Abstract
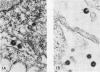
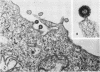
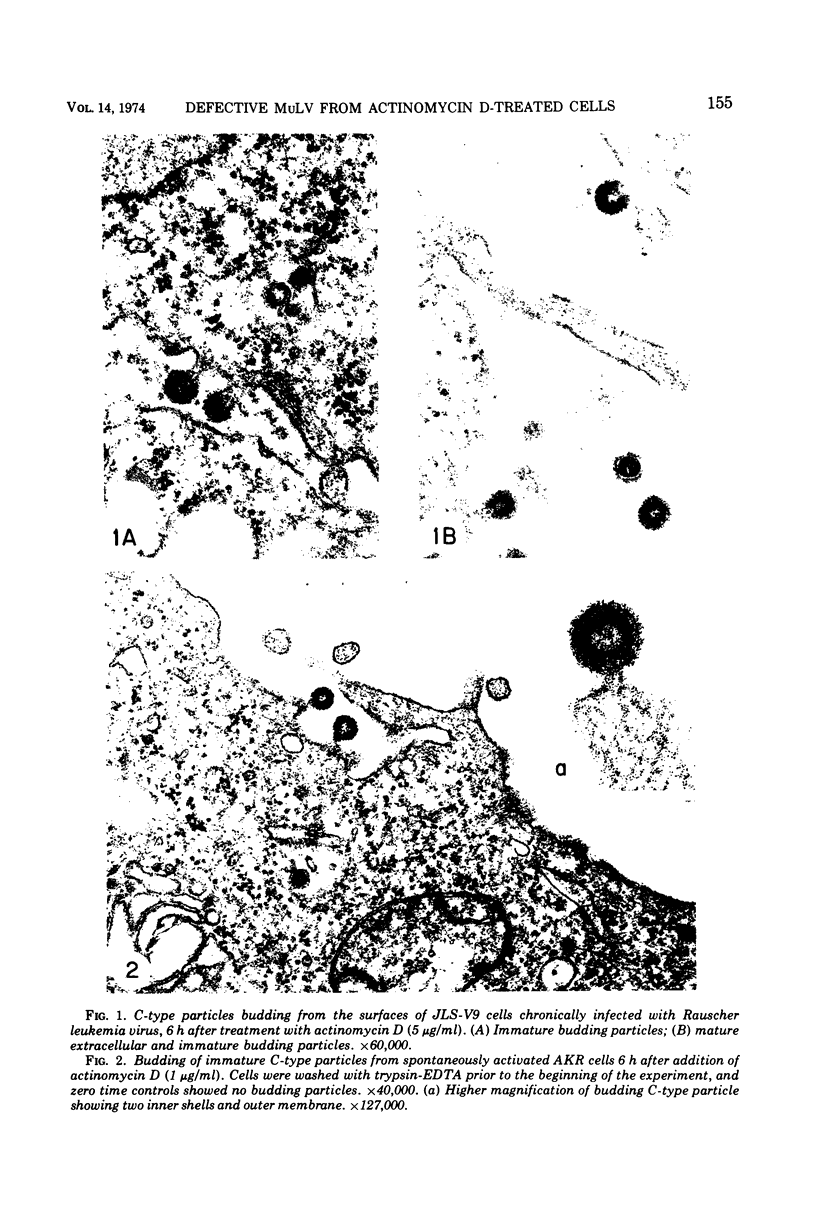
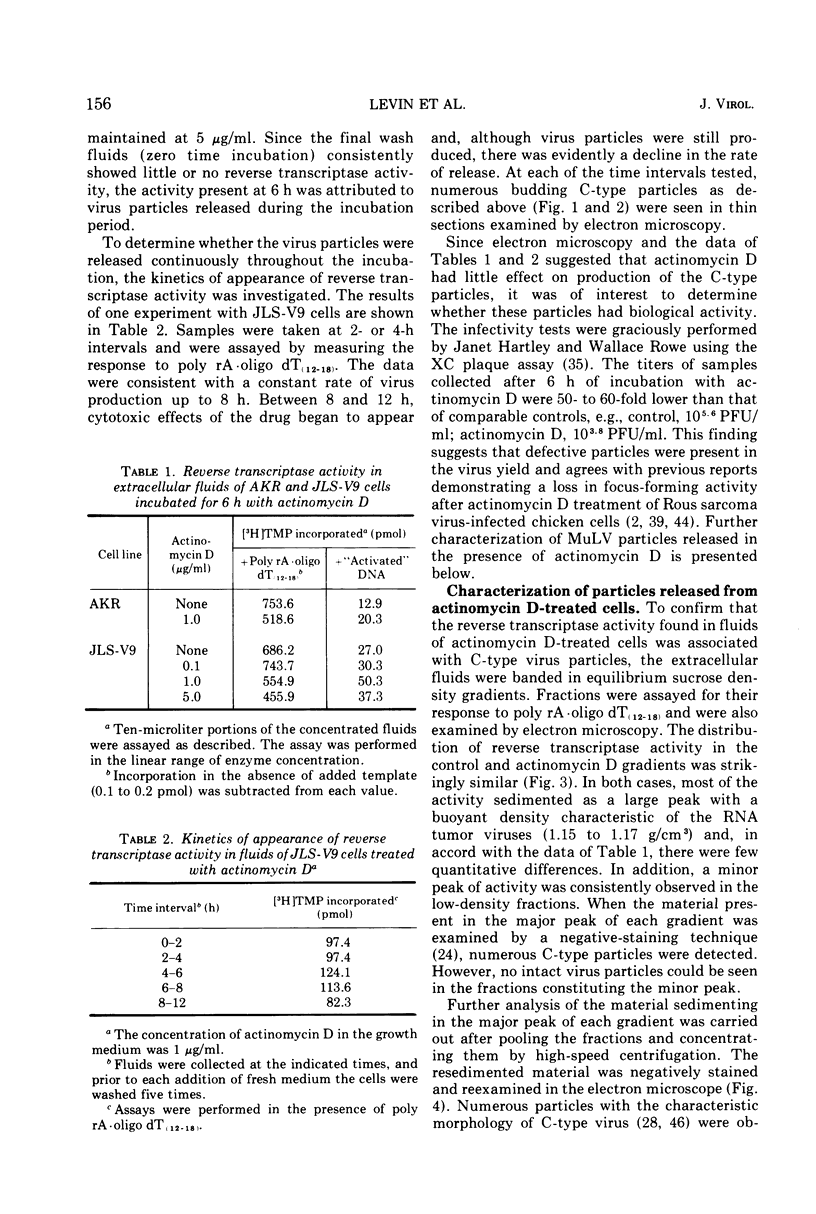
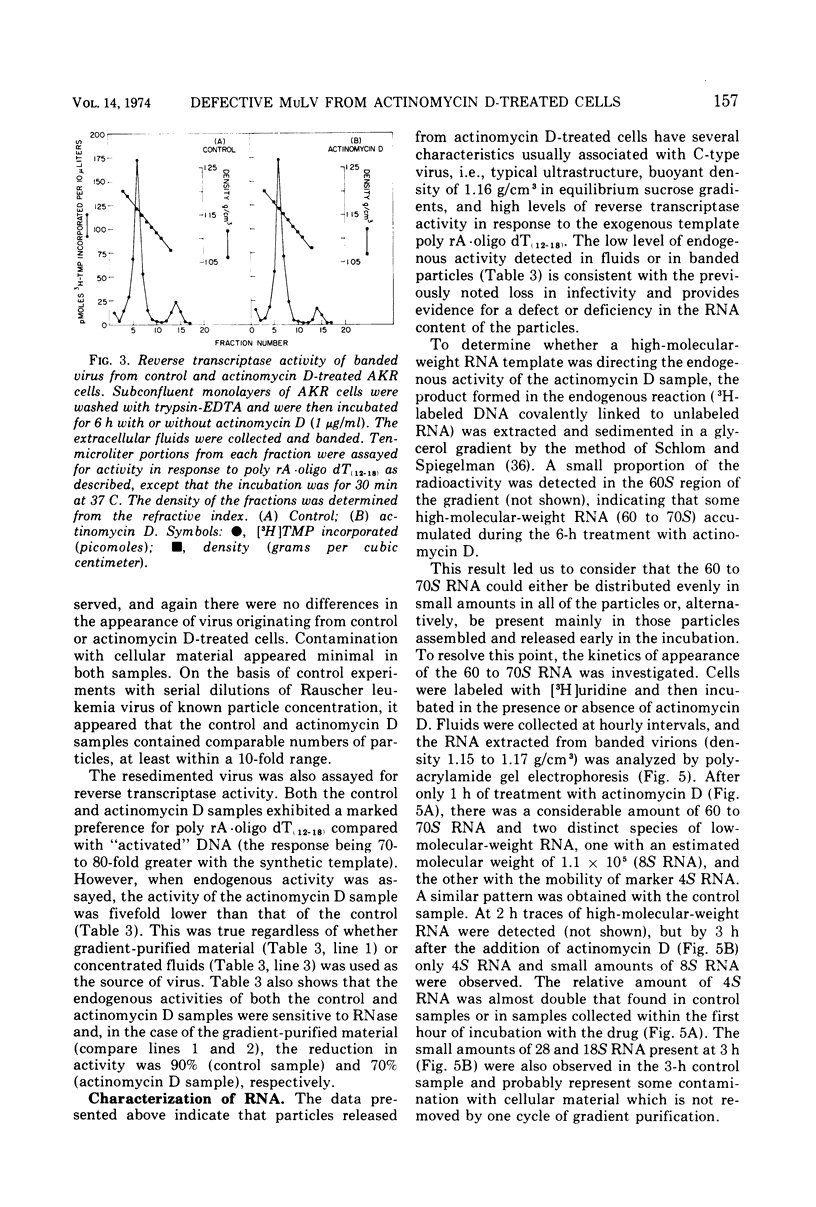
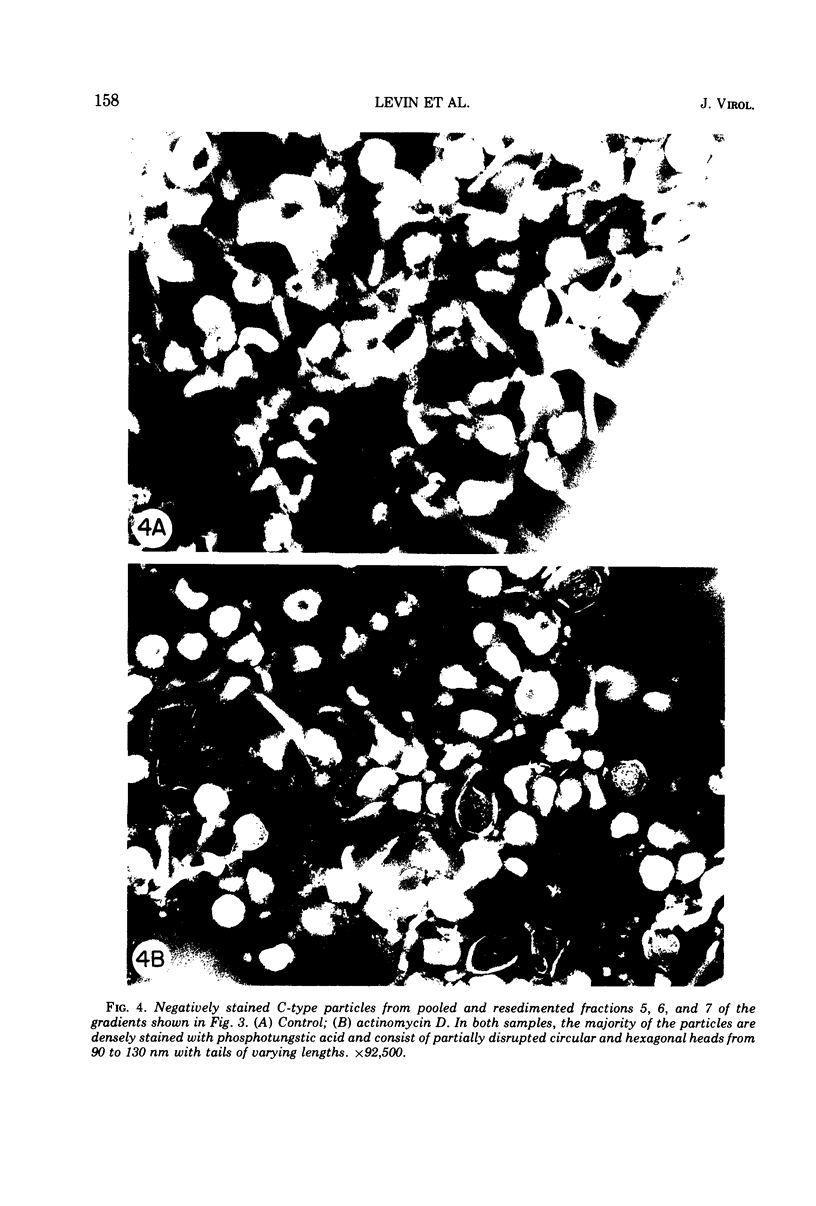
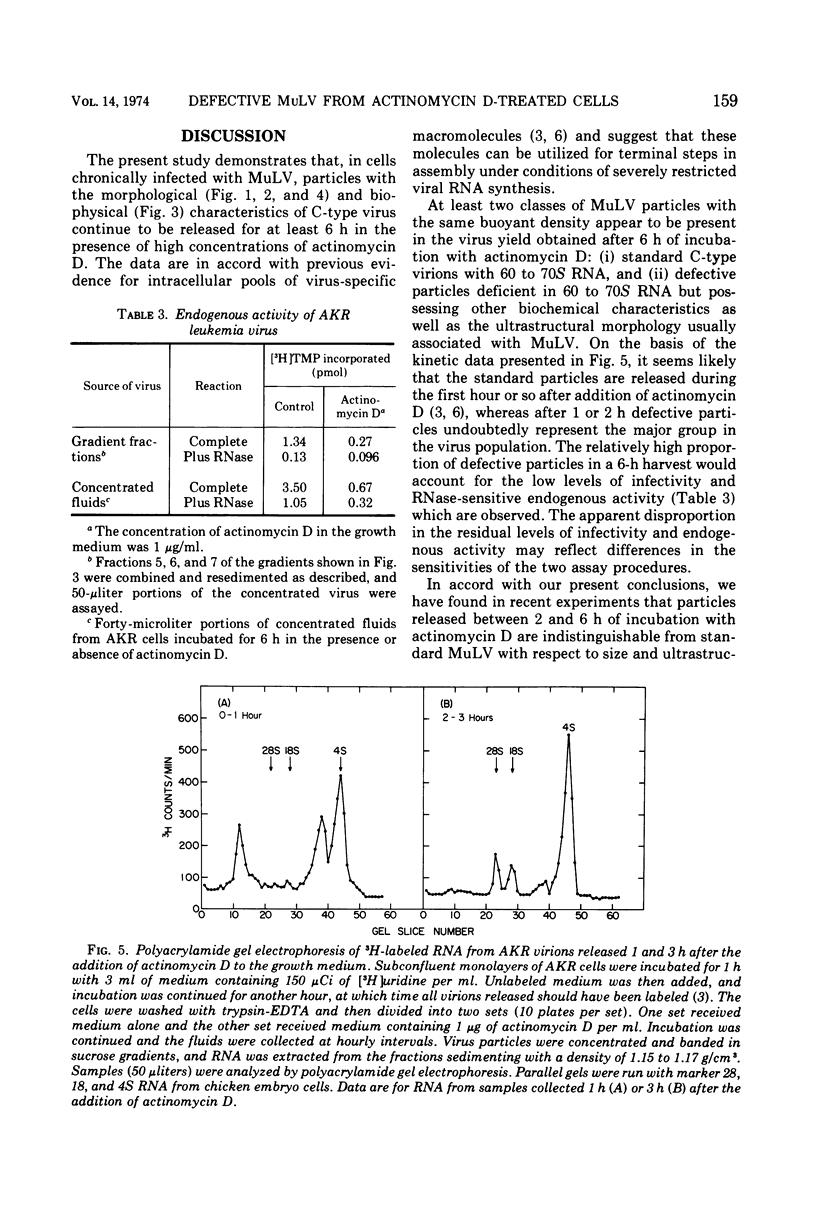
Production of particles with the ultrastructural appearance of C-type virions persisted for at least 6 h in actinomycin D-treated cells infected with murine leukemia virus. This phenomenon occurred despite severe inhibition of viral RNA synthesis. Virus particles present in a 6-h harvest sedimented in sucrose gradients with the buoyant density characteristic of RNA tumor viruses (1.16 g/cm3) and exhibited high levels of reverse transcriptase activity in response to the exogenous template polyriboadenylic acid·oligo deoxythymidylic acid in the range of untreated controls. However, RNase-sensitive endogenous activity was only ⅕ the level found in controls. This observation correlated with a marked reduction in infectivity. Kinetic studies on the appearance of labeled RNA in banded virions revealed that within the first hour after addition of actinomycin D, particles contained 60 to 70S RNA and two low-molecular-weight RNA species corresponding to 8 and 4S RNA. After approximately 1 h of incubation with actinomycin D, 60 to 70S RNA could not be detected and 4S RNA was the predominant species. These findings suggest that murine leukemia virus particles assembled in the presence of actinomycin D are deficient in 60 to 70S viral RNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- BADER J. P. THE ROLE OF DEOXYRIBONUCLEIC ACID IN THE SYNTHESIS OF ROUS SARCOMA VIRUS. Virology. 