T., Hisano Y., Fukui H., Yamaguchi A., Mochizuki N. (2009) The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 323, 524–527 [DOI] [PubMed] [Google Scholar]
- 18. Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995) Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- 19. Lawson N. D., Weinstein B. M. (2002) In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307–318 [DOI] [PubMed] [Google Scholar]
- 20. Chi N. C., Shaw R. M., De Val S., Kang G., Jan L. Y., Black B. L., Stainier D. Y. (2008) Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 22, 734–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blum Y., Belting H. G., Ellertsdottir E., Herwig L., Lüders F., Affolter M. (2008) Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev. Biol. 316, 312–322 [DOI] [PubMed] [Google Scholar]
- 22. Traver D., Paw B. H., Poss K. D., Penberthy W. T., Lin S., Zon L. I. (2003) Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 4, 1238–1246 [DOI] [PubMed] [Google Scholar]
- 23. Matsui T., Raya A., Callol-Massot C., Kawakami Y., Oishi I., Rodriguez-Esteban C., Izpisúa Belmonte J. C. (2007) Miles-apart-mediated regulation of cell-fibronectin interaction and myocardial migration in zebrafish. Nat. Clin. Pract. Cardiovasc. Med. 4, S77-S82 [DOI] [PubMed] [Google Scholar]
- 24. Robu M. E., Larson J. D., Nasevicius A., Beiraghi S., Brenner C., Farber S. A., Ekker S. C. (2007) p53 activation by knockdown technologies. PLoS Genet. 3, e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexander J., Stainier D. Y., Yelon D. (1998) Screening mosaic F1 females for mutations affecting zebrafish heart induction and patterning. Dev. Genet. 22, 288–299 [DOI] [PubMed] [Google Scholar]
- 26. Pedroso G. L., Hammes T. O., Escobar T. D., Fracasso L. B., Forgiarini L. F., da Silveira T. R. (2012) Blood collection for biochemical analysis in adult zebrafish. J. Vis. Exp. 63, e3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hisano Y., Kobayashi N., Yamaguchi A., Nishi T. (2012) Mouse SPNS2 functions as a sphingosine 1-phosphate transporter in vascular endothelial cells. PLoS One 7, e38941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kohno M., Momoi M., Oo M. L., Paik J. H., Lee Y. M., Venkataraman K., Ai Y., Ristimaki A. P., Fyrst H., Sano H., Rosenberg D., Saba J. D., Proia R. L., Hla T. (2006) Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol. Cell. Biol. 26, 7211–7223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langheinrich U., Hennen E., Stott G., Vacun G. (2002) Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr. Biol. 12, 2023–2028 [DOI] [PubMed] [Google Scholar]
- 30. Choi J., Mouillesseaux K., Wang Z., Fiji H. D., Kinderman S. S., Otto G. W., Geisler R., Kwon O., Chen J. N. (2011) Aplexone targets the HMG-CoA reductase pathway and differentially regulates arteriovenous angiogenesis. Development 138, 1173–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amores A., Force A., Yan Y. L., Joly L., Amemiya C., Fritz A., Ho R. K., Langeland J., Prince V., Wang Y. L., Westerfield M., Ekker M., Postlethwait J. H. (1998) Zebrafish hox clusters and vertebrate genome evolution. Science 282, 1711–1714 [DOI] [PubMed] [Google Scholar]
- 32. Meyer A., Van de Peer Y. (2005) From 2R to 3R. Evidence for a fish-specific genome duplication (FSGD). BioEssays 27, 937–945 [DOI] [PubMed] [Google Scholar]
- 33. Taylor J. S., Braasch I., Frickey T., Meyer A., Van de Peer Y. (2003) Genome duplication, a trait shared by 22000 species of ray-finned fish. Genome Res. 13, 382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmelz E. M., Crall K. J., Larocque R., Dillehay D. L., Merrill A. H., Jr. (1994) Uptake and metabolism of sphingolipids in isolated intestinal loops of mice. J. Nutr. 124, 702–712 [DOI] [PubMed] [Google Scholar]
- 35. Im D. S., Clemens J., Macdonald T. L., Lynch K. R. (2001) Characterization of the human and mouse sphingosine 1-phosphate receptor, S1P5 (Edg-8). Structure-activity relationship of sphingosine 1-phosphate receptors. Biochemistry 40, 14053–14060 [DOI] [PubMed] [Google Scholar]
- 36. Schwab S. R., Pereira J. P., Matloubian M., Xu Y., Huang Y., Cyster J. G. (2005) Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 [DOI] [PubMed] [Google Scholar]
- 37. Hla T., Venkataraman K., Michaud J. (2008) The vascular S1P gradient-cellular sources and biological significance. Biochim. Biophys. Acta 1781, 477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pappu R., Schwab S. R., Cornelissen I., Pereira J. P., Regard J. B., Xu Y., Camerer E., Zheng Y. W., Huang Y., Cyster J. G., Coughlin S. R. (2007) Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine 1-phosphate. Science 316, 295–298 [DOI] [PubMed] [Google Scholar]
- 39. Massberg S., Schaerli P., Knezevic-Maramica I., Köllnberger M., Tubo N., Moseman E. A., Huff I. V., Junt T., Wagers A. J., Mazo I. B., von Andrian U. H. (2007) Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 131, 994–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tobia C., Chiodelli P., Nicoli S., Dell'era P., Buraschi S., Mitola S., Foglia E., van Loenen P. B., Alewijnse A. E., Presta M. (2012) Sphingosine 1-phosphate receptor-1 controls venous endothelial barrier integrity in zebrafish. Arterioscler. Thromb. Vasc. Biol. 32, e104–116 [DOI] [PubMed] [Google Scholar]
- 41. Paik J. H., Skoura A., Chae S. S., Cowan A. E., Han D. K., Proia R. L., Hla T. (2004) Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 18, 2392–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gaengel K., Niaudet C., Hagikura K., Siemsen B. L., Muhl L., Hofmann J. J., Ebarasi L., Nyström S., Rymo S., Chen L. L., Pang M. F., Jin Y., Raschperger E., Roswall P., Schulte D., Benedito R., Larsson J., Hellström M., Fuxe J., Uhlén P., Adams R., Jakobsson L., Majumdar A., Vestweber D., Uv A., Betsholtz C. (2012) The sphingosine 1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev. Cell 23, 587–599 [DOI] [PubMed] [Google Scholar]