1964 Apr;22:462–468. doi: 10.1016/0042-6822(64)90067-4. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Synthesis of the RNA of RNA-containing tumor viruses. I. The interval between synthesis and envelopment. Virology. 1970 Mar;40(3):494–504. doi: 10.1016/0042-6822(70)90192-3. [DOI] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. Incorporation of precursors into ribonucleic acid, protein, glycoprotein, and lipoprotein of avian myeloblastosis virions. J Virol. 1969 Nov;4(5):554–566. doi: 10.1128/jvi.4.5.554-566.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bases R. E., King A. S. Inhibition of Rauscher murine leukemia virus growth in vitro by actinomycin D. Virology. 1967 Jun;32(2):175–183. doi: 10.1016/0042-6822(67)90268-1. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Phillips L. A., Kramer M. J., Haapala D. K., Peebles P. T., Nomura S., Fischinger P. J. Transformation of mouse 3T3 cells by murine sarcoma virus: release of virus-like particles in the absence of replicating murine leukemia helper virus. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1520–1524. doi: 10.1073/pnas.68.7.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonar R. A., Sverak L., Bolognesi D. P., Langlois A. J., Beard D., Beard J. W. Ribonucleic acid components of BAI strain A (myeloblastosis) avian tumor virus. Cancer Res. 1967 Jun;27(6):1138–1157. [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnegie J. W., Deeney A. O., Olson K. C., Beaudreau G. S. An RNA fraction from myeloblastosis virus having properties similar to transfer RNA. Biochim Biophys Acta. 1969 Oct 22;190(2):274–284. doi: 10.1016/0005-2787(69)90079-3. [DOI] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Inhibition of mouse leukemia virus (MLV) replication by actinomycin D. Virology. 1967 Apr;31(4):742–746. doi: 10.1016/0042-6822(67)90211-5. [DOI] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Association of 4S ribonucleic acid with oncornavirus ribonucleic acids. J Virol. 1971 Aug;8(2):254–256. doi: 10.1128/jvi.8.2.254-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Isolation of amino acid acceptor RNA from purified avian myeloblastosis virus. J Mol Biol. 1970 Sep 14;52(2):387–390. doi: 10.1016/0022-2836(70)90038-0. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., Levinson W. E., Goodman H. M., Bishop J. M. RNA-directed DNA polymerase of Rous sarcoma virus: initiation of synthesis with 70 S viral RNA as template. J Mol Biol. 1973 Sep 5;79(1):163–183. doi: 10.1016/0022-2836(73)90277-5. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Protein synthesis directed by an arbovirus. J Virol. 1968 Jan;2(1):26–32. doi: 10.1128/jvi.2.1.26-32.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., Varmus H. E., Faras A. J., Levinson W. E., Bishop J. M. RNA-directed DNA synthesis by virions of Rous sarcoma virus: further characterization of the templates and the extent of their transcription. Virology. 1973 Mar;52(1):264–274. doi: 10.1016/0042-6822(73)90414-5. [DOI] [PubMed] [Google Scholar]
- Goodman N. C., Spiegelman S. Distinguishing reverse transcriptase of an RNA tumor virus from other known DNA polymerases. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2203–2206. doi: 10.1073/pnas.68.9.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P. M., Berezesky I. K., Levin J. G. Morphogenesis of C-type and A-type particles in cells infected by an arbovirus. J Natl Cancer Inst. 1973 Jan;50(1):275–279. doi: 10.1093/jnci/50.1.275. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Complement fixation and tissue culture assays for mouse leukemia viruses. Proc Natl Acad Sci U S A. 1965 May;53(5):931–938. doi: 10.1073/pnas.53.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelloff G. J., Hatanaka M., Gilden R. V. Assay of C-type virus infectivity by measurement of RNA-dependent DNA polymerase activity. Virology. 1972 Apr;48(1):266–269. doi: 10.1016/0042-6822(72)90135-3. [DOI] [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Ramseur J. M., Grimley P. M. Host effect on arbovirus replication: appearance of defective interfering particles in murine cells. J Virol. 1973 Dec;12(6):1401–1406. doi: 10.1128/jvi.12.6.1401-1406.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Kilham S. S. An electron microscope study of Rauscher leukemia virus. Virology. 1971 Nov;46(2):277–297. doi: 10.1016/0042-6822(71)90030-4. [DOI] [PubMed] [Google Scholar]
- Nichols J. L., Waddell M. Comparison of free and 80S RNA-associated RNAs of mouse L cell virions. Nat New Biol. 1973 Jun 20;243(129):236–238. doi: 10.1038/newbio243236a0. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Ross J., Todaro G. J., Aaronson S. A. Immunological relationships of reverse transcriptases from ribonucleic acid tumor viruses. J Virol. 1972 Jan;9(1):110–115. doi: 10.1128/jvi.9.1.110-115.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L. A., Hollis V. W., Jr, Bassin R. H., Fischinger P. J. Characterization of RNA from noninfectious virions produced by sarcoma positive-leukemia negative transformed 3T3 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):3002–3006. doi: 10.1073/pnas.70.10.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert M. S., Smith R. G., Gallo R. C., Sarin P. S., Abrell J. W. Viral and cellular DNA polymerase: comparison of activities with synthetic and natural RNA templates. Science. 1972 May 19;176(4036):798–800. doi: 10.1126/science.176.4036.798. [DOI] [PubMed] [Google Scholar]
- Ross J., Scolnick E. M., Todaro G. J., Aaronson S. A. Separation of murine cellular and murine leukaemia virus DNA polymerases. Nat New Biol. 1971 Jun 9;231(23):163–167. doi: 10.1038/newbio231163a0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Lander M. R., Pugh W. E., Teich N. Noninfectious AKR mouse embryo cell lines in which each cell has the capacity to be activated to produce infectious murine leukemia virus. Virology. 1971 Dec;46(3):866–876. doi: 10.1016/0042-6822(71)90087-0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science. 1971 Nov 19;174(4011):840–843. doi: 10.1126/science.174.4011.840. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Reynolds R. K., Aaronson S. A. Isolation of temperature-sensitive mutants of murine leukemia virus. Virology. 1972 Jun;48(3):749–756. doi: 10.1016/0042-6822(72)90158-4. [DOI] [PubMed] [Google Scholar]
- TAYLOR J. STUDIES ON THE MECHANISM OF ACTION OF INTERFERON. I. INTERFERON ACTION AND RNA SYNTHESIS IN CHICK EMBRYO FIBROBLASTS INFECTED WITH SEMLIKI FOREST VIRUS. Virology. 1965 Mar;25:340–349. doi: 10.1016/0042-6822(65)90053-x. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. THE EFFECTS OF ACTINOMYCIN D ON GROWTH OF ROUS SARCOMA VIRUS IN VITRO. Virology. 1963 Aug;20:577–582. doi: 10.1016/0042-6822(63)90282-4. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. THE PARTICIPATION OF DNA IN ROUS SARCOMA VIRUS PRODUCTION. Virology. 1964 Aug;23:486–494. doi: 10.1016/0042-6822(64)90232-6. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Trávnícek M. RNA with amino acid-acceptor activity isolated from an oncogenic virus. Biochim Biophys Acta. 1968 Oct 29;166(3):757–759. [PubMed] [Google Scholar]
- VIGIER P., GOLDE A. EFFECTS OF ACTINOMYCIN D AND OF MITOMYCIN C ON THE DEVELOPMENT OF ROUS SARCOMA VIRUS. Virology. 1964 Aug;23:511–519. doi: 10.1016/0042-6822(64)90235-1. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Bromfeld E., Manly K. F., Baltimore D. Covalently linked RNA-DNA molecule as initial product of RNA tumour virus DNA polymerase. Nat New Biol. 1971 Sep 29;233(39):131–134. doi: 10.1038/newbio233131a0. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]
- ZEIGEL R. F., RAUSCHER F. J. ELECTRON MICROSCOPIC AND BIOASSAY STUDIES ON A MURINE LEUKEMIA VIRUS (RAUSCHER). I. EFFECTS OF PHYSICOCHEMICAL TREATMENTS ON THE MORPHOLOGY AND BIOLOGICAL ACTIVITY OF THE VIRUS. J Natl Cancer Inst. 1964 Jun;32:1277–1307. doi: 10.1093/jnci/32.6.1277. [DOI] [PubMed] [Google Scholar